Abstract
Male Manduca sexta moths are attracted to a mixture of two components of the female’s sex pheromone at the natural concentration ratio. Deviation from this ratio results in reduced attraction. Projection neurons innervating prominent male-specific glomeruli in the male’s antennal lobe produce maximal synchronized spiking activity in response to synthetic mixtures of the two components centering around the natural ratio, suggesting that behaviorally effective mixture ratios are encoded by synchronous neuronal activity. We investigated the physiological activity and morphology of downstream protocerebral neurons that responded to antennal stimulation with single pheromone components and their mixtures at various concentration ratios. Among the tested neurons, only a few gave stronger responses to the mixture at the natural ratio whereas most did not distinguish among the mixtures that were tested. We also found that the population response distinguished among the two pheromone components and their mixtures, prior to the peak population response. This observation is consistent with our previous finding that synchronous firing of antennal-lobe projection neurons reaches its maximum before the firing rate reaches its peak. Moreover, the response patterns of protocerebral neurons are diverse, suggesting that the representation of olfactory stimuli at the level of protocerebrum is complex.








Similar content being viewed by others
References
Aertsen A, Diesmann M, Gewaltig MO (1996) Propagation of synchronous spiking activity in feedforward neural networks. J Physiol 90:243–247
Anton S, Hansson BS (1995) Sex-pheromone and plant-associated odor processing in antennal lobe interneurons of male Spodoptera littoralis (Lepidoptera, Noctuidae). J Comp Physiol A 176(6):773–789
Anton S, Löfstedt C, Hansson BS (1997) Central nervous processing of sex pheromones in two strains of the European corn borer Ostrinia nubilalis (Lepidoptera: Pyralidae). J Exp Biol 200:1073–1087
Bell RA, Joachim FG (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms (Lepidoptera-Sphingidae-Gelechiidae). Ann Entomol Soc Am 69:365–373
Brévault T, Quilici S (2010) Interaction between visual and olfactory cues during host finding in the tomato fruit fly Neoceratitis cyanescens. J Chem Ecol 36(3):249–259
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Carlsson MA, Chong KY, Daniels W, Hansson BS, Pearce TC (2007) Component information is preserved in glomerular responses to binary odor mixtures in the moth Spodoptera littoralis. Chem Senses 32:433–443
Cha DH, Nojima S, Hesler SP, Zhang A, Linn CE, Roelofs WL, Loeb GM (2008) Identification and field evaluation of grape shoot volatiles attractive to female grape berry moth (Paralobesia viteana). J Chem Ecol 34:1180–1189
Cha DH, Linn CE, Teal PEA, Zhang A, Roelofs WL, Loeb GM (2011) Eavesdropping on plant volatiles by a specialist moth: significance of ratio and concentration. PLoS One 6(2):e17033
Chapin JK, Nicolelis MAL (1999) Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods 94:121–140
Chelaru MI, Dragoi V (2008) Efficient coding in heterogeneous neuronal populations. Proc Natl Acad Sci USA 105:16344–16349
Chow DM, Theobald JC, Frye MA (2011) An olfactory circuit increases the fidelity of visual behavior. J Neurosci 31(42):15035–15047
Christensen TA, Hildebrand JG (1987) Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol A 160:553–569
Couto A, Alenius M, Dickson BJ (2005) Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 15:1535–1547
Domingue MJ, Musto CJ, Linn CE Jr, Roelofs WL, Baker TC (2007) Evidence of olfactory antagonistic imposition as a facilitator of evolutionary shifts in pheromone blend usage in Ostrinia spp. (Lepidoptera: Crambidae). J Insect Physiol 53:488–496
Domingue MJ, Musto CJ, Linn J, Roelofs WL, Baker TC (2008) Olfactory neuron responsiveness and pheromone blend preference in hybrids between Ostrinia furnacalis and Ostrinia nubilalis (Lepidoptera: Crambidae). J Insect Physiol 54:1261–1270
El-Sayed AM, Mitchell VJ, Manning LA, Suckling DM (2011) New sex pheromone blend for the lightbrown apple moth, Epiphyas postvittana. J Chem Ecol 37:640–646
Fang N, Teal PEA, Doolittle RE, Tumlinson JH (1995) Biosynthesis of conjugated olefinic systems in the sex pheromone gland of female tobacco hornworm moths, Manduca sexta (L.). Insect Biochem 25:39–48
Fujiwara T, Kazawa T, Haupt SS, Kanzaki R (2009) Ca2+ imaging of identifiable neurons labeled by electroporation in insect brains. NeuroReport 20:1061–1065
Gao Q, Yuan B, Chess A (2000) Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci 3:780–785
Goyret J, Markwell PM, Raguso RA (2007) The effect of decoupling olfactory and visual stimuli on the foraging behavior of Manduca sexta. J Exp Biol 210(8):1398–1405
Haddad R, Weiss T, Khan R, Nadler B, Mandairon N, Bensafi M, Schneidman E, Sobel N (2010) Global features of neural activity in the olfactory system form a parallel code that predicts olfactory behavior and perception. J Neurosci 30:9017–9026
Hagström ÅK, Liénard MA, Groot AT, Hedenström E, Löfstedt C (2012) Semi-selective fatty acyl reductases from four heliothine moths influence the specific pheromone. PLoS One 7(5):e37230
Han Q, Hansson BS, Anton S (2005) Interactions of mechanical stimuli and sex pheromone information in antennal lobe neurons of a male moth Spodoptera littoralis. J Comp Physiol A 191(6):521–528
Hillier NK, Vickers NJ (2011) Mixture interactions in moth olfactory physiology: examining the effects of odorant mixture, concentration, distal stimulation, and antennal nerve transection on sensillar responses. Chem Senses 36:93–108
Homberg U, Montague RA, Hildebrand JG (1988) Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res 254(2):255–281
Iwano M, Hill ES, Mori A, Mishima T, Mishima T, Ito K, Kanzaki R (2010) Neurons associated with the flip-flop activity in the lateral accessory lobe and ventral protocerebrum of the silkworm moth brain. J Comp Neurol 518(3):366–388
Jefferis GSXE, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Luo L (2007) Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128:1187–1203
Kanno H, Kuenen LPS, Klingler KA, Millar JG, Cardé RT (2010) Attractiveness of a four-component pheromone blend to male navel orangeworm moths. J Chem Ecol 36:584–591
Kanzaki R, Shibuya T (1992) Long-lasting excitation of protocerebral bilateral neurons in the pheromone-processing pathways of the male moth Bombyx mori. Brain Res 587(2):211–215
Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG (1989) Physiology and morphology of projection neurons in the antennal lobe of the male moth Manduca sexta. J Comp Physiol A 165(4):427–453
Kanzaki R, Ikeda A, Shibuya T (1994) Morphological and physiological-properties of pheromone-triggered flipflopping descending interneurons of the male silkworm moth, Bombyx mori. J Comp Physiol A 175(1):1–14
Kanzaki R, Soo K, Seki Y, Wada S (2003) Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem Senses 28:113–130
Kay LM, Lowry CA, Jacobs HA (2003) Receptor contributions to configural and elemental odor mixture perception. Behav Neurosci 117:1108–1114
Kirschner S, Kleineidam CJ, Zube C, Rybak J, Grunewald B, Rossler W (2006) Dual olfactory pathway in the honeybee, Apis mellifera. J Comp Neurol 499(6):933–952
Kisley MA, Gerstein GL (1999) The continuum of operating modes for a passive model neuron. Neural Comput 11:1139–1154
Leary GP, Allen JE, Bunger PL, Luginbill JB, Linn CE Jr, Macallister IE, Kavanaugh MP, Wanner KW (2012) Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc Natl Acad Sci USA 109:14081–14086
Lei H, Vickers N (2008) Central processing of natural odor mixtures in insects. J Chem Ecol 34:915–927
Lei H, Anton S, Hansson BS (2001) Olfactory protocerebral pathways processing sex pheromone and plant odor information in the male moth Agrotis segetum. J Comp Neurol 432:356–370
Lei H, Christensen TA, Hildebrand JG (2002) Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci 5:557–565
Lei H, Christensen TA, Hildebrand JG (2004) Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci 24:11108–11119
Løfaldli BB, Kvello P, Kirkerud N, Mustaparta H (2012) Activity in neurons of a putative protocerebral circuit representing information about a 10 component plant odor blend in Heliothis virescens. Front Syst Neurosci 6:64
Martin JP, Lei H, Riffell JA, Hildebrand JG (2013) Synchronous firing of antennal-lobe neurons encodes the behaviorally effective ratio of sex-pheromone components in male Manduca sexta. J Comp Physiol A. doi:10.1007/s00359-013-0849-z
Matoušková P, Pichová I, Svatoš A (2007) Functional characterization of a desaturase from the tobacco hornworm moth (Manduca sexta) with bifunctional Z11- and 10,12-desaturase activity. Insect Biochem 37:601–610
Najar-Rodriguez AJ, Galizia CG, Stierle J, Dorn S (2010) Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J Exp Biol 213:3388–3397
Nikonov A, Leal W (2002) Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle, Popillia japonica. J Chem Ecol 28:1075–1089
Nojima S, Linn C Jr, Morris B, Zhang A, Roelofs W (2003) Identification of host fruit volatiles from hawthorn (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. J Chem Ecol 29:321–336
Ochieng SA, Park KC, Baker TC (2002) Host plant volatiles synergize responses of sex pheromone-specific olfactory receptors neurons in male Helicoverpa zea. J Comp Physiol A 188:325–333
Person AL, Raman IM (2012) Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481:502–505
Pickett J, Bruce T, Chamberlain K, Hassanali A, Khan Z, Napier JA, Smart LE, Wadhams LJ, Woodcock CM (2006) Plant volatiles yielding new ways to exploit plant defence. In: Dicke M, Takken W (eds) Chemical ecology: from gene to ecosystem. Springer, Wageningen, pp 161–173
Piñero JC, Dorn S (2007) Synergism between aromatic compounds and green leaf volatiles derived from the host plant underlies female attraction in the oriental fruit moth. Entomol Exp Appl 125:185–194
Pregitzer P, Schubert M, Breer H, Hansson BS, Sachse S, Krieger J (2012) Plant odorants interfere with detection of sex pheromone signals by male Heliothis virescens. Front Cell Neurosci 6 (article 42):1–12
Riffell JA, Lei H, Hildebrand JG (2009a) Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc Natl Acad Sci USA 106:19219–19226
Riffell JA, Lei H, Christensen TA, Hildebrand JG (2009b) Characterization and coding of behaviorally significant odor mixtures. Curr Biol 19:335–340
Rudolph M, Destexhe A (2003) Tuning neocortical pyramidal neurons between Integrators and coincidence detectors. J Comput Neurosci 14:239–251
Sakurai Y (1999) How do cell assemblies encode information in the brain? Neurosci Biobehav Rev 23:785–796
Sanes JR, Hildebrand JG (1976) Structure and development of antennae in a moth, Manduca sexta. Dev Biol 51:282–299
Seki Y, Aonuma H, Kanzaki R (2005) Pheromone processing center in the protocerebrum of Bombyx mori revealed by nitric oxide-induced anti-cGMP immunocytochemistry. J Comp Neurol 481(4):340–351
Shields VDC, Hildebrand JG (2001) Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J Comp Physiol A 186:1135–1151
Stocker RF, Lienhard MC, Borst A, Fischbach KF (1990) Neural architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 262:9–34
Sun XJ, Tolbert LP, Hildebrand JG (1997) Synaptic organization of the uniglomerular projection neurons of the antennal lobe of the moth Manduca sexta: a laser scanning confocal and electron microscopic study. J Comp Neurol 379(1):2–20
Tanaka NK, Suzuki E, Dye L, Ejima A, Stopfer M (2012) Dye fills reveal additional olfactory tracts in the protocerebrum of wild-type Drosophila. J Comp Neurol 520(18):4131–4140
Tasin M, Backman AC, Bengtsson M, Ioriatti C, Witzgall P (2006) Essential host plant cues in the grapevine moth. Naturwiss 93:141–144
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem 29:481–514
Tolbert LP, Matsumoto SG, Hildebrand JG (1983) Development of synapses in the antennal lobes of the moth Manduca sexta during metamorphosis. J Neurosci 3:1158–1175
Tumlinson JH, Brennan MM, Doolittle RE, Mitchell ER, Brabham A, Mazomenos BE, Baumhover AH, Jackson DM (1989) Identification of a pheromone blend attractive to Manduca sexta (L.) males in a wind tunnel. Arch Insect Biochem Physiol 10:255–271
Tumlinson JH, Mitchell ER, Doolittle RE, Jackson DM (1994) Field tests of synthetic Manduca sexta sex pheromone. J Chem Ecol 20:579–591
Vickers NJ, Poole K, Linn CE (2003) Consequences of interspecies antennal imaginal disc transplantation on organization of olfactory glomeruli and pheromone blend discrimination. J Comp Neurol 466:377–388
Vosshall LB, Wong AM, Axel R (2000) An olfactory sensory map in the fly brain. Cell 102:147–159
Webster B, Bruce T, Dufour S, Birkemeyer C, Birkett M, Hardie J, Pickett J (2008) Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J Chem Ecol 34:1153–1161
Wong AM, Wang JW, Axel R (2002) Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109:229–241
Yao CA, Ignell R, Carlson JR (2005) Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci 25(37):8359–8367
Acknowledgments
The authors thank Teresa Gregory for technical assistance and Drs. Robert Mitchell, Jeffrey Riffell and Joshua Martin for valuable comments. This work was supported by NSF grant DMS-1200004 to HL and NIH grant R01-DC02751 to JGH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
359_2013_844_MOESM1_ESM.eps
Supplemental Figure 1. Examination of response consistency, measured by coefficient of variation (CV). The responses are quantified using Mean instantaneous firing rate within response window (a), Response delay (b) and Number of spikes within response window (c). Each matrix is consisted of the CV values (expressed as a color scale) from 34 neurons that are arranged symmetrically. The primary diagonal represents the CV values based on the multiple trials within each neuron and other parallel diagonals are based on pairwise combinations of all neurons. The mean of each diagonal is assigned to the bar graph that is placed at the top right corner. The middle section of the bar graph is indicated by double arrows and the mean ± 2 standard deviation of the bar values is indicated red and black dashed lines. (EPS 9757 kb)
359_2013_844_MOESM2_ESM.eps
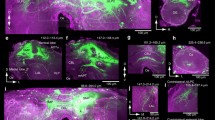
Supplemental Figure 2. Dye fill reveals a partially stained protocerebral neuron (labeled as VC110804) with arborization in lateral protocerebrum (a). This neuron showed nearly identical responses to EEZ, BAL and the Mixture, as demonstrated by the peristimulus time histograms (b). The black bar below each histogram indicates olfactory stimulation. D: dorsal; V: ventral; L: lateral; M: medial; O: optic lobe; SOG: suboesophageal ganglion; EEZ: E10-E12-Z14-hexadecatrienal; BAL: bombykal, i.e. E10-Z12-hexadecadienal. (EPS 7579 kb)
359_2013_844_MOESM3_ESM.eps
Supplemental Figure 3. Two neurons were partially stained with their somata seen lateral to the ipsilateral calyx and their main neurites crossing the calyx anteriorly (arrows) toward contralateral hemisphere (a). These two neurons had sparse arborization in the ipsilateral ventral protocerebrum. The recorded neuron showed nearly identical responses to single pheromone components and their blend of different ratios (b). When the quantity of both components was raised 100 times, the response appeared to be weaker. D: dorsal; V: ventral; L: lateral; M: medial; SOG: suboesophageal ganglion; EEZ: E10-E12-Z14-hexadecatrienal; BAL: bombykal, i.e. E10-Z12-hexadecadienal. (EPS 8105 kb)
359_2013_844_MOESM4_ESM.eps
Supplemental Figure 4. A protocerebral neuron, labeled as VC100714, bilaterally connects lateral protocerebrum of both hemispheres and send information to the mushroom calyx that is ispilateral to its soma (a). The branches in the calyx display blebby processes, typical of neuronal terminals. This neuron exhibited triphasic response pattern to single pheromone components and their blend of different ratios (b). The pattern can be characterized as a short burst of spikes followed by a period of spike suppression than followed by a 2nd burst of spikes. Hisbiscus oil, containing many plant derived volatiles, evoked strong excitatory response on this neuron. D: dorsal; V: ventral; L: lateral; M: medial; SOG: suboesophageal ganglion; EEZ: E10-E12-Z14-hexadecatrienal; BAL: bombykal, i.e. E10-Z12-hexadecadienal. (EPS 9002 kb)
Rights and permissions
About this article
Cite this article
Lei, H., Chiu, HY. & Hildebrand, J.G. Responses of protocerebral neurons in Manduca sexta to sex-pheromone mixtures. J Comp Physiol A 199, 997–1014 (2013). https://doi.org/10.1007/s00359-013-0844-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-013-0844-4