Abstract
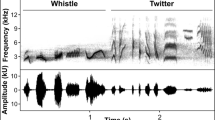
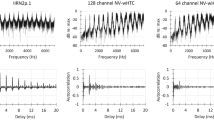
We examined temporal processing of harmonic tone complexes in two woodland species (tufted titmice and white-breasted nuthatches) and two open-habitat species (house sparrows and white-crowned sparrows). Envelope and fine-structure processing were quantified using the envelope following response (EFR) and frequency following response (FFR). We predicted stronger EFRs in the open-habitat species based on broader auditory filters and greater amplitude modulation of vocal signals in this group. We predicted stronger FFRs in woodland species based on narrower auditory filters. As predicted, EFR amplitude was generally greatest in the open habitat species. FFR amplitude, in contrast, was greatest in white-crowned sparrows with no clear difference between habitats. This result cannot be fully explained by species differences in audiogram shape and might instead reflect greater acoustic complexity of songs in the white-crowned sparrow. Finally, we observed stronger FFRs in woodland species when tones were broadcast with the next higher harmonic in the complex. Thus, species such as nuthatches that have songs with strong harmonics may process these sounds using enhanced spectral processing instead of enhanced amplitude-envelope processing. The results suggest coevolution between signal design and temporal processing of complex signals and underscore the need to study auditory processing with a diversity of signals.





Similar content being viewed by others
Abbreviations
- ABR:
-
Auditory brainstem response
- AEP:
-
Auditory evoked potential
- EFR:
-
Envelope following response
- FFR:
-
Frequency following rate
- AM:
-
Amplitude modulation
References
Airey DC, Buchanan KL, Székeley T, Catchpole CK, DeVoogd TJ (2000) Song, sexual selection, and a song control nucleus (HVc) in the brains of European sedge warblers. J Neurobiol 44:1–6
Boersma P, Weenink D (2014) Praat: doing phonetics by computer [Computer program]. Version 5.1.07. Retrieved from http://www.praat.org/
Boston JR, Møller AR (1985) Brainstem auditory evoked potentials. Crit Rev Biomed Engineer 13:97–123
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer, Sunderland
Brittan-Powell EF, Dooling RJ (2004) Development of auditory sensitivity in budgerigars (Melopsittacus undulatus). J Acoust Soc Am 15:3092–3102
Brittan-Powell EF, Dooling RJ, Gleich O (2002) Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus). J Acoust Soc Am 112:999–1008
Brown-Borg HM, Beck MM, Jones TA (1987) Origin of peripheral and brainstem auditory responses in the white leghorn chick. Comp Biochem Physiol 88A:391–396
Canady RA, Kroodsma DE, Nottebohm F (1984) Population differences in complexity of a learned skill are correlated with the brain space involved. Proc Natl Acad Sci USA 81:6232–6234
Caras ML, Brenowitz E, Rubel EW (2010) Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol A 196:581–599
Catchpole C, Slater PJB (1995) Bird song: biological themes and variations. Cambridge University Press, New York
de Boer E, Kruidenier C (1990) On ringing limits of the auditory periphery. Biol Cybern 63:433–442
DeVoogd TJ, Krebs JR, Healy SD, Purvis A (1993) Relations between sing repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc R Soc Lond B 254:75–82
Dooling RJ (1982) Auditory perception in birds. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds. Academic Press, New York, pp 95–130
Dooling RJ, Lohr B, Dent ML (2000) Hearing in birds and reptiles. In: Dooling RJ, Popper AN, Fay RR (eds) Comparative hearing: birds and reptiles. Springer, New York, pp 308–359
Dooling RJ, Leek MR, Gleich O, Dent ML (2002) Auditory temporal resolution in birds: discrimination of harmonic complexes. J Acoust Soc Am 112:748–759
Elliot L, Stokes D, Stokes L (1997) Stokes field guide to bird songs: Eastern Region. Time Warner Audio Books (compact disc), New York
Farabaugh SM, Dooling RJ (1996) Acoustic communication in parrots: laboratory and field studies of budgerigars, Melopsittacus undulatus. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Cornell University Press, Ithaca, pp 97–117
Fletcher H (1940) Auditory patterns. Rev Modern Phys 12:47–65
Gall MD, Brierley LE, Lucas JR (2011) Species and sex effects on auditory processing in brown-headed cowbirds and red-winged blackbirds. Anim Behav 81:973–982
Gall MD, Brierley LE, Lucas JR (2012a) The sender-receiver matching hypothesis: support from the peripheral coding of acoustic features in songbirds. J Exp Biol 215:3742–3751
Gall MD, Henry KS, Lucas JR (2012b) Two measures of temporal resolution in brown-headed cowbirds (Molothrus ater). J Comp Physiol A 198:61–68
Gall MD, Salameh TS, Lucas JR (2013) Songbird frequency selectivity and temporal resolution vary with sex and season. Proc R Soc B 280:20122296
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hall JW III (2007) Handbook of auditory-evoked responses. Allyn and Bacon, Boston
Henry KR (1997) Sharply tuned cochlear nerve ensemble periodicity responses to sonic and ultrasonic frequencies. J Comp Physiol A 181:239–246
Henry KS, Lucas JR (2008) Coevolution of auditory sensitivity and temporal resolution with acoustic signal space in three songbirds. Anim Behav 76:1659–1671
Henry KS, Lucas JR (2010) Habitat-related differences in the frequency selectivity of auditory filters in songbirds. Funct Ecol 24:614–624
Henry KS, Gall MD, Bidelman G, Lucas JR (2011) Songbirds trade off auditory frequency resolution and temporal resolution. J Comp Physiol A 197:351–359
Konishi M (1970) Comparative neurophysiological studies of hearing and vocalizations in songbirds. Zeitschrift Vergleichende Physiol 66:257–272
Krishnan A (2002) Human frequency-following responses: representation of steady-state synthetic vowels. Hear Res 166:192–201
Kubke MF, Massoglia DP, Carr CE (2004) Bigger brains or bigger nuclei? Regulating the size of auditory structures in birds. Brain Behav Evol 63:169–180
Lohr B, Dooling RJ (1998) Detection of changes in timbre and harmonicity in complex sounds by zebra finches (Taeniopygia guttata) and budgerigars (Melopsittacus undulatus). J Comp Psychol 112:36–47
Lohr B, Wright TF, Dooling RJ (2003) Detection and discrimination of natural calls in masking noise by birds: estimating the active space of a signal. Anim Behav 65:763–777
Lucas JR, Peterson LJ, Boudinier RL (1993) The effects of time constraints and changes in body mass and satiation on the simultaneous expression of caching and diet-choice decisions. Anim Behav 45:639–658
Lucas JR, Freeberg TM, Krishnan A, Long GR (2002) A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A 188:981–992
Lucas JR, Freeberg TM, Long GR, Krishnan A (2007) Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A 192:201–215
Marler P, Tamura M (1962) Song “dialects” in three populations of white-crowned sparrows. Condor 64:368–377
McIver EL, Marchaterre MA, Rice AN, Bass AH (2014) Novel underwater soundscape: acoustic repertoire of plainfin midshipman fish. J Exp Biol 217:2377–2389
Møller AR (2006) Hearing: anatomy, physiology, and disorders of the auditory system, 2nd edn. Academic Press, Amsterdam
Moore BCJ (1993) Frequency analysis and pitch perception. In: Yost WA, Popper AN, Fay RR (eds) Human psychophysics. Springer, New York, pp 58–89
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Nelson DA, Marler P (1990) The perception of birdsong and an ecological concept of signal space. In: Stebbins WC, Berkley MA (eds) Comparative perception, vol II., Complex signalsWiley, New York, pp 443–478
Nottebohm F, Kasparian S, Pandazis C (1981) Brain space for a learned task. Brain Res 213:99–109
Okanoya K, Dooling RJ (1990) Detection of gaps in noise by budgerigars (Melopsittacus undulatus) and zebra finches (Poephila guttata). Hear Res 50:185–192
Owens JL, Freeberg TM (2007) Variation in chick-a-dee calls of tufted titmice, Baeolophus bicolor: note type and individual distinctiveness. J Acoust Soc Am 122:1216–1226
Pyle P (1997) Identification guide to North American birds. Slate Creek Press, Bolinas
Ramsier MA, Cunningham AJ, Finneran JJ, Dominy NJ (2012) Social drive and the evolution of primate hearing. Phil Trans R Soc B 367:1860–1868
Ritchison G (1983) Vocalizations of the white-breasted nuthatch. Wilson Bull 95:440–451
Schneider DM, Woolley SMN (2011) Extra-classical tuning predicts stimulus-dependent receptive fields in auditory neurons. J Neurosci 31:11867–11878
Simmons AM, Buxbaum RC (1996) Neural cues for “pitch” processing in a unique vertebrate auditory system. In: Moss CF, Shettleworth SJ (eds) Neuroethological studies of cognitive and perceptual processes. Westview Press, Boulder CO, pp 185–228
Sisneros JA, Bass AH (2003) Seasonal plasticity of peripheral auditory frequency selectivity. J Neurosci 23:1049–1058
Smith ZM, Delgutte B, Oxenham AJ (2002) Chimeric sounds reveal dichotomies in auditory perception. Nature 416:87–90
SzékeleyT Catchpole CK, DeVoogd A, Marchl Z, DeVoogd TJ (1996) Evolutionary changes in a song control area of the brain (HVC) are associated with evolutionary changes in song repertoire among European warblers (Sylviidae). Proc R Soc Lond B 263:607–610
Szymanski MD, Bain DE, Kiehl K, Pennington S, Wong S, Henry KR (1999) Killer whale (Orcinus orca) hearing: auditory brainstem response and behavioral audiograms. J Acoust Soc Am 106:1134–1141
Tchernichovski O, Nottebohm F, Ho CE, Bijan P, Mitra PP (2000) A procedure for an automated measurement of song similarity. Anim Behav 59:1167–1176
Theunissen FE, Doupe AJ (1998) Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. J Neurosci 18:3786–3802
Vélez A, Gall MD, Fu J, Lucas JR (2015) Song structure, not high-frequency song content, determines high-frequency auditory sensitivity in nine species of New World sparrows (Passeriformes: Emberizidae). Funct Ecol
Vernaleo BA, Dooling RJ (2011) Relative salience of envelope and fine structure cues in zebra finch song. J Acoust Soc Am 129:3373–3383
Viemeister NF, Plack CJ (1993) Time analysis. In: Yost WA, Popper AN, Fay RR (eds) Human psychophysics. Springer, New York, pp 116–154
Wiley RH (1991) Associations of song properties with habitats for territorial oscine birds of eastern North America. Am Nat 138:973–993
Wilkins MR, Seddon N, Safran RJ (2013) Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol Evol 28:156–166
Woolley SMN, Portfors CV (2013) Conserved mechanisms of vocalization coding in mammalian and songbird auditory midbrain. Hear Res 305:45–56
Zann R (1984) Structural variation in the zebra finch distance call. Zeitschrift Tierpsychol. 66:328–345
Acknowledgments
Thanks to Lauren Brierley, Kerry Fanson, Mark Nolen, and Aravindakshan Parthasarathy for reviewing the manuscript. Ravi Krishnan lent us the TDT II and sound level meter. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (Purdue University IACUC no. 05-058). This study was funded by the National Science Foundation [NSF IOS 1121728 to J.R.L.].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lucas, J.R., Vélez, A. & Henry, K.S. Habitat-related differences in auditory processing of complex tones and vocal signal properties in four songbirds. J Comp Physiol A 201, 395–410 (2015). https://doi.org/10.1007/s00359-015-0986-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-015-0986-7