Abstract
Purpose
Recent advancements in screening, prostate MRI, robotic surgery, and active surveillance have influenced the profile of patients undergoing radical prostatectomy (RP). We sought to examine their impact on trends in clinicodemographic, risk classification, and adverse pathology in men undergoing surgery.
Methods
We queried the National Cancer Database for clinicodemographic, risk group, and pathology data in men undergoing upfront RP between 2006 and 2020. Patients were categorized by NCCN risk groups, and trends were assessed among 2006–2010, 2011–2015, and 2016–2020 periods. Endpoints included rates of pT3, positive surgical margins (PSM), pathologic upstaging, and Gleason grade group (GG) upgrading.
Results
610,762 patients were included. There were significant increases in African Americans (9.8–14.1%), comorbidities (2.1–5.2% with Charlson scores > 1), and robot-assisted RP (78–84%). Over the three time periods, high-risk cases increased from 15 to 20 to 27%, and intermediate-risk from 54 to 51 to 60%. Overall rates of pT3 rose from 20 to 38%, and PSM from 20 to 27% (p < 0.001). Pathologic upstaging increased in low (6–15%), intermediate (20–33%), and high-risk groups (42–58%) –p < 0.001. Gleason upgrading rose in low-risk (45–59%, p < 0.001), with slight reductions in the intermediate and high-risk groups.
Conclusions
Recent trends in RP indicate a shift towards more advanced disease, evidenced by increasing rates of pT3, PSM, and pathologic upstaging across all NCCN risk groups. These findings emphasize the need for a careful balance in applying fascia and nerve-sparing techniques to avoid compromising oncological safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate Cancer (PCa) has experienced significant changes in both diagnostic and treatment methods over the past fifteen years. Screening practices that initially involved widespread, non-selective methods, then shifted following the US Preventive Services Task Force recommendations for a more selective approach [1], emphasizing a shared decision-making process [2]. On top of policy swings, adherence to guidelines differs in time and across specialties, societies, and providers [3, 4]. Management strategies have undergone notable changes, including the increasing adoption of active surveillance (AS), the introduction of focal therapy, and the transition from open to robot-assisted radical prostatectomy (RP) [5, 6]. As RP has evolved, with reduced morbidity, accumulated surgical experience, and advancements in technology, its use has become more prevalent, particularly among high-risk patients [3, 7].
These transformations may modify the profile of patients undergoing RP. In fact, retrospective series have indicated increasing age, comorbidities, and PCa risk [8,9,10] among RP candidates. These shifts are not merely of demographic or descriptive significance; they also hold the potential to impact the functional and oncological outcomes experienced by patients. Additionally, the presence of residual or recurrent disease after RP implies a greater need for advanced imaging, multidisciplinary care, and additional treatment such as radiation therapy (RT) and androgen deprivation therapy [8, 11]. This changing scenario is relevant to clinicians who must provide patients with up-to-date data on oncological and functional outcomes of RP and later define the most appropriate surgical strategy.
To our knowledge, this is the first study to address these changes in a large contemporary RP population. We sought to characterize temporal trends in sociodemographic, risk profiles, adverse pathologic features, and the need for additional therapy in men undergoing RP. We hypothesized that RP is increasingly being performed on men with higher-risk disease, potentially influencing tumor upstaging and positive surgical margins rates.
Materials and methods
Data source
The National Cancer Database (NCDB) is the largest oncology registry in the United States, capturing over 70% of cancers diagnosed and treated in hospitals annually [12]. It is the product of, and maintained by, a joint initiative between the Commission on Cancer of the American College of Surgeons and the American Cancer Society. Deidentified sociodemographic, clinical, and treatment information is collected from more than 1,500 accredited hospitals by trained personnel on a standard methodology [12].
Cohort selection
Men > 18 years of age diagnosed with prostate adenocarcinoma who underwent radical prostatectomy as their primary treatment between 2006 and 2020 were identified using the International Classification of Diseases for Oncology, 3rd edition (topography code C61.9). This period was intentionally set after the major revision in the Gleason scoring system introduced in 2005 to allow for a more consistent evaluation of temporal trends, thus avoiding possible confusion with earlier versions. Patients who had received previous radiation or hormone therapy, presented with distant metastasis, or had missing data regarding the procedure, risk group determinants (PSA, Gleason, clinical T stage), or surgical margin status were excluded. The study population selection process is detailed in Fig. 1. Selecting a broad time frame and applying few exclusion criteria were deliberate choices to enable a real-world evaluation of radical prostatectomy (RP) utilization across various risk groups and disease scenarios.
Data extraction and endpoints
We collected sociodemographic data including age, race, ethnicity, Charlson-Deyo comorbidity score, insurance status, and rurality status. Treatment information, including year, facility type (academic or community), and RP approach (robot-assisted vs. other), were extracted. Preoperative clinical risk determinants including Gleason score (GS), PSA levels, and clinical T staging were compiled and patients were classified according to a modified version of the NCCN Guidelines into low risk (cT1c–T2a, GS ≤ 6, and PSA < 10 ng/mL), intermediate risk (cT2b–T2c, GS 7, or PSA 10–20 ng/mL), and high risk (cT3–T4, GS 8–10, or PSA > 20 ng/mL). Finally, the study population was divided into three sequential time periods (2006–2010, 2011–2015, and 2016–2020) to assess temporal trends of the above-mentioned variables.
The endpoints were rates of pathologic T3 (pT3) disease, positive surgical margins, pathologic upstaging, and Gleason upgrading on surgical specimen analysis. Pathologic upstaging was defined as cT1-T2 tumors that were ultimately found to be pT3-T4 (pT3+) upon surgical specimen analysis. Upstaging rates were calculated by dividing the number of upstaged men by all patients with cT1-T2 (i.e. exposed to upstaging as per this definition) in each NCCN risk group. Gleason upgrading was defined as any increase in Grade Group (GG 1–5) – including GG2 to GG3 in intermediate-risk patients – at the surgical specimen compared to pre-operative (biopsy) values. Similarly, upgrading rates were calculated separately for each risk group by dividing the number of upstaged cases by the total of cases in each NCCN category. Temporal trends were evaluated across the different NCCN risk groups by year and across the three time periods described above. As both robotic surgery and post-operative Gleason scores were not significantly captured until 2010, these two variables were exceptionally analyzed considering only the two latest time periods (i.e. 2011–2015 and 2016–2020).
Statistical analysis
We compared characteristics among the three time periods using an ANOVA test for continuous variables and a Chi-squared test for proportions. Changes in the relative frequency of variables and primary endpoints over time were assessed using the Mantel–Haenszel test for trends. Statistical analysis was performed using SAS v. 9.4 software (SAS Institute, Cary, NC, USA). A P value < 0.05 was considered significant.
Results
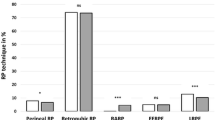
A total of 610,762 patients underwent RP between 2006 and 2020 and met the inclusion criteria. Baseline sociodemographic, clinical, and treatment characteristics are detailed in Table 1. There were no clinically relevant changes in median age across the different eras. We found a statistically significant increase in the frequency of comorbidity scores (2.1–5.2% with Charlson scores > 1) and the use of robot-assisted RP (78–84%).
Over the three time periods, the percentages of high-risk patients were 15%, 20%, and 27%, while intermediate risk were 54%, 51%, and 60%, respectively. As shown in Fig. 2A, these shifts corresponded with a 58% drop in low-risk cases (p < 0.001 for all). The analysis of individual components of the NCCN risk classification reveals an increase in GS ≥ 8 from 9 to 22%, and GS 7 from 47 to 64% between 2006 and 2020 (p < 0.001). During the same period, levels of PSA ranging from 10–20ng/dL increased from 10 to 17%, while no clinically significant changes occurred in cT3 + stage and PSA > 20 ng/mL categories. Correspondingly, there were reductions in the percentages of low-risk features, specifically in GS 6 and PSA levels < 10 ng/dL. These temporal trends are illustrated in Fig. 2b and d.
Overall, pT3 rates rose from 20 to 38%, and PSM rates from 20 to 27% across the studied time periods (p < 0.001 for both). Elevations in both pT3 rates and PSM were significant across all NCCN risk groups, although growth rates differed among them (Fig. 3). Considering the three time periods, PSM rates were 15%, 16%, and 18% for pT2 (p < 0.001), and 42%, 41%, and 43% for pT3 cases (p = 0.0164). Similarly, rising tumor upstaging rates were observed in low (6–15%), intermediate (20–33%), and high-risk patients (42–58%) between the first and last time periods (p < 0.001 for all, Fig. 4).
Gleason group upgrading showed different trends according to NCCN risk groups. As illustrated in Fig. 5, upgrading rates in the low-risk group rose from 45% in 2011–2015 to 59% in 2016–2020 (p < 0.001). Conversely, intermediate and high-risk patients demonstrated slight reductions, respectively, from 20 to 19% (p < 0.001), and 19–17% in the same time periods (p < 0.001).
Discussion
Our study shows a trend towards the surgical treatment of more advanced PCa, in particular, due to higher grade disease, more lymph node metastases, and rising rates of PSM and pT3 across all NCCN risk groups. Radical prostatectomy has been performed in slightly older men with more comorbidities. A higher representation of African American patients was observed, likely as a result of selection bias due to their higher rates of aggressive disease; yet other competing factors (e.g. disparities in access to health care) cannot be ruled out. We also observed a notable and steady rise in pathologic upstaging across all risk groups after radical prostatectomy between 2006 and 2020.
The elevation in pT3 rates is likely multifactorial: in addition to the growing utilization of RP for treating high-risk PCa, more low and favorable intermediate-risk cases have been managed with active surveillance or focal therapy, rather than immediate surgery [13, 14]. Since 2021, AS has become the preferred treatment modality according to the NCCN guidelines for very low and low-risk PCa. Moreover, the shift towards less intense screening strategies might delay diagnosis and result in more advanced disease stages at the time of radical prostatectomy [8, 15]. The increase in pT3 rates within intermediate and especially low-risk categories suggests a shift towards higher-risk PCa among men undergoing surgery, both across and within risk groups. In fact, contemporary low-risk patients undergoing RP likely have more adverse features than in the past. These factors might also have contributed to the more than two-fold increase in pathologic node-positive disease over the last 15 years. As a result, fewer patients might be cured with surgery alone, necessitating more multimodal therapy, which adversely impacts functional outcomes.
The notable increase in tumor pathologic upstaging across all NCCN groups is primarily a consequence of the growing discrepancy between stable rates of clinical and rising pathologic T3 stages. This trend persisted despite greater availability and refined expertise with prostate MRI [16]. This observation may reflect MRI’s limited sensitivity in detecting extraprostatic extension [17]. Additionally, persistent use of DRE alone for clinical staging may understage cases with limited extraprostatic extension. Still, such observations merit further investigation as they raise concern for unanticipated upstaging in this population, which is associated with lower rates of Pentafecta (functional, oncologic, and perioperative success) [18].
Gleason upgrading exhibited differing trends across NCCN risk groups that reflect the evolution of PCa management in the last years. The significant elevation in upgrading among low-risk cases suggests a more selective indication of RP for those with unfavorable features within this category, such as high-volume Gleason 6 or elevated PSA density. Also, Gleason 6 (Grade Group 1) tumors are less visible in multiparametric MRI. In other words, trends in NCCN low-risk group indicate a more selective application of surgery to cases in the highest spectrum of risk. Conversely, the minimal reduction in upgrading rates in intermediate and high-risk patients is relevant as they would be expected to rise amidst the major shift to higher-risk disease observed in RPs. This suggests an improved detection of the highest-grade disease within the prostate before surgery, likely due to the expansion of imaging and targeted biopsies. This hypothesis is supported by evidence demonstrating that multiparametric MRI not only improves the detection of clinically significant PCa [19]but also reduces pathologic upstaging in the RP specimen [20].
Rising PSM rates have been previously reported and largely mirror the significant increase in pT3 rates across all risk groups [8, 10]. PSM increased despite the growing adoption and accumulated experience with the robotic platform over the years. Combined with enhanced precision and magnified visualization, one would expect stable or even declining PSM rates, particularly for organ-confined (pT2) disease. Thus, the observed 20% increase in PSM rates in pT2 cases is concerning for surgical decision-making because more conservative approaches, such as fascia and nerve-sparing techniques, might increase the incidence and extension of PSM, potentially risking RP oncological effectiveness. As surgeons may choose to perform less fascia and nerve-sparing procedures, our findings carry implications on functional outcomes as well. Indeed, this was highlighted by a single-center study of 10,000 RPs which showed a decrease in nerve-sparing procedures as pT3 and PSM rates went up [8]. Consequently, the current rates of continence and potency may no longer be comparable to the early robot-assisted RP series [9, 21, 22]. Although still frequently cited as reference standards for functional outcomes, these series contain higher proportions of low-risk and favorable cases. Therefore, our findings suggest that surgical protocols and patient counseling may need to be revised to more accurately reflect these recent changes.
The strengths of our study lie in its detailed information on demographics, cancer features, and postoperative outcomes coupled with a large hospital-based cohort. This allows for a broader understanding of how the RP population has changed over time. While not a population-based registry, NCDB captures more than 70% of cancers diagnosed and treated annually in the United States across more than 1,500 commission on cancer-accredited facilities [12, 23]. This makes it particularly valuable for assessing real-world practices, in contrast to previous studies addressing changes in RP patient profiles from academic centers, known for achieving superior outcomes [24].
The main limitations of our study are linked to the retrospective analysis of large datasets, which inherently includes risks of bias. However, the NCDB is a substantial national dataset that is well-suited for analyzing trends. Another limitation is the 2005 ISUP major review of the Gleason grading system, which experienced a delay in adoption. Consequently, Gleason scores, particularly from the initial years following this review, may not be entirely comparable even after limiting analysis to the period post-modifications [25]. Lastly, NCDB does not capture MRI utilization and whether clinical tumor stage was based on digital rectal examination (DRE) or MRI findings. As MRI has superior local staging accuracy [26], persistent utilization of DRE can lead to rising pathologic upstaging rates particularly when the population is shifting to higher-risk disease.
Conclusions
Contemporary data from a large hospital-based registry indicate that RP is increasingly performed on men with more comorbidities and higher risk PCa. This trend is marked by rising rates of PSM, pT3, and pathologic upstaging across all NCCN risk groups, despite growing experience with prostate MRI and robotic surgery. Gleason upgrading rates suggest a more selective indication for RP in low-risk cases and improved detection of high-grade cancer in the prostate before surgery, likely due to the expansion of targeted biopsies. These trends should guide surgical decision-making, emphasizing the need for a careful balance between the use of fascia and nerve-sparing techniques to avoid compromising oncological safety.
Data availability
This study used data made available by the National Cancer Database (NCDB) through the Participant User Data File (PUF), a HIPAA-compliant, de-identified dataset. The PUF includes patient-level data submitted to the NCDB by Commission on Cancer (CoC)-accredited programs. Access to NCDB PUFs is restricted to participating programs and requires an application process. Therefore, the original dataset cannot be disclosed.
References
Moyer VA, Force USPST (2012) Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157:120
Grossman DC, Curry SJ, Owens DK et al (1901) Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA, 319: 2018
Loeb S, Byrne NK, Wang B et al (2020) Exploring variation in the Use of Conservative Management for low-risk prostate Cancer in the Veterans affairs Healthcare System. Eur Urol 77:683
Cooperberg MR, Meeks W, Fang R et al (2023) Time trends and Variation in the use of active surveillance for management of low-risk prostate Cancer in the US. JAMA Netw Open 6:e231439
Cooperberg MR, Carroll PR (2015) Trends in Management for patients with localized prostate Cancer, 1990–2013. JAMA 314:80
Logan CD, Mahenthiran AK, Siddiqui MR et al (2023) Disparities in access to robotic technology and perioperative outcomes among patients treated with radical prostatectomy. J Surg Oncol 128:375
Agrawal V, Ma X, Hu JC et al (2020) Trends in diagnosis and disparities in initial management of high-risk prostate Cancer in the US. JAMA Netw Open 3:e2014674
Onol FF, Ganapathi P, Rogers H (2019) Changing clinical trends in 10 000 robot-assisted laparoscopic prostatectomy patients and impact of the 2012 US Preventive Services Task Force’s statement against PSA screening. BJU Int 124:1014
Schiffmann J, Haese A, Boehm K et al (2017) Ten-year experience of robot-assisted radical prostatectomy: the road from cherry-picking to standard procedure. Minerva Urol Nefrol 69:69
Ahlering T, Huynh LM, Kaler KS et al (2019) Unintended consequences of decreased PSA-based prostate cancer screening. World J Urol 37:489
Yossepowitch O, Briganti A, Eastham JA et al (2014) Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 65:303
Boffa DJ, Rosen JE, Mallin K et al (2017) Using the National Cancer Database for Outcomes Research: a review. JAMA Oncol 3:1722
Kaps B, Leapman M, An Y (2020) Trends in prostatectomy utilization: increasing upfront prostatectomy and postprostatectomy radiotherapy for high-risk prostate cancer. Cancer Med 9:8754
Gray PJ, Lin CC, Cooperberg MR et al (2017) Temporal trends and the impact of race, insurance, and socioeconomic status in the management of localized prostate Cancer. Eur Urol 71:729
Kelly SP, Anderson WF, Rosenberg PS et al (2018) Past, current, and Future Incidence Rates and Burden of metastatic prostate Cancer in the United States. Eur Urol Focus 4:121
Giganti F, Rosenkrantz AB, Villeirs G et al (2019) The evolution of MRI of the prostate: the past, the Present, and the future. AJR Am J Roentgenol 213:384
de Rooij M, Hamoen EH, Witjes JA et al (2016) Accuracy of magnetic resonance imaging for local staging of prostate Cancer: a diagnostic Meta-analysis. Eur Urol 70:233
Afferi L, Moschini M, Baumeister P et al (2021) Trends in risk-group distribution and Pentafecta outcomes in patients treated with nerve-sparing, robot-assisted radical prostatectomy: a 10-year low-intermediate volume single-center experience. World J Urol 39:389
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-Targeted or standard biopsy for prostate-Cancer diagnosis. N Engl J Med 378:1767
Weinstein IC, Wu X, Hill A et al (2023) Impact of magnetic resonance imaging targeting on pathologic upgrading and downgrading at Prostatectomy: a systematic review and Meta-analysis. Eur Urol Oncol 6:355
Patel VR, Coelho RF, Chauhan S et al (2010) Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int 106:696
Menon M, Shrivastava A, Kaul S et al (2007) Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol 51:648
Lerro CC, Robbins AS, Phillips JL et al (2013) Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol 20:1759
Trinh QD, Schmitges J, Sun M et al (2011) Radical prostatectomy at academic versus nonacademic institutions: a population based analysis. J Urol 186:1849
Danneman D, Drevin L, Robinson D et al (2015) Gleason inflation 1998–2011: a registry study of 97,168 men. BJU Int 115:248
Soeterik TFW, van Melick HHE, Dijksman LM et al (2021) Multiparametric magnetic resonance imaging should be Preferred over Digital rectal examination for prostate Cancer Local Staging and Disease Risk classification. Urology 147:205
Acknowledgements
Data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Funding
Additionally, no external or specific funding was granted or used in this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Competing interests
The authors declare that there are no direct or indirect competing interests with regards to the subjects addressed in this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freitas, P.F.S., Blachman-Braun, R., Soodana-Prakash, N. et al. Changing times: trends in risk classification, tumor upstaging, and positive surgical margins after radical prostatectomy - results from a contemporary National Cancer Database study. World J Urol 42, 551 (2024). https://doi.org/10.1007/s00345-024-05262-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05262-0