Summary
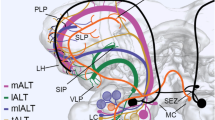
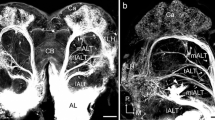
In the moth Manduca sexta, the number and morphology of neuronal connections between the antennal lobes and the protocerebrum were examined. Cobalt injections revealed eight morphological types of neurons with somata adjacent to the AL neuropil that project in the inner, middle, and outer antenno-cerebral tracts to the protocerebrum. Neurons innervating the macroglomerular complex and many neurons with fibers in the inner antennocerebral tract have uniglomerular antennal-lobe arborizations. Most neurons in the middle and outer antenno-cerebral tracts, on the other hand, seem to innervate more than one glomerulus. Protocerebral areas receiving direct input from the antennal lobe include the calyces of the mushroom bodies, and circumscribed areas termed “olfactory foci” in the lateral horn of the protocerebrum and several other regions, especially areas in close proximity to the mushroom bodies. Fibers in the inner antenno-cerebral tract that innervate the male-specific macroglomerular complex have arborizations in the protocerebrum that are distinct from the projections of sexually non-specific neurons. Protocerebral neurons projecting into the antennal lobe are much less numerous than antennal-lobe output cells. Most of these protocerebral fibers enter the antennal lobe in small fiber tracts that are different from those described above. In the protocerebrum, these centrifugal cells arborize in olfactory foci and also in the inferior median protocerebrum and the lateral accessory lobes. The morphological diversity of connections between the antennal lobes and the protocerebrum, described here for the first time on a single-cell level, suggests a much greater physiological complexity of the olfactory system than has been assumed so far.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Arnold G, Masson C, Budharugsa S (1985) Comparative study of the antennal lobes and their afferent pathways in the worker bee and the drone (Apis mellifera). Cell Tissue Res 242:593–60
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res 138:359–363
Bell RA, Joachim FA (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Ent Soc Am 69:365–373
Boeckh J, Boeckh V (1979) Threshold and odor specificity of pheromone-sensitive neurons in the deutocerebrum of Antheraea pernyi and A. polyphemus. J Comp Physiol 132:235–242
Boeckh J, Ernst KD (1987) Contribution of single unit analysis in insects to an understanding of olfactory function. J Insect Physiol A 161:549–565
Boeckh J, Ernst KD, Sass H, Waldow U (1984) Anatomical and physiological characteristics of individual neurons in the central antennal pathway of insects. J Insect Physiol 30:15–26
Borst A (1984) Untersuchungen zur zentralnervösen Verarbeitung olfaktorischer Reize bei Drosophila melanogaster. PhD Dissertation, Universität Würzburg, FRG
Boyan GS (1983) Postembryonic development in the auditory system of the locust. Anatomical and physiological characterization of interneurons ascending to the brain. J Comp Physiol 151:499–513
Bretschneider F (1924) Über die Gehirne des Eichenspinners und des Seidenspinners (Lasiocampa quercus L.) und Bombyx mori L. Jena Z Nat 60:563–578
Burrows M, Boeckh J, Esslen J (1982) Physiological and morphological properties of interneurons in the deutocerebrum of male cockroaches which respond to female pheromone. J Comp Physiol 145:447–457
Camazine SM, Hildebrand JG (1979) Central projections of antennal sensory neurons in mature and developing Manduca sexta. Soc Neurosci Abstr 5:155
Chambille I, Rospars JP (1985) Neurons and identified glomeruli of antennal lobes during postembryonic development in the cockroach Blaberus craniifer Burm. (Dictyoptera: Blaberidae). Int J Insect Morphol Embryol 14:203–226
Christensen TA, Hildebrand JG (1984) Functional anatomy and physiology of male-specific pheromone-processing interneurons in the brain of Manduca sexta. Soc Neurosci Abstr 10:862
Christensen TA, Hildebrand JG (1987a) Functions, organization, and physiology of the olfactory pathways in the lepidopteran brain. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure and functions. Wiley, New York, pp 457–484
Christensen TA, Hildebrand JG (1987b) Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol 160:553–569
Erber J, Homberg U, Gronenberg W (1987) The functional roles of the mushroom bodies in insects. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure, and functions. Wiley, New York, pp 485–511
Ernst KD, Boeckh J (1983) A neuroanatomical study on the organization of the central antennal pathways in insects. III. Neuroanatomical characterization of physiologically defined response types of deutocerebral neurons in Periplaneta americana. Cell Tissue Res 229:1–22
Ernst KD, Boeckh J, Boeckh V (1977) A neuroanatomical study on the oganization of the central antennal pathways in insects. II. Deutocerebal connections in Locusta migratoria and Periplaneta americana. Cell Tissue Res 176:285–308
Fischbach KF, Heisenberg M (1984) Neurogenetics and behaviour in insects. J Exp Biol 112:65–93
Frontali N, Mancini G (1970) Studies on the neuronal organisation of cockroach corpora pedunculata. J Insect Physiol 16:2239–2301
Goll W (1967) Strukturuntersuchungen am Gehirn von Formica. Z Morphol Ökol Tiere 59:143–210
Goodman LJ (1981) Organisation and physiology of the insect dorsal ocellar system. In: Autrum H, Jung R, Loewenstein WR, MacKay DM, Teuber HL (eds) Handbook of sensory physiology VII/6C. Springer, Berlin Heidelberg New York, pp 201–287
Gregory GE (1980) The Bodian protargol technique. In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques. Springer, Berlin Heidelberg New York, pp 75–95
Gronenberg W (1986) Physiological and anatomical properties of optical input fibers to the mushroom body in the bee brain. J Insect Physiol 32:695–704
Groth U (1971) Vergleichende Untersuchungen über die Topographie und Histologie des Gehirns der Dipteren. Zool Jb Anat 88:203–319
Hanström B (1928) Vergleichende Anatomie des Nervensystems der wirbellosen Tiere. Springer, Berlin Heidelberg New York
Heisenberg M, Borst A, Wagner S, Byers D (1985) Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet 2:1–30
Hildebrand JG (1985) Metamorphosis of the insect nervous system. Influences of the periphery on the postembryonic development of the antennal sensory pathway in the brain of Manduca sexta. In: Selverston AI (ed) Model neural networks and behavior. Plenum, New York, pp 129–148
Hildebrand JG, Montague RA (1985) Functional organization of olfactory pathways in the central nervous system of Manduca sexta. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Clarendon Press, Oxford, pp 279–285
Hildebrand JG, Matsumoto SG, Camazine SM, Tolbert LP, Blank S, Ferguson H, Ecker V (1980) Organisation and physiology of antennal centers in the brain of the moth Manduca sexta. In: Insect neurobiology and pesticide action (Neurotox 79). Soc Chem Ind, London, pp 375–382
Homberg U (1984) Processing of antennal information in extrinsic mushroom body neurons of the bee brain. J Comp Physiol A 154:825–836
Homberg U (1987) Structure and functions of the central complex in insects. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure and functions. Wiley, New York, pp 347–367
Homberg U, Kingan TG, Hildebrand JG (1987) Immunocytochemistry of GABA in the brain and suboesophageal ganglion of Manduca sexta. Cell Tissue Res 248:1–24
Hoskins SG, Homberg U, Kingan TG, Hildebrand JG (1986) Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res 244:243–252
Humason GL (1972) Animal Tissue Techniques. Freeman, San Francisco
Jawlowski H (1948) Studies on the insect brain. Ann Univ M Curie-Sklodowska C 3:1–37
Jawlowski H (1954) Über die Struktur des Gehirns bei Saltatoria. Ann Univ M Curie-Sklodowska C 8:403–434
Jawlowski H (1957) Nerve tracts in the bee (Apis mellifica) running from the light and antennal organs to the brain. Ann Univ M Curie-Sklodowska C 12:307–323
Kaissling K-E, Priesner E (1970) Die Riechschwelle des Seidenspinners. Naturwissenschaften 57:23–28
Kanzaki R, Shibuya T (1983) Olfactory neural pathway and sexual pheromone responses in the deutocerebrum of the male silkworm moth, Bombyx mori (Lepidoptera, Bombycidae). Appl Ent Zool 18:131–133
Kanzaki R, Shibuya T (1986a) Identification of the deutocerebral neurons responding to the sexual pheromone in the male silkworm moth brain. Zool Sci 3:409–418
Kanzaki R, Shibuya T (1986b) Descending protocerebral neurons related to the mating dance of the male silkworm moth. Brain Res 377:378–382
Kent KS (1985) Metamorphosis of the antennal center and the influence of sensory innervation on the formation of glomeruli in the hawkmoth Manduca sexta. PhD Dissertation, Harvard University, Cambridge, USA
Kent KS, Harrow ID, Quartararo P, Hildebrand JG (1986) An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res 245:237–245
Kent KS, Hoskins SG, Hildebrand JG (1987) A novel serotonin-immunoreactive neuron in the antennal lobe of the sphinx moth Manduca sexta persists throughout postembryonic life. J Neurobiol 189:451–465
Kenyon FC (1896) The brain of the bee. A preliminary contribution to the morphology of the nervous system of the Arthropoda. J Comp Neurol 6:133–210
Koontz MA, Schneider D (1987) Sexual dimorphism in neuronal projections from the antennae of silk moths (Bombyx mori, Antherea polyphemus) and the gypsy moth (Lymantria dispar). Cell Tissue Res 249:39–50
Light DM (1986) Central integration of sensory signals: an exploration of processing of pheromonal and multimodal information in lepidopteran brain. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Clarendon Press, Oxford, pp 287–301
Matsumoto SG, Hildebrand JG (1981) Olfactory mechanisms in the moth Manduca sexta: response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond [Biol] 213:249–277
Mobbs PG (1982) The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Philos Trans R Soc Lond [Biol] 298:309–354
Mobbs PG (1985) Brain structure. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. V. Nervous system, structure and motor function. Pergamon, Oxford, pp 299–370
Mollenhauer HH (1964) Plastic embedding mixtures for use in electron microscopy. Stain Technol 39:111–114
Montague RA, Kent KS, Imperato MT, Hildebrand JG (1983) Projections of antennal-lobe output neurons in the brain of Manduca sexta. Soc Neurosci Abstr 9:216
Olberg RM (1983a) Interneurons sensitive to female pheromone in the deutocerebrum of the male silkworm moth Bombyx mori. Physiol Entomol 8:419–428
Olberg RM (1983b) Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol 152:297–307
Pearson L (1971) The corpora pedunculata of Sphinx ligustri L. and other Lepidoptera, an anatomical study. Philos Trans R Soc Lond [Biol] 259:477–516
Prillinger L (1981) Postembryonic development of the antennal lobes in Periplaneta americana L. Cell Tissue Res 215:563–575
Power ME (1946) The antennal centers and their connections with the brain of Drosophila melanogaster. J Comp Neurol 85:485–517
Rospars JP (1983) Invariance and sex-specific variations of the glomerular organization in the antennal lobes of a moth, Mamesta brassicae and a butterfly, Pieris brassicae. J Comp Neurol 220:80–96
Schildberger K (1983) Local interneurons associated with the mushroom bodies and the central body in the brain of Acheta domesticus. Cell Tissue Res 230:573–586
Schildberger K (1984a) Multimodal interneurons in the cricket brain: properties of identified extrinsic mushroom body cells. J Comp Physiol A 154:71–79
Schildberger K (1984b) Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A 155:171–185
Schneiderman AM (1984) Postembryonic development of a sexually dimorphic sensory pathway in the central nervous system of the sphinx moth, Manduca sexta. PhD Dissertation, Harvard University, Cambridge, USA
Schürmann FW (1974) Bemerkungen zur Funktion der Corpora pedunculata im Gehirn der Insekten aus morphologischer Sicht. Exp Brain Res 19:406–432
Steiger U (1967) Über den Feinbau des Neuropils im Corpus pedunculatum der Waldameise. Z Zellforsch 81:511–536
Stengl M (1985) Elektrophysiologische Ableitungen im Deutocerebrum von Calliphora erythrocephala. Diploma Thesis, Universität Würzburg, FRG
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strausfeld NJ, Bacon JP (1983) Multimodal convergence in the central nervous system of dipterous insects. Fortschr Zool 28:47–76
Strausfeld NJ, Bassemir UK (1985) Lobula plate and ocellar interneurons converge onto a cluster of descending neurons leading to neck and motor neuropil in Calliphora erythrocephala. Cell Tissue Res 240:617–640
Strausfeld NJ, Hausen K (1977) The resolution of neuronal assemblies after cobalt injection into neuropil. Proc R Soc Lond [Biol] 199:463–476
Strausfeld NJ, Seyan HS (1985) Convergence of visual, haltere, and prosternai inputs at neck motor neurons of Calliphora erythrocephala. Cell Tissue Res 240:601–615
Strausfeld NJ, Bassemir U, Singh RN, Bacon JP (1984) Organizational principles of outputs from dipteran brains. J Insect Physiol 30:73–93
Trujillo-Cenóz O, Melamed J (1962) Electron microscope observations on the calyces of the insect brain. J Ultrastruct Res 7:389–398
Trump BF, Smuckler EA, Benditt EP (1961) A method for staining epoxy sections for light microscopy. J Ultrastruct Res 5:343–348
Tyrer NM, Shaw MK, Altman JS (1980) Intensification of cobalt-filled neurons in sections (light and electron microscopy). In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques. Springer, Berlin Heidelberg New York, pp 429–446
Weiss MJ (1974) Neuronal connections and the function of the corpora pedunculata in the brain of the American cockroach Periplaneta americana. J Morphol 142:21–70
Weiss MJ (1981) Structural patterns in the corpora pedunculata of Orthoptera: A reduced silver analysis. J Comp Neurol 203:515–553
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Homberg, U., Montague, R.A. & Hildebrand, J.G. Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta . Cell Tissue Res. 254, 255–281 (1988). https://doi.org/10.1007/BF00225800
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00225800