Abstract
In mammals, the membrane-based protein Prestin confers unique electromotile properties to cochlear outer hair cells, which contribute to the cochlear amplifier. Like mammals, the ears of insects, such as those of Drosophila melanogaster, mechanically amplify sound stimuli and have also been reported to express Prestin homologs. To determine whether the D. melanogaster Prestin homolog (dpres) is required for auditory amplification, we generated and analyzed dpres mutant flies. We found that dpres is robustly expressed in the fly’s antennal ear. However, dpres mutant flies show normal auditory nerve responses, and intact non-linear amplification. Thus we conclude that, in D. melanogaster, auditory amplification is independent of Prestin. This finding resonates with prior phylogenetic analyses, which suggest that the derived motor function of mammalian Prestin replaced, or amended, an ancestral transport function. Indeed, we show that dpres encodes a functional anion transporter. Interestingly, the acquired new motor function in the phylogenetic lineage leading to birds and mammals coincides with loss of the mechanotransducer channel NompC (=TRPN1), which has been shown to be required for auditory amplification in flies. The advent of Prestin (or loss of NompC, respectively) may thus mark an evolutionary transition from a transducer-based to a Prestin-based mechanism of auditory amplification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hearing is a physiologically delicate process. The sensitivity of vertebrate ears results from the intricate and unique molecular transduction machinery that resides in the hair bundles of their sensory hair cells. Within the bundles, the interplay between force-gated transducer channels and adaptation motors generates forces that amplify sound-induced bundle movements and thereby boost vertebrate auditory performance while in mammals, an additional amplification mechanism is provided by a Prestin-mediated electromotile activity (Hudspeth 2008). Understanding the molecular, and mechanistic, details of auditory transduction is essential for understanding the bases of hearing and the causes of deafness.
In mammals, the exquisite sensitivity and frequency selectivity is achieved through an active amplification of sound-evoked vibrations of the basilar membrane (BM) within the cochlea. BM vibrations deflect specialized microvilli, or stereocilia, of inner ear hair cells and thereby directly open mechano-electric transducer channels (Fettiplace and Hackney 2006; Peng et al. 2011). Two types of auditory hair cells exist, both of which harbor mechanosensory transducer channels: (1) inner hair cells (IHCs) represent the ‘sensory arm’ of cochlear function and send the auditory information, created by the BM vibrations, to the brain; (2) outer hair cells (OHCs) represent the ‘motor arm’ of cochlear function and respond to transduction-induced changes in membrane potential by contractions and expansions of their cell bodies, thereby amplifying the BM vibrations. The molecular motor responsible for this electromotility of OHCs is Prestin, a member of the SLC26 anion transporter family (Dallos and Fakler 2002). Loss of Prestin in mice raises the threshold at which they respond to sound, and a mutation in Prestin may cause hereditary hearing loss in humans (Liberman et al. 2002; Liu et al. 2003).
Like vertebrate hair cells, the auditory neurons of the fruit fly D. melanogaster are endowed with an active, force-generating process that involves mechanically gated transducer channels and adaptation motors acting in concert to boost hearing (Göpfert et al. 2005; Nadrowski et al. 2008). The macroscopic performance of the entire D. melanogaster ear is governed by the properties of these active transducer modules, and the fly’s external antennal sound receiver can be used to directly probe auditory transducer function in vivo in both wild-type and mutant flies (Albert et al. 2007; Kamikouchi et al. 2010).
D. melanogaster responds to sound through the Johnston’s organ (JO), which is made up of an array of ciliated neurons in function analogous to the IHCs of the mammalian cochlea (Eberl 1999). The motor mechanism by which sound-induced vibrations are actively amplified by the JO is unknown but the origin of this amplification is attributable to JO neurons (Göpfert et al. 2005). JO does not have OHCs to amplify vibrations. Instead, the dendritic cilia of JO neurons may contract to boost the vibratory response to faint sounds (Göpfert and Robert 2002; Kernan 2007; Lee et al. 2008). A transducer-based, quantitative model, which assumes that (as yet molecularly unknown) motor proteins act in series with the actual transducer channels, was found to explain the mechanical and electrical responses of the fly’s ear to small disturbances (Nadrowski et al. 2008). An alternative hypothesis as to how JO neurons might amplify sound is through a Prestin-based electromotility. In this hypothesis, the D. melanogaster homolog Prestin (dpres) may contribute to ciliary motility by causing contraction in the membranes of the JO neurons.
The possibility that dpres contributes to transducer-based active amplification of the sound-induced vibrations has escaped rigorous molecular evolutionary analysis of Prestin orthologs in representative species which suggested that Prestin-based electromotility exists only in mammals (Okoruwa et al. 2008; Tan et al. 2011). In fact, specific residues have been identified as being essential for Prestin’s evolution from an anion transporter to a protein with electromotility responsible for OHC electromotility necessary for sound amplification (Schaechinger et al. 2011; Tan et al. 2011). New research, however, questions the time at which this electromotility evolved in metazoans. While an early study in chickens revealed no Prestin-based electromotility (He et al. 2003), a more recent study showed Prestin-like signatures in chicken short hair cell stiffness (orthologous function to mammalian outer hair cells), active amplification and frequency tuning of the auditory organ (Beurg et al. 2013). This suggests that the amplifying function of Prestin could be more highly conserved than previously supposed.
The goal of this study was to determine whether dpres is necessary for non-linear mechanical amplification in the hearing organ of D. melanogaster. This was accomplished by examining the expression pattern of dpres, and by analyzing the phenotypes of newly generated dpres loss-of-function mutants. The results demonstrate that dpres is expressed in JO neurons yet is not required for auditory amplification in D. melanogaster. Instead, dpres function may be restricted to the role as anion antiporter, consistent with previous reports (Hirata et al. 2012a, b).
Methods
Fly lines
The D. melanogaster strains carrying w 1118, dpres-Gal4, and dpres mutants (engineered from homologous recombination and P-element mobilization described below) were used in the analysis. All flies were raised at 25 °C and 60 % humidity.
Expression analysis
The 5′ regulatory sequences of dpres were cloned into the pPTGal vector (Sharma et al. 2002) and injected into w 1118 embryos to produce a transgenic line. The resulting line was crossed to a UAS-EGFP reporter to determine the dpres expression pattern.
Homologous recombination
We used an ends-out targeting approach using the homologous recombination techniques in D. melanogaster (Rong and Golic 2000, 2001). First, PCR-based site-directed mutagenesis using mutant primers was used to engineer two in-frame stop codons each in the first and third exons of the dpres gene (Fig. 1b, asterisks; Fig. S1). An SceI cut site was created by overlapping oligonucleotides and inserted into a BamHI site between the engineered mutations, approximately 500 bp from each to avoid loss of the mutations due to degradation from the cut site (Rong et al. 2002). The mutant dpres construct was ligated into the pTV2 NotI plasmid. pTV2 contains the sequence necessary for insertion of the P-element into the genome, FRT sites for excision of the circular donor DNA molecule, and a modified w + gene (w hs) for tracking of the insertion.
Generation of mutations in dpres. a The genomic organization of the dpres gene on the left arm of chromosome 3 at polytene chromosome position 75A9. Gene annotations (blue) are shown below the sequence coordinates (top line) of 3L, with flanking genes Tsp74F and CG14353. The dpres gene (shown as Prestin) is encoded on the minus strand with the transcript structure shown below (coding exons in orange, untranslated regions in gray). The sites of P-element insertions EY04256 and KG05103 are shown as triangles, and extent of the dpres 51C deletion derived from an imprecise excision of KG05103 is shown by the labeled black line. The location of the lesion in homologous recombination allele dpres 339 is shown as a triangle with the w hs insertion (red box). The extent of the enhancer fragment used in generating the dpres-Gal4 line is also depicted. The upper part of the panel was obtained from GBrowse, accessed through Flybase. b Transcript structure of dpres in wild type and homologous recombination mutant. The wild-type transcript contains all four coding exons (colored) and encodes a 742 amino acid protein. Ends-out homologous recombination generated an insertion of the w hs element (providing w+ eye color), with flanking duplications of the 3′ end of exon 3 and the 5′ end of exon 3 (black asterisks) containing engineered stop codons. RT-PCR amplified several dpres transcripts, none of which are wild type. Splicing past the w hs insert causes a frame shift leading to tandem stop codons near the 3′ end of exon 2 (red asterisks)
The construct was injected into fly embryos to make a transgenic line that was then crossed to FLP, SceI for targeted mutagenesis. Transgenic larvae were heat-shocked at 37 °C for 30 min to produce a circularized DNA element that recombined with dpres. Stable lines were produced from the progeny and screened using PCR to identify lines with a disrupted dpres gene. Line 339 was confirmed as a dpres mutant by long-template PCR and therefore renamed dpres 339.
P-element mobilization
A P-element transposase line [Dr, P(Δ2–3)] was crossed to the P-element insertion lines KG05103 and EY04256 to generate EY04256 Dr, P(Δ2–3/KG05103 dysgenic flies, which were then crossed to CxD/TM3. Offspring were collected to create stable lines that were screened by PCR to identify imprecise excisions that removed dpres. Of the 101 balanced lines isolated, 92 were homozygous viable. Using PCR on 72 viable or lethal lines, we determined that one viable line, 51C, was a dpres deficiency spanning from the KG05103 insertion site past exon two (Fig. 1).
RT-PCR
RT-PCR primers were designed against the dpres gene. PCR was performed on mRNA from late-stage embryos, larvae and adults to determine expression pattern of dpres mRNA from controls and homologous recombinants.
Electrophysiology
Electrophysiology was performed on the antenna with methods described by Eberl et al. (2000). Briefly, the fly was immobilized in a pipette tip trimmed to allow the head to protrude. The pulse song, a component of the fly’s courtship song, was played on a loudspeaker and delivered to the fly through Tygon tubing to preserve near-field sound properties. The sound stimulus intensity was measured at 5.3 mm/s at the position of the antennae, using a calibrated Emkay NR3158 particle velocity microphone (Knowles). One electrolytically sharpened tungsten electrode was inserted in the junction between the first and second antennal segments while a second electrode penetrated the dorsal head cuticle as a reference. The signals were subtracted with a DAM50 differential amplifier (World Precision Instruments) and then digitized and normalized using a virtual instrument designed with Superscope 3.0 (G. W. Instruments) software. Responses to ten presentations of the stimulus were averaged, and the peak amplitudes of sound-evoked potentials (SEPs) were measured.
Confocal microscopy
Confocal microscopy was performed using a Zeiss LSM510 microscope. Whole mount flies were prepared by immersing in 50 % glycerol and mounting on depression slides. Antennae were visualized by dissection and staining with rhodamine phalloidin.
Laser Doppler vibrometry
Adult D. melanogaster aged 1–5 days after eclosion were mounted on a Teflon rod and immobilized using dental glue. A laser Doppler vibrometer (LDV; PSV-400 with an OFV-70 close-up unit and a DD-5000 displacement decoder, Polytec GmbH, Waldbronn, Germany) was used to record the oscillations of the arista. Signals were generated and recorded in the PSV 8.6 Scanning Vibrometer Software (GmbH, Waldbronn, Germany) and later processed in Sigmaplot (Systat Software Inc) to extract best frequencies and energy gain (more details in Göpfert et al. 2005). Mutant and control data were tested for normality (Shapiro–Wilk) and equal variance (rejection thresholds set to p < 0.05 in both cases) prior to further statistical analysis. All data presented here passed both the normality and equal variance test and were analyzed using a two-tailed t test.
Anion transport measurements
A dpres-GFP fusion construct (in pEGFP-N2 vector) was transfected into CHO cells using JetPEI transfection reagent (Polyplus). Patch-clamp recordings of electrogenic transport currents were performed as described (Schaechinger and Oliver 2007; Schaechinger et al. 2011). Briefly, cells with unequivocal membrane fluorescence were selected (24–48 h after transfection). Whole-cell patch-clamp recordings were carried out at room temperature (20–22 °C) with an EPC10 amplifier (HEKA electronics, Lambrecht, Germany) controlled by Patchmaster software (HEKA). Electrogenic anion transport was measured as the ionic transport current in response to command voltage ramps (−100 to +100 mV; 300 ms). Background currents recorded before application of extracellular transport substrates were subtracted from current traces. For comparison between cells, current densities were calculated by normalizing to whole-cell capacitance as a measure of membrane area.
Patch electrodes were filled with intracellular solution containing 160 mM CsCl, 1 mM HEPES, 1 mM K2EGTA, adjusted to pH 7.3 with KOH. Extracellular transport substrates were locally applied to the cells via a gravity-fed application capillary. Extracellular solution contained 10 mM NaCl, 150 mM Na-gluconate, 10 mM HEPES, and 2 mM Mg-gluconate, adjusted to pH 7.4 with NaOH. For extracellular application of divalent anions, 10 mM SO4 or oxalate substituted for chloride. For application of bicarbonate, 25 mM HCO3 − was exchanged for equimolar Cl− and the solution was equilibrated with 5 % CO2.
Results
dpres is expressed in auditory neurons
The D. melanogaster gene CG5845 encodes dpres (Fig. 1a) (Weber et al. 2003). dpres is located at polytene chromosome position 75A9 on chromosome arm 3L (Fig. 1a). The predicted gene product is a 742 amino acid protein with sulfate transporter domains that overall shares 18 % identity and 31 % similarity with mammalian Prestin (Fig. S1).
If dpres were functionally orthologous to human Prestin, acting as a piezo crystal-like motor that augments the ears’ mechanical responses to sound, we would expect dpres to be expressed in the neurons of JO, and possibly other chordotonal organs. Therefore, we first characterized the dpres expression pattern.
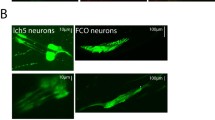
RT-PCR demonstrated expression in embryos, larvae and adults (Fig. 2a). To obtain evidence for dpres expression pattern, the 5′ regulatory region was cloned upstream of Gal4 using the P-element vector pPTGal (Sharma et al. 2002) to produce a dpres-Gal4 transgenic line (Fig. 1a). The dpres expression pattern was visualized by crossing the Gal4 line to a UAS-EGFP reporter. dpres is expressed in the chordotonal neurons of embryos and adults (Fig. 2b, c). dpres is also expressed in mechanosensitive neurons of the legs and wings indicating possible anion antiporter functions in these sensory organs as well (Fig. 2d, e). In particular, dpres is expressed in the neurons of the JO in the second antennal segment, which are the auditory mechanoreceptors (Eberl 1999).
dpres promoter region drives expression in chordotonal organs. a RT-PCR amplifies dpres mRNA from embryos, larvae, and adults. Without reverse transcriptase (RT) the longer genomic DNA contamination amplifies, but with RT, the shorter spliced RNA product is distinguished. b A 6.1 kb fragment of the dpres regulatory region (shown in Fig. 1) connected to Gal4 drives expression of GFP in the peripheral nervous system of embryos. Expression is seen in the neurons of the embryonic lateral chordotonal organs (lch5′s) (arrow and inset). c–e dpres-Gal4 also drives strong expression in JO neurons (c) in the second antennal segment (green: GFP, magenta: rhodamine phalloidin) and in the femoral chordotonal organ of the leg (d arrow) and the sensory organs of the anterior wing margin (e). Scale bars represent 40 μm in (b), 10 μm in (c), and 100 μm in (d) and (e)
dpres mutants have no effect on SEPs
Because dpres is the homolog of mammalian Prestin and is expressed in JO neurons, we expected reduced hearing in dpres mutants in response to sound stimuli. We took a reverse genetics approach to engineer mutations in the dpres gene. Homologous recombination was used to disrupt dpres with 5 kb insertion containing the w hs gene inserted in the middle of dpres. The recombination resulted in a small duplication of the 3′ end of exon two and the 5′ end of exon three, flanking the insert. RT-PCR amplified several dpres transcripts, none of which are wild type (Fig. 1b) and were very short or contained frameshifts to early stop codons. Electrophysiological recordings were conducted on the antennal nerve to determine whether sound-evoked potentials were affected in dpres mutant flies. SEPs from homozygous dpres 339/dpres 339 flies responded to the pulse stimulus and were indistinguishable from dpres 339/+ heterozygous sibling controls (Fig. 3).
dpres mutants have no effect on auditory sound-evoked potentials. Average peak amplitudes of sound-evoked potentials (SEPs), extracellular potentials recorded from the vicinity of the antennal nerve in response to simulated pulse song. Two-tailed unpaired t tests showed no significant difference between the dpres mutants and their heterozygous controls, (p = 0.8295 for dpres 339, p = 0.8306 for dpres 51C). Numbers of antennae recorded are indicated at the bottom of each bar. Error bars indicate standard error of the mean (SEM)
To reconcile the lack of electrophysiological phenotype in the homologous recombinant allele dpres 339, we wondered whether the truncated proteins, up to 250 residues, from the aberrant transcripts could be partially functional. Therefore, we wanted to generate a deletion that completely removes the dpres coding sequences. To this end, we took two approaches. We first made use of the w hs element inserted into the homologous recombinant allele, providing w+ eye color, as a substrate for gamma-ray mutagenesis to select for w− derivatives. Two w− derivatives recovered were found to have cytologically visible deletions in the 75AB region of polytene chromosomes, and to form a lethal complementation group with Df(3L)BSC8. Because these deletions in all combinations removed other vital genes in addition to dpres, they were not useful for testing dpres function. In the second approach, we made use of two P-element insertions near dpres to generate imprecise excisions. To allow the possibility of recovering a synthetic deletion between these two elements by P-induced recombination, we generated flies containing both P-elements and the P-element transposase. From this we recovered dpres 51C, an imprecise excision of the KG05103 element with a deletion extending from the KG05103 insertion site into the dpres gene, thus removing the entire upstream regulatory region and the first two exons. As with dpres 339, we found that SEPs were unaffected in the dpres 51C allele (Fig. 3).
Mechanical amplification of sound is independent of dpres function
To estimate the extent of mechanical feedback amplification in dpres loss-of-function mutations more directly, we analyzed the free fluctuations of unstimulated antennal sound receivers of mutants and control flies (see Göpfert et al. 2005 for details). In the receivers of wild-type flies, these recordings reflect two distinct phenomena: (1) the thermal energy of the environment and (2) the additional energy injected by JO neurons (Göpfert et al. 2005). Through their energy contributions, JO neurons cause characteristic shifts of both the frequency tuning and damping characteristics of the D. melanogaster antennal ear (Göpfert et al. 2005; Nadrowski and Göpfert 2009; Riabinina et al. 2011). A potential reduction (or loss) of mechanical feedback amplification in dpres mutants would thus result in predictable changes of the flies’ antennal mechanics. Our laser Doppler vibrometric analyses of the sound receivers’ free fluctuations (Fig. 4, top), however, showed no significant difference between dpres 339 /dpres 339 homozygotes and dpres 339 /+ control flies. Receiver best frequencies were 184 ± 18 Hz for control flies and 167 ± 31 Hz for mutant flies (two-tailed test: p = 0.112, n = 13, Fig. 4); a loss of amplification, for comparison, would have resulted in best frequency values of ~800 Hz [as for example seen in the ‘passive’ antennae of tilB null mutant flies (Göpfert et al. 2005)]. The values for the quality factor Q (a dimensionless, reciprocal measure of the antennae’s damping, with higher Q values indicating a less damped system) was 1.3 ± 0.4 in control flies and 1.4 ± 0.4 in mutants (p = 0.439, n = 13, Fig. 4). Auditory amplification in flies coincides with an undamping of the antennal receiver; a loss of amplification would have thus yielded lower Q values of ~0.8–1 (as characteristic of the passive antennal joint). Estimated energy gain values, finally, were 7.5 ± 4.1 kBT for controls and 8.8 ± 3.0 kBT for mutants (p = 0.357, n = 13, Fig. 4). A loss of amplification would have abolished all energy gain, leading to values of 0 kBT. Taken together these results demonstrate that dpres is not required for auditory feedback amplification.
dpres mutants have normal mechanical amplification of sound. Free sound receiver fluctuations were recorded in dpres 339 mutants (left, green) and homozygous controls (right, blue). Shown are the FFT amplitudes of receiver velocities (top). No detectable difference was found in the receivers’ energy gain due to active mechanical amplification (two-tailed t test p = 0.357), the quality factor of the receivers’ mechanical tuning (two-tailed t test p = 0.439) and their best frequency (two-tailed t test p = 0.112) between dpres 339 mutants and the dpres 339/+ heterozygote control flies. The boundaries of the boxes closest to zero mark the 25th percentiles. The lines within the boxes mark the median, and the boundaries of the boxes farthest from zero mark the 75th percentiles. Error bars above and below the box mark the 90th and 10th percentiles, respectively. n = 13 for all values
The absence of a contribution to active mechanics rather suggested a function as an anion transporter. Vertebrate Prestin (SLC26A5) orthologs (Schaechinger and Oliver 2007; Gorbunov et al. 2014) and a closely related transporter from the ascidian Ciona intestinalis (Deng et al. 2013) are electrogenic anion antiporters that exchange sulfate and oxalate against chloride. We therefore explored the transport function in a heterologous expression system using whole-cell patch-clamp methods. When chloride was the only small cytoplasmic and extracellular anion, no currents above the background level of non-transfected CHO cells were observed, even in the presence of a chloride concentration gradient (not shown). Thus dpres does not mediate the chloride channel-like transport mode prevalent in the mammalian Prestin relatives SLC26A7 and SLC26A9 (Kim et al. 2005; Dorwart et al. 2007). However, as shown in Fig. 5, application of either sulfate or oxalate to the extracellular solution induced sizeable outward transport currents, consistent with stoichiometric exchange of the divalent anion against chloride, as in non-mammalian Prestin (Schaechinger and Oliver 2007). A recent study using Xenopus oocytes as the expression system suggested that dpres also mediates electrogenic exchange of bicarbonate versus chloride (Hirata et al. 2012b). We therefore attempted to measure transport currents with extracellular bicarbonate (25 mM) in the presence of an outward chloride gradient. In this situation, only negligible currents above background were observed (Fig. 5a, b), indicating that electrogenic exchange of n HCO3 − against 1 Cl− occurs either at a low rate or is absent. Probably, dpres mediates electroneutral Cl−:HCO3 − exchange (i.e., with 1:1 stoichiometry), as found for the C. intestinalis Prestin homolog Ci-Slc26aα (Deng et al. 2013).
Electrogenic anion transport mediated by dpres. a Representative transport currents recorded from the same cell expressing dpres-GFP during subsequent applications of extracellular sulfate, oxalate (10 mM each) and HCO3 − (25 mM). Intra- and extracellular concentrations of Cl− were 160 and 10 mM, respectively. Currents shown were recorded in response to voltage ramps (−100 to +100 mV) and background currents measured immediately before application of each transport substrate were subtracted. b Average currents at +80 mV from experiments as in (a), normalized to membrane surface. Bars represent means, error bars represent standard error, n = 10 cells, each tested sequentially for all three anions
Discussion
The role of dpres in mechanical amplification of sound
Of the nine mammalian members of the SLC26 family, only Prestin is known to confer electromotility when transfected into mammalian cells (Zheng et al. 2000; Oliver et al. 2001; Lohi et al. 2002). Recent structural and functional analysis of Prestin suggests that the molecular mechanism underlying electromotility was derived evolutionarily from a primordial anion transport mechanism. Thus the protein domains that mediate anion transport, including a central anion binding site, are also critically involved in the electromotile function (Schaechinger et al. 2011; Gorbunov et al. 2014). Yet, by generating chimeric molecules between mammalian mechanically active Prestin and a non-mammalian transporter ortholog, it was shown that in principle, Prestin can mediate both anion transport and electromechanical activity at the same time (Schaechinger et al. 2011). It is thought that the movement of a voltage sensor, most likely related to the interaction of anions with the protein, changes the area occupied by the Prestin molecule within the plane of the membrane (Oliver et al. 2001; Dallos and Fakler 2002; Song and Santos-Sacchi 2013). When many Prestin molecules are clustered in the baso-lateral membrane and change shape at the same time, the outer hair cell length changes (Dallos et al. 1993; Iwasa 2001). An increase in cilia membrane tension in response to decreases in sound intensity would be an analogous function in D. melanogaster. We found evidence for specific dpres expression in the auditory sensory neurons. However, our study with flies lacking dpres found that dpres is dispensable for fly hearing.
The anion antiporter activity of dpres
The primordial function of mammalian Prestin as an anion transporter, rather than a motor, is further supported by Albert et al. (2007) who show that the zebrafish Prestin ortholog does not possess the same electromotility as mammalian Prestin, along with altered kinetics and voltage dependence. In fact, non-mammalian Prestin orthologs are electrogenic anion transporters that exchange monovalent versus divalent anions (Schaechinger and Oliver 2007; Deng et al. 2013). The explanation for lack of a dpres role in D.melanogaster hearing is supported by the studies of Okoruwa et al. (2008), who surveyed the electromotility of Prestin homologs among vertebrates, by looking at protein sequence conservation. They found that the electromotility appears to be a derived character that is distinctly mammalian, especially in eutherian mammals. They argue that two insertion/deletion events and subsequent evolution conferred fast electromotility in the transmembrane anion transporter domain of Prestin. While this divergence to electromotility in mammalian Prestin was thought at the time to coincide with anatomical innovations in the outer hair cells and surrounding cochlear tissue of eutherian lineage, namely the reduction in number of rows of IHCs and in OHCs and changes in the tectorial membrane structure, the inference of chick Prestin’s role in short hair cell amplification (Beurg et al. 2013) suggests that electromotile properties may have predated the mammalian anatomical arrangement.
Because the dpres gene has been functionally retained in the D. melanogaster genome, is expressed in sense organs at all major stages of its life cycle, and its product mediates robust anion transport (Fig. 5) (Hirata et al. 2012a, b), we expect that dpres acts as an anion transporter in the normal function of the neuron. If that is the case, one might still expect to find that dpres mutants have reduced hearing due to defects in functional neurons. A possible explanation for the lack of a quantifiable dpres mutant phenotype is the presence of other proteins that may serve a redundant function to dpres in the chordotonal organ. A search of genes differentially expressed in the JO (Senthilan et al. 2012) identified ten solute carriers, though none closely related to dpres. The possibility remains, though, that one of these transporter genes serves a redundant function throughout the mechanosensory tissues in which it is expressed and can thus compensate for the loss of dpres function, or that functionally equivalent solute carriers replacing dpres are not selectively expressed in the mechanosensory organ. Alternatively, it is possible that functional consequences of dpres mutation in the JO can only be revealed under certain conditions that differ from the standard laboratory environment, such as at the fringes of tolerable temperature or under conditions of cell stress.
Are acquisition of Prestin electromotility and loss of nompC coeval?
An interesting evolutionary relationship may occur between Prestin and the gene no mechanoreceptor potential C (nompC or TRPN1). NompC is a mechanotransduction channel (Yan et al. 2013) and integral component of the D. melanogaster auditory mechanotransducer complex (Effertz et al. 2012) and has been shown to be essential for mechanical amplification in D. melanogaster hearing (Göpfert et al. 2006; Effertz et al. 2011). nompC homologs also are expressed in zebrafish (Albert et al. 2007) and Xenopus tropicalis (Shin et al. 2005), indicating widespread occurrence in lower vertebrates. Meanwhile, and in accordance with prior attempts (Delmas and Coste 2013), we have been unable to identify a mammalian or avian nompC ortholog. The search was conducted through Blastp using the full-length NompC isoform AAF52248.3 against the refseq protein database of representative mammals and birds (Fig. 6). In this search we defined an ortholog as having both the N-terminal ankyrin repeats and the C-terminal transmembrane domain.
Evolution of Prestin-mediated somatic electromotility coincides with disappearance of NompC/TRPN1. Data from published work and Blastp searches of the representative protein databases derived from model organism genomes show that Prestin somatic electromotility coincides with the absences of nompC orthologs. Representative genomes available from NCBI were blasted with the D. melanogaster NompC isoform AAF52248.3 to identify whether there were nompC orthologs. Only those TRP channels with both the transmembrane and N-terminal ankyrin repeat structure representative of NompC were considered orthologous proteins. Gallus gallus and Anas platyrhynchos were used as representative birds. Among reptiles, the Anolis carolinensis genome appears to be devoid of nompC homologs, while Chrysemys picta, Chelonia mydas, Pelodiscus sinensis, Alligator mississippiensis, and Alligator sinensis each had recognizable nompC homologs. Xenopus tropicalis represented amphibians (Tang et al. 2013). The Caenorhabditis elegans nompC ortholog Trp-4 was used as the basis for this figure (Kang et al. 2010). Information on Prestin electromotility from mammals, birds, reptiles, amphibians and fish was taken from published work (Dallos and Fakler 2002; Albert et al. 2007; Beurg et al. 2013; Tang et al. 2013). Question marks denote those organisms that have Prestin homologs with no (C. elegans) or conflicting (birds and lizards) data regarding putative somatic electromotility (Stewart and Hudspeth 2000; Tang et al. 2013)
Prestin’s derived cochlear amplifier function meanwhile has been reported to have evolved in both avian and mammalian lineages—lineages that do not contain nompC orthologs, while its sole (ancestral) function as an anion transporter is present in those species that have retained NompC as a functional mechanotransduction channel (Fig. 6). The association of Prestin’s derived function with loss of the nompC gene suggests an interesting hypothesis in which the non-linear sound amplification function of NompC has been replaced by Prestin. This replacement may be facilitated by the fact that nompC mutations in D. melanogaster reduce, but do not eliminate, auditory function (Eberl et al. 2000; Effertz et al. 2011). We are excited to see further functional and evolutionary analysis on both Prestin and nompC to test if this is an accurate reflection of the evolution of auditory sound amplification. Key progress in confirming an association between Prestin electromotility and loss of NompC will be made by analysis of reptiles, among which NompC homologs can be found in turtles and alligators, but so far not in lizards (Fig. 6). Reports of active amplification mechanisms in reptiles (Stewart and Hudspeth 2000) have yet to be linked to, or distinguished from, Prestin-based mechanisms (Fig. 6).
Abbreviations
- BM:
-
Basilar membrane
- FFT:
-
Fast Fourier transform
- IHC:
-
Inner hair cell
- JO:
-
Johnston’s organ
- OHC:
-
Outer hair cell
- PCR:
-
Polymerase chain reaction
- SEP:
-
Sound-evoked potential
- RT:
-
Reverse transcriptase
References
Albert JT, Winter H, Schaechinger TJ, Weber T, Wang X, He DZZ, Hendrich O, Geisler H-S, Zimmermann U, Oelmann K, Knipper M, Göpfert MC, Oliver D (2007) Voltage-sensitive prestin orthologue expressed in zebrafish hair cells. J Physiol 580:451–461
Beurg M, Tan X, Fettiplace R (2013) A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron 79:69–81
Dallos P, Fakler B (2002) Prestin, a new type of motor protein. Nature Rev Molec Cell Biol 3:104–111
Dallos P, Hallworth R, Evans BN (1993) Theory of electrically driven shape changes of cochlear outer hair cells. J Neurophysiol 70:299–323
Delmas P, Coste B (2013) Mechano-gated ion channels in sensory systems. Cell 155:278–284
Deng W, Nies F, Feuer A, Bocina I, Oliver D, Jiang D (2013) Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis. Proc Natl Acad Sci USA 110:14972–14977
Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S (2007) SLC26A9 is a Cl(−) channel regulated by the WNK kinases. J Physiol 584:333–345
Eberl DF (1999) Feeling the vibes: chordotonal mechanisms in insect hearing. Curr Opin Neurobiol 9:389–393
Eberl DF, Hardy RW, Kernan M (2000) Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci 20:5981–5988
Effertz T, Wiek R, Göpfert MC (2011) NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol 21:592–597
Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Göpfert MC (2012) Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci 15:1198–1200
Fettiplace R, Hackney CM (2006) The sensory and motor roles of auditory hair cells. Nature Rev Neurosci 7:19–29
Göpfert MC, Robert D (2002) The mechanical basis of Drosophila audition. J Exp Biol 205:1199–1208
Göpfert MC, Humphris ADL, Albert JT, Robert D, Hendrich O (2005) Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc Natl Acad Sci USA 102:325–330
Göpfert MC, Albert JT, Nadrowski A, Kamikouchi A (2006) Specification of auditory sensitivity by Drosophila TRP channels. Nature Neurosci 9:999–1000
Gorbunov D, Sturlese M, Nies F, Kluge M, Bellanda M, Battistutta R, Oliver D (2014) Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat Commun 5:3622
He DZ, Beisel KW, Chen L, Ding DL, Jia S, Fritzsch B, Salvi R (2003) Chick hair cells do not exhibit voltage-dependent somatic motility. J Physiol 546:511–520
Hirata T, Cabrero P, Berkholz DS, Bondeson DP, Ritman EL, Thompson JR, Dow JA, Romero MF (2012a) In vivo Drosophila genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol 303:F1555–F1562
Hirata T, Czapar A, Brin L, Haritonova A, Bondeson DP, Linser P, Cabrera P, Thompson J, Dow JAT, Romero MF (2012b) Ion and solute transport by Prestin in Drosophila and Anopheles. J Insect Physiol 58:563–569
Hudspeth AJ (2008) Making an effort to listen: mechanical amplification in the ear. Neuron 59:530–545
Iwasa KH (2001) A two-state piezoelectric model for outer hair cell motility. Biophys J 81:2495–2506
Kamikouchi A, Albert JT, Göpfert MC (2010) Mechanical feedback amplification in Drosophila hearing is independent of synaptic transmission. Eur J Neurosci 31:697–703
Kang L, Gao J, Schafer WR, Xie Z, Xu XZS (2010) C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67:381–391
Kernan MJ (2007) Mechanotransduction and auditory transduction in Drosophila. Pflügers Arch Eur J Physiol 454:703–720
Kim KH, Shcheynikov N, Wang Y, Muallem S (2005) SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280:6463–6470
Lee E, Sivan-Loukianova E, Eberl DF, Kernan MJ (2008) An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr Biol 18:1899–1906
Liberman MC, Gao J, He DZZ, Wu X, Jia S, Zuo J (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419:300–304
Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJH, Webb BT, Yan D, Arnos KS, Corey D, Dallos P, Nance WE, Chen ZY (2003) Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Molec Genet 12:1155–1162
Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J (2002) Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem 277:14246–14254
Nadrowski B, Göpfert MC (2009) Level-dependent auditory tuning: transducer-based active processes in hearing and best-frequency shifts. Commun Integr Biol 2:7–10
Nadrowski B, Albert JT, Göpfert MC (2008) Transducer-based force generation explains active process in Drosophila hearing. Curr Biol 18:1365–1372
Okoruwa OE, Weston MD, Sanjeevi DC, Millemon AR, Fritzsch B, Hallworth R, Beisel KW (2008) Evolutionary insights into the unique electromotility motor of mammalian outer hair cells. Evol Dev 10:300–315
Oliver D, He DZZ, Klöcker NJ, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B (2001) Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292:2340–2343
Peng AW, Salles FT, Pan B, Ricci AJ (2011) Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction. Nat Commun 2:523
Riabinina O, Dai M, Duke T, Albert JT (2011) Active process mediates species-specific tuning of Drosophila ears. Curr Biol 21:658–664
Rong YS, Golic KG (2000) Gene targeting by homologous recombination in Drosophila. Science 288:2013–2018
Rong YS, Golic KG (2001) A targeted gene knockout in Drosophila. Genetics 157:1307–1312
Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P, Olivera BM, Brodsky M, Rubin GM, Golic KG (2002) Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev 16:1568–1581
Schaechinger TJ, Oliver D (2007) Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci USA 104:7693–7698
Schaechinger TJ, Gorbunov D, Halaszovich CR, Moser T, Kugler S, Fakler B, Oliver D (2011) A synthetic prestin reveals protein domains and molecular operation of outer hair cell piezoelectricity. EMBO J 30:2793–2804
Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, Winkler M, Möbius W, Howard J, Göpfert MC (2012) Drosophila auditory organ genes and genetic hearing defects. Cell 150:1042–1054
Sharma Y, Cheung U, Larsen EW, Eberl DF (2002) pPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis 34:115–118
Shin J-B, Adams D, Paukert M, Siba M, Sidi S, Levin M, Gillespie PG, Gründer S (2005) Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc Natl Acad Sci USA 102:12572–12577
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539
Song L, Santos-Sacchi J (2013) Disparities in voltage-sensor charge and electromotility imply slow chloride-driven state transitions in the solute carrier SLC26a5. Proc Natl Acad Sci USA 110:3883–3888
Stewart CE, Hudspeth AJ (2000) Effects of salicylates and aminoglycosides on spontaneous otoacoustic emissions in the Tokay gecko. Proc Natl Acad Sci USA 97:454–459
Tan X, Pecka JL, Tang J, Okoruwa OE, Zhang Q, Beisel KW, He DZ (2011) From zebrafish to mammal: functional evolution of prestin, the motor protein of cochlear outer hair cells. J Neurophysiol 105:36–44
Tang J, Pecka JL, Fritzsch B, Beisel KW, He DZ (2013) Lizard and frog prestin: evolutionary insight into functional changes. PLoS One 8:e54388
Weber T, Göpfert MC, Winter H, Zimmermann U, Kohler H, Meier A, Hendrich O, Rohbock K, Robert D, Knipper M (2003) Expression of prestin-homologous solute carrier (SLC26) in auditory organs of nonmammalian vertebrates and insects. Proc Natl Acad Sci USA 100:7690–7695
Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN (2013) Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493:221–225
Zheng J, Shen W, He DZZ, Long KB, Madison LD, Dallos P (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155
Acknowledgments
We would like to thank the University of Iowa Central Microscopy Research Facility for use of the Zeiss LSM510 confocal microscope (Grant Number 1 S10 RR12916-01). Dominic Evans performed the laser vibrometry on dpres mutants. Katie Bunce conducted the P-element mobilization experiments that led to the 51C mutation. Matthew Topping provided constructive criticism during manuscript preparation. Fly stocks were obtained from the Bloomington Drosophila Stock Center at Indiana University and P-element constructs were originally obtained from the Drosophila Genome Research Center. This work was supported by NIH RO1 DC004848 to Daniel F. Eberl, a Ruth L. Kirschstein NRSA Post-Doctoral Research Fellowship DC006550 to Janice Fritz and grants from the HFSP (RGY0070/2011), BBSRC (BB/L02084X/1) to Joerg Albert, and grants from the DFG (GO 1092/2, SPP1608 and OL 240/4-1, SPP1608) to Martin C. Göpfert and Dominik Oliver. This work was also facilitated by the Iowa Center for Molecular Auditory Neuroscience supported by NIH P30 core grant DC010362 to Steven Green. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
359_2014_960_MOESM1_ESM.pdf
Supplementary material 1 Fig. S1: Alignment of dPres with human Prestin amino acid sequence. The amino acid sequences of dPres (AAF49285.1) and human Prestin (AAP31417.1) were aligned using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) (Sievers et al. 2011). The ion transporter domain is underlined. Identical residues are shown by (*) while conserved substitutions are shown by (:) and semi-conserved substitutions are shown by (.). Red - small hydrophobic residues, blue– acidic residues, magenta– basic residues, green - hydroxyl + amine + basic + Q. The locations of engineered stop codons are highlighted in gray. (PDF 44 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kavlie, R.G., Fritz, J.L., Nies, F. et al. Prestin is an anion transporter dispensable for mechanical feedback amplification in Drosophila hearing. J Comp Physiol A 201, 51–60 (2015). https://doi.org/10.1007/s00359-014-0960-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0960-9