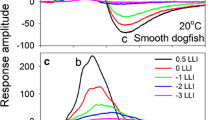
Abstract
Rod and cone visual pigments of 11 marine carnivores were evaluated. Rod, middle/long-wavelength sensitive (M/L) cone, and short-wavelength sensitive (S) cone opsin (if present) sequences were obtained from retinal mRNA. Spectral sensitivity was inferred through evaluation of known spectral tuning residues. The rod pigments of all but one of the pinnipeds were similar to those of the sea otter, polar bear, and most other terrestrial carnivores with spectral peak sensitivities (λmax) of 499 or 501 nm. Similarly, the M/L cone pigments of the pinnipeds, polar bear, and otter had inferred λmax of 545 to 560 nm. Only the rod opsin sequence of the elephant seal had sensitivity characteristic of adaptation for vision in the marine environment, with an inferred λmax of 487 nm. No evidence of S cones was found for any of the pinnipeds. The polar bear and otter had S cones with inferred λmax of ∼440 nm. Flicker-photometric ERG was additionally used to examine the in situ sensitivities of three species of pinniped. Despite the use of conditions previously shown to evoke cone responses in other mammals, no cone responses could be elicited from any of these pinnipeds. Rod photoreceptor responses for all three species were as predicted by the genetic data.



Similar content being viewed by others
References
Adrian ED (1946) Rod and cone components in the electric response of the eye. J Physiol 105:24–37
Asenjo AB, Rim J, Oprian DD (1994) Molecular determinants of human red:green color discrimination. Neuron 12:1131–1138
Bininda-Emonds ORP (2000) Factors influencing phylogenetic inference: a case study using the mammalian carnivores. Mol Phylo Evol 16:113–126
Bowmaker JK (1995) The visual pigments of fish. Prog Retinal Eye Res 15:1–31
Bowmaker JK (1998) Evolution of colour vision in vertebrates. Eye 12:541–547
Calderone JB, Jacobs GH (2003) Spectral properties and retinal distributions of ferret cones. Vis Neurosci 20:11–17
Chiu MI, Zack DJ, Wang Y, Nathans J (1994) Murine and bovine blue cone pigment genes: cloning and characterization of two new members of the S family of visual pigments. Genomics 21:440–443
Collins FD, Morton RA (1950). Studies on rhodopsin. I. Methods of extraction and the absorption spectrum. Biochem J 47:3–10
Cowing JA, Poopalasundaram S, Wilkie SE, Bowmaker JK, Hunt DM (2002) Spectral tuning and evolution of short wave-sensitive cone pigments in cottoid fish from Lake Baikal. Biochem 41:6019–6025
Crescitelli F (1958) The natural history of visual pigments. Ann New York Acad Sci 74:230–255
Crognale MA, Levenson D, Ponganis PJ, Deegan JFII, Jacobs GH (1998) Cone spectral sensitivity in the harbour seal (Phoca vitulina) and implications for colour vision. Can J Zool 76:2114–2118
Dehnhardt G, Mauck B, Hanke W, Bleckmann H (2001) Hydrodynamic trail-following in harbor seals (Phoca vitulina). Science 293:102–104
Fasick JI, Applebury ML, Oprian DD (2002) Spectral tuning in the mammalian short-wavelength sensitive cone pigments. Biochem 41:6860–6865
Fasick JI, Lee N, Oprian DD (1999) Spectral tuning in the human blue cone pigment. Biochem 38:11593–11596
Fasick JI, Robinson PR (1998) Mechanism of spectral tuning in the dolphin visual pigments. Biochem 37:433–438
Fasick JI, Robinson PR (2000) Spectral-tuning mechanisms of marine mammal rhodopsins and correlations with foraging depth. Vis Neurosci 17:781–788
Govardovskii VI, Fyhrquist N, Reuter T, Kuzimin DG, Donner K (2001) In search of the visual pigment template. Vis Neurosci 17:509–528
Griebel U, Peichl L (2003) Color vision in aquatic mammals: facts and open questions. Aquat Mammals 29:18–30
Hargrave PA (1982) Rhodopsin chemistry, structure, and topography. Prog Retinal Eye Res 1:1–51
Hillis DM, Moritz C, Mable BK (1996) Molecular systematics, 2nd edn. Sinauer, Sunderland
Hunt DM, Dulai KS, Partridge JC, Cottrill P, Bowmaker JK (2001) The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J Exp Biol 204:3333–3344
Hunt DM, Fitzgibbon J, Slobodyanyuk SJ, Bowmaker JK (1996) Spectral tuning and molecular evolution of rod visual pigments in the species flock of Cottoid fish in Lake Baikal. Vis Res 36:1217–1224
Jacobs GH (1993) The distribution and nature of colour vision among the mammals. Biol Rev 68:413–471
Jacobs GH, Neitz J, Deegan JFII (1991) Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353:655–656
Jacobs GH, Deegan JFII, Crognale MA, Fenwick JA (1993) Photopigments of dogs and foxes and their implications for canid vision. Vis Neurosci 10:173–180
Jacobs GH, Neitz J, Krogh K (1996a) Electroretinogram flicker photometry and its applications. J Opt Soc Am A13:641–648
Jacobs GH, Neitz M, Neitz J (1996b) Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc R Soc Lond Biol Sci 263:705–710
Jacobs GH, Fenwick JA, Williams GA (2001) Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol 204:2439–2446
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Lavigne DM, Bernholz CD, Ronald K (1977) Functional aspects of pinniped vision. In: Harrison RJ (ed) Functional anatomy of marine mammals, vol 3. Academic, London, pp 135–173
Lavigne DM, Ronald K (1972) The harp seal, Pagophilus groenlandicus, part 23—spectral sensitivity. Can J Zool 50:1197–1206
Lavigne DM, Ronald K (1975) Pinniped visual pigments. Comp Biochem Physiol 52B:325–329
Levenson DH, Schusterman RJ (1997) Pupillometry in seals and sea lions: ecological implications. Can J Zool 75:2050–2057
Levenson DH, Dizon A (2003) Genetic evidence for the ancestral loss of short-wavelength-sensitive cone pigments in mysticete and odontocete cetaceans. Proc R Soc Lond Biol Sci B270:673–679
Lythgoe JN, Dartnall HJA (1970) A “deep sea rhodopsin” in a mammal. Nature 227:955–956
Marmor MF, Zrenner E (1999) Standard for clinical electroretinography, 1999 update. Doc Ophth 97:143–156
McFarland WN (1971) Cetacean visual pigments. Vis Res 11:1065–1076
Nathans J (1990) Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochem 29:937–942
Oprian DD, Asenjo AA, Lee N, Pelletier S (1991) Design, chemical synthesis, and expression of genes for the three human color vision pigments. Biochem 30:11367–11372
Peichl L, Behrmann G, Kroger RHH (2001) For whales and seals the ocean is not blue: a visual pigment loss in marine mammals. Eur J Neurosci 13:1520–1528
Peichl L, Moutairou K (1998). Absence of short-wavelength sensitive cones in the retinae of seals (Carnivora) and African giant rats (Rodentia). Eur J Neurosci 10:2586–2594
Rice DW (1998) Marine mammals of the world: systematics and distribution. Society for Marine Mammalogy, Lawrence, KS
Robinson PR, Griffith K, Gross JM, O’Neil MC (1999) A back-propagation neural network predicts absorption maxima of chimeric human red:green visual pigments. Vis Res 39:1707–1712
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA 74:5463–5467
Schusterman RJ, Kastak D, Levenson DH, Reichmuth CJ, Southall BL (2000) Why pinnipeds don’t echolocate. J Acoust Soc Am 107:2256–2264
Shi Y, Yokoyama S (2003) Molecular analysis of the evolutionary significance of ultraviolet vision in vertebrates. Proc Nat Acad Sci USA 100:8308–8313
Southall KD, Oliver GW, Lewis JW, Le Boeuf BJ, Levenson DH, Southall BL (2002) Visual pigment sensitivity in three deep diving marine mammals. Mar Mam Sci 18:275–281
Swofford DL (2001) Phylogenetic analysis using parsimony, version 4. Sinauer, Sunderland
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucl Acid Res 22:4673–4680
Tremblay F, Parkinson JE (2003) Alteration of electroretinographic recordings when performed under sedation or halogenate anesthesia in a pediatric population. Doc Opthalm 107:271–279
Walls G (1942) The vertebrate eye and its adaptive radiation. The Cranbrook Institute of Science, Bloomfield Hills
Wasserschaff M, Schmidt JGH (1986) Electroretinographic responses to the additional of nitrous oxide to halothane in rats. Doc Ophthalm 64:347–354
Yokoyama S, Radlwimmer FB (1998) The “five sites” rule and the evolution of red and green color vision in mammals. Mol Biol Evol 15:5660–5667
Yokoyama S, Radlwimmer FB (1999) The molecular genetics of red and green color vision in mammals. Genetics 153:919–932
Yokoyama S, Radlwimmer FB (2001) The molecular genetics and evolution of red and green color vision in vertebrates. Genetics 158:1697–1710
Yokoyama S, Yokoyama R (1996) Adaptive evolution of photoreceptors and visual pigments in vertebrates. Ann Rev Ecol Sys 27:543–567
Acknowledgments
Our principal thanks go to the marine mammal stranding program of SeaWorld, San Diego, CA for providing rehabilitated animals for electroretinographic (ERG) examination, as well as tissue samples from several species for genetic study. Representatives of the U.S. Fish and Wildlife Service (Fairbanks, AK), Alaska Department of Fish and Game (Anchorage, AK), California Department of Fish and Game (Santa Cruz, CA), and Dr. Siniff’s Antarctic research group (McMurdo station, Antarctica) also provided valuable samples for genetic examination. We appreciate the valuable input of the dissertation committee members of DHL who reviewed earlier versions of this manuscript. We thank the graduate student support for DHL provided by the NSF Office of Polar Programs (NSF 98-14794). Financial support for this project provided by the San Diego Achievement Rewards for College Scientists (ARCS) program to DHL, NSF (IBN 00-78540) and the UCSD Chancellors Fund grants to PJP, and an NIH grant (EY002052) to GHJ. All animal husbandry and experimental procedures were conducted following protocols approved by the National Marine Fisheries Service (NMFS) Office of Protected Resources (permit 732–1487), the University of California, San Diego Institutional Animal Care and Use Committee (IACUC; permit S00092), and the NIH Principles of animal care, publication No. 86-23 (1985).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Levenson, D.H., Ponganis, P.J., Crognale, M.A. et al. Visual pigments of marine carnivores: pinnipeds, polar bear, and sea otter. J Comp Physiol A 192, 833–843 (2006). https://doi.org/10.1007/s00359-006-0121-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0121-x