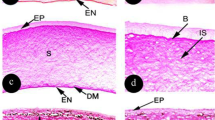
Abstract
The cornea is the first optical element in the path of light entering the eye, playing a role in image formation and protection. Corneas of vertebrate simple camera-type eyes possess microprojections on the outer surface in the form of microridges, microvilli, and microplicae. Corneas of invertebrates, which have simple or compound eyes, or both, may be featureless or may possess microprojections in the form of nipples. It was previously unknown whether cephalopods (invertebrates with camera-type eyes like vertebrates) possess corneal microprojections and, if so, of what form. Using scanning electron microscopy, we examined corneas of a range of cephalopods and discovered nipple-like microprojections in all species. In some species, nipples were like those described on arthropod compound eyes, with a regular hexagonal arrangement and sizes ranging from 75 to 103 nm in diameter. In others, nipples were nodule shaped and irregularly distributed. Although terrestrial invertebrate nipples create an antireflective surface that may play a role in camouflage, no such optical function can be assigned to cephalopod nipples due to refractive index similarities of corneas and water. Their function may be to increase surface-area-to-volume ratio of corneal epithelial cells to increase nutrient, gas, and metabolite exchange, and/or stabilize the corneal mucous layer, as proposed for corneal microprojections of vertebrates.




Similar content being viewed by others
References
Bernhard CG (1971) Evidence for visual function of corneal interference filters. J Insect Physiol 17:2287–2300
Bernhard CG, Miller WH, Møller AR (1963) Function of the corneal nipples in the compound eyes of insects. Acta Phys Scand 58:381–382
Bernhard CG, Gemne G, Sällström J (1970) Comparative ultrastructure of corneal surface topography in insects with aspects on phylogenesis and function. J Comp Physiol A 67:1–25
Collin HB, Collin SP (1988) The cornea of the sand lance, Lymnichthyes fasciatus (Creeiidae). Cornea 7:190–203
Collin HB, Collin SP (2000a) The corneal surface of aquatic vertebrates: microstructures with optical and nutritional function? Philos Trans R Soc Lond B 355:1171–1176
Collin SP, Collin HB (2000b) A comparative SEM study of the vertebrate corneal epithelium. Cornea 19:218–230
Collin SP, Collin HB (2001) The fish cornea: adaptations for different aquatic environments. In: Kapoor BG, Hara TJ (eds) Sensory biology of jawed fishes—new insights. Science Publishers Inc, Enfield, pp 57–96
Collin SP, Collin HB (2006) The corneal epithelial surface in the eyes of vertebrates: environmental and evolutionary influences on structure and function. J Morphol 267:273–291
Denton EJ (1970) Review lecture: on the organization of reflecting surfaces in some marine animals. Phil Trans Roy Soc Lond B 258:285–313
Deparis O, Khuzayim N, Parker A, Vigneron JP (2009) Assessment of the antireflection property of moth wings by three-dimensional transfer-matrix optical simulators. Phys Rev E 79:041910
Dingerkus G, Santoro E (1981) Cornea regeneration in the Pacific giant octopus, Octopus dofleini, and the common octopus O. vulgaris. Experientia 37:368–369
Douglas RH, Marshall NJ (1999) A review of vertebrate and invertebrate ocular filter. In: Archer SN, Djamgoz MBA, Loew ER, Partridge JC, Vallerga S (eds) Adaptive mechanisms in the ecology of vision. Kluwer, London, pp 95–162
Gao H, Liu Z, Zhang J, Zhang G, Xie G (2007) Precise replication of antireflective nanostructures from biotemplates. Appl Phys Lett 90:123115
Johnsen S (2011) The optics of life: a biologist’s guide to light in nature, chapter 5. Princeton University Press, Princeton, pp 116–150
Johnsen S, Widder EA (1999) The physical basis of transparency in biological tissue: ultrastructure and the minimization of light scattering. J Theor Biol 199:181–198
Jordan TM, Partridge JC, Roberts NW (2012) Non-polarizing broadband multilayer reflectors in fish. Nat Photonics (in press)
Kikuta H, Toyota H, Wanji Y (2003) Optical elements with sub wavelength structures. Opt Rev 10:63–73
Lythgoe JN (1975) The structure and function of iridescent corneas in teleost fishes. Proc R Soc Lond B Biol Sci 188:437–457
Meyer RA (1979) Light scattering from biological cells: dependence of backscatter radiation on membrane thickness and refractive index. Appl Opt 18:585–588
Miller WH (1979) Ocular optical filtering. In: Autrum H, Jung R, Loewenstein WR, MacKay DM, Teuber H-L (eds) Handbook of sensory physiology–comparative physiology and evolution of vision in invertebrates a: invertebrate photoreceptors volume VII/6A. Springer, Berlin, pp 69–143
Miller WH, Bernhard CG, Møller AR (1964) Insect corneal nipple array—natural impedance transformer. J Opt Soc Am 54:581
Miller WH, Møller AR, Bernhard CG (1966) The corneal nipple array. In: Bernhard CG (ed) The functional organisation of the compound eye. Pergamon Press, Oxford, pp 21–33
Moody M, Parriss J (1961) The discrimination of polarized light by Octopus: a behavioural and morphological study’. Z Vergl Physiol 44:268–291
Norman M (2003) Cephalopods: a world guide. ConchBooks, Heckenhaim
Parker AR, Hegedus Z, Watts RA (1998) Solar-absorber antireflector on the eye of an Eocene fly (45 Ma). Proc R Soc Lond B Biol Sci 265:811–815
Peisker H, Gorb SN (2010) Always on the bright side of life: anti-adhesive properties of insect ommatidia grating. J Exp Biol 213:3457–3462
Pfister RR (1973) The normal surface of corneal epithelium: a scanning electron microscopic study. Invest Ophthalmol 12:654–668
Pignatelli V, Temple SE, Chiou TH, Roberts NW, Collin SP, Marshall NJ (2011) Behavioural relevance of polarization sensitivity as a target detection mechanism in cephalopods and fishes. Philos Trans Roy Soc Lond B 366:734–741
Quan XH, Fry ES (1995a) Empirical-equation for the index of refraction of seawater. Appl Opt 34:3477–3480
Quan XH, Fry ES (1995b) Empirical-equation for the index of refraction of seawater. Appl Opt 34:3477–3480
Shashar N, Milbury C, Hanlon R (2001) Polarization vision in cephalopods: neuroanatomical and behavioural features that illustrate aspects of form and function. Mar Fresh Behav Physiol 35:57–68
Siebeck UE, Collin SP, Ghoddusi M, Marshall NJ (2003) Occlusable corneas in toadfishes: light transmission, movement and ultrastructure of pigment during light and dark adaptation. J Exp Biol 206:2177–2190
Simmich J, Temple SE, Collin SP (2012) A fish eye out of water: epithelial surface projections on aerial and aquatic corneas of the ‘four eyed fish’ Anableps anableps. Clin Exp Optom 95:140–145
Stavenga DG (2006) Partial coherence and other optical delicacies of lepidopteran superposition eyes. J Exp Biol 209:1904–1913
Stavenga DG, Foletti S, Palasantzas G, Arikawa K (2006) Light on the moth-eye corneal nipple array of butterflies. Proc R Soc Lond B Biol Sci 273:661–667
Sun M, Watson GS, Zheng Y, Watson J, Liang A (2009) Wetting properties on nanostructured surfaces of cicada wings. J Exp Biol 212:3148–3155
Talbot CM, Marshall J (2010a) Polarization sensitivity in two species of cuttlefish—Sepia plangon (Gray 1849) and Sepia mestus (Gray 1849)—demonstrated with polarized optomotor stimuli. J Exp Biol 213:3364–3370
Talbot CM, Marshall J (2010b) Polarization sensitivity and retinal topography of the striped pyjama squid (Sepioloidea lineolata—Quoy/Gaimard 1832). J Exp Biol 213:3371–3377
Temple SE (2007) Effect of salinity on the refractive index of water: considerations for archer fish aerial vision. J Fish Biol 70:1626–1629
Temple SE (2011) Why different regions of the retina have different spectral sensitivities: a review of mechanisms and functional significance of intraretinal variations in spectral sensitivity in vertebrates. Vis Neurosci 28:281–293
Temple SE, Pignatelli V, Cook T, How MJ, Chiou T-H, Roberts NW, Marshall NJ (2012) High-resolution polarisation vision in a cuttlefish. Curr Biol 22:R121–R122
Thorpe A, Douglas RH (1993) Spectral transmission and short-wave absorbing pigments in the fish lens: II Effects of age. Vision Res 33:301–307
Thorpe A, Douglas RH, Truscott RJW (1993) Spectral transmission and short-wave absorbing pigments in the fish lens: I phylogenetic distribution and identity. Vision Res 33:289–300
Vukusic P, Sambles JR (2003) Photonic structures in biology. Nature 424:852–855
Wilson SJ, Hutley MC (1982) The optical properties of’moth eye’ antireflection surfaces. J Mod Opt 29:993–1009
Yoshida A, Motoyama M, Kosaku A, Miyamoto K (1996) Nanoprotuberance array in the transparent wing of a hawkmoth, Cephonodes hylas. Zool Sci 13:525–526
Acknowledgments
We thank Joshua Simmich for assistance with SEM and useful discussions, Michael Archer (UWA) and Lynn Tolley (UQ) for conducting EM services, and Wen-Sung for input on identification and phylogeny. We thank two anonymous referees for their contributions towards improving this manuscript. S.E.T. was supported by postdoctoral fellowships from The University of Queensland and the Natural Sciences and Engineering Research Council of Canada. TMJ was funded by EPSRC (Grant no. EP/E501214/1). Part of this work was supported by the Australian Research Council (SPC; NJM), the Biotechnology and Biological Sciences Research Council (Grant no. BB/G022917/1 to NWR), and the Engineering and Physical Sciences Research Council (Grant no. EP/E501214/1 to NWR), and the Asian Office of Aerospace and Research and Development (NJM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talbot, C., Jordan, T.M., Roberts, N.W. et al. Corneal microprojections in coleoid cephalopods. J Comp Physiol A 198, 849–856 (2012). https://doi.org/10.1007/s00359-012-0755-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-012-0755-9