Abstract
The edible part of citrus fruit is composed of juice vesicles/sacs which develop from the endocarp, the two to three inner cell layers of the white spongy peel termed albedo. Juice sac primordia usually appear 1 week after anthesis. Hormones, especially auxin and gibberellin, play a role in pericarp development during the ovary-to-fruit transition, but their effect on juice vesicle induction has not been studied. Here, hormone profiling in the pericarp and changes in the expression of their corresponding genes in the endocarp and pericarp were compared between two citrus cultivars: Calabria citron, in which juice sacs develop normally, and Yemenite citron, in which juice sac formation does not initiate. Most of the identified hormones, abscisic acid, gibberellin A4, indole-3-acetic acid, isopentenyladenine, jasmonic acid and zeatin riboside, were at higher levels in Yemenite than in Calabria. Overall, changes in abscisic acid levels in the pericarp were very well correlated with changes in the expression of abscisic acid-related genes in the endocarp. However, the application of various hormones, including abscisic acid, to Calabria flowers failed to arrest juice sac initiation. The possible involvement of abscisic acid and other hormones in the process of juice vesicle initiation and pericarp growth is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is a major crop worldwide, consumed fresh and in the juice industry (Spreen et al. 2020). According to classical taxonomy, the genus Citrus and its related genera belong to the angiosperm subfamily Aurantioideae of the family Rutaceae. Trees belonging to this subfamily are characterized by a unique type of true berry fruit, the hesperidium (Esau 1965; Fahn 1990). Its pericarp originates from the mature ovary wall, composed of the exocarp (flavedo—outer skin), the mesocarp (albedo—spongy white inner peel) and the endocarp (two to three inner cell layers of the mesocarp) (Schneider 1968). The edible part of the fruit—the pulp—is composed of juice vesicles/sacs (JSs), which develop from the endocarp during early stages of fruit development (Tadeo et al. 2020) (Fig. 1a, c). The primordia of the JSs develop by anticlinal divisions of the endocarp cells and periclinal divisions of the adjoining subepidermal cells (Nii and Coombe 1988; Tisserat et al. 1990; Burns et al. 1994). In a few cases, such as parthenocarpic fruit, JS primordia are visible even before fertilization and fruit set (Burns et al. 1992). The JSs develop into the ovary locules, defined as sections in the fruitlet, and they are connected to the section wall by a stalk. The epidermis covering the JSs also covers the section generating continuous layers of cells. Three major vascular bundles reach each section and feed the vesicles: a dorsal vascular bundle and two side vascular bundles. Most of the JSs develop from the dorsal wall of the section, but some develop from the side wall, adjacent to the side vascular bundle (Koch and Avigne 1990). JSs are not directly connected to the vascular bundle; therefore, translocation of photoassimilates into the JS stalk most likely occurs by diffusion (Sadka et al. 2019). If present, the seeds develop on the inner side of the fruit, where the sections merge, or along the ovary wall. Growth of the JSs usually occurs during stage II of fruit development. Cellular growth is characterized by massive enlargement of the vacuole, which occupies over 90% of the total cell volume in the mature fruit, and its content is released as juice when the fruit is squeezed.
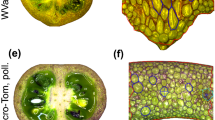
Yemenite citron does not contain juice vesicles. Cross sections of fruit of ‘Calabria’ (a) and ‘Yemenite’ (b) citrons at the end of Stage I (cell division) of fruit development. While exocarp, mesocarp and exocarp (written in white letters) refer to the botanical definitions of fruit tissues, other terms are those commonly used for citrus (written in red letters). Scanning electron micrographs of ‘Calabria’ (c) and ‘Yemenite’ (d) Sects. 3–4 weeks after anthesis
The ovary-to-fruit transition, during which the JSs initiate, is thought to be controlled by hormones, especially auxin and gibberellins (GAs). Increases in their endogenous levels have been detected in the fertilized egg/embryo and ovary wall/pericarp following pollination and fertilization (Garcia-Papi and Garcia-Martinez 1984; Ali-Dinar et al. 1988; Talón et al. 1990a, b), and their external application can lead to parthenocarpic fruit development (Mesejo et al. 2016). It is commonly accepted that induced indole-3-acetic acid (IAA) levels lead to GA biosynthesis, involved in cell division in the ovary wall/pericarp (Bermejo et al. 2018). In contrast to the positive relationships between IAA and GA and the ovary-to-fruit transition, abscisic acid (ABA) has been suggested to antagonize GA action (Ruan et al. 2012). The levels of the two most abundant cytokinins (CTKs) in citrus—trans-zeatin (tZ) and N6-(Δ2-isopentenyl) adenine (iP)—increase during anthesis (Bermejo et al. 2015).
When removed from the fruit and transferred to synthetic liquid or solid media, such as Murashige and Skoog (MS), JSs show substantial growth (Kordan 1974; Unger and Feng 1978; Einset 1978; Gülsen et al. 1981; Altman et al. 1982; Tisserat and Galletta 1987; Tisserat et al. 1988; Harada et al. 2001). Their growth can also be induced from pre-initiated primordia by incubation on synthetic media with segments of very young peel-containing fruitlets of lemon, grapefruit, mandarin, citron and orange (Gülsen et al. 1981; Altman et al. 1982; Tisserat et al. 1988). Increasing sugar content and the addition of some hormones (auxins, CTKs and GAs), or natural orange juice, promote callus development from the JSs (Kordan 1974; Erner et al. 1975; Unger and Feng 1978). It should be noted that hormones are not necessarily required for the growth of JSs in culture; however, the addition of auxin (IAA or 2,4-dichlorophenoxyacetic acid [2,4-D]), GA3 or CTK (benzyladenine) may increase the fresh weight of lemon, grapefruit, orange and mandarin JSs originating from small fruitlets (Einset 1978; Gülsen et al. 1981; Altman et al. 1982).
Despite these studies, the regulation of JS primordium induction has never been described. Considering their major roles in numerous fundamental processes, plant hormones may well control this process, either separately or in concert. As mentioned above, various hormones—auxin, CTK and GA—affect JS growth and callus development. Moreover, application of GA3 and CTK (benzyladenine) during citrus flowering induces fruitlet size (both hormones) and the number of JSs (GA only), due to a transient increase in cell division in the ovary wall (Guardiola et al. 1993; Mesejo et al. 2016). In fact, GA is commonly applied during citrus flowering to enhance fruitlet survival. Depending on its time of application, synthetic auxin, such as trichlorophenoxyacetic acid (2,4,5-TP), results in opposite outcomes; when it is applied during flowering peak or fruit set, it reduces fruitlet size due to depressed cell expansion (Guardiola et al. 1988, 1993; Bons et al. 2015). However, a later application, i.e., on fruits of about 30 mm in diameter, results in induced fruit growth (Guardiola et al. 1988). Changes in endogenous hormone homeostasis may well indicate its function. Therefore, the aim of this work was to perform hormonal profiling along with expression analyses of hormone-related genes. The analyses were conducted in two citrus cultivars: Calabria citron, in which JSs develop normally, and Yemenite citron, in which JSs do not initiate or develop (Fig. 1). In addition, an in-vivo experiment was performed to test the effect of different plant growth regulators (PGRs) on JS initiation in Calabria. The analyses showed significant differences between the two cultivars in hormone levels in the pericarp and in the expression of their corresponding genes in the endocarp and pericarp. Higher ABA levels and increased expression of its corresponding genes in Yemenite citron as compared to Calabria citron suggest its involvement in suppressing JS initiation in Yemenite. However, hormone application to Calabria ovaries/fruitlets failed to affect JS development.
Materials and Methods
Plant Material
Ovaries and fruitlets of citrons Calabria (Citrus medica var. vulgaris), with JSs, and Yemenite (Citrus medica var. etrog), without JSs, were collected from adult trees grown in homogeneous commercial orchards located in the central coastal region of Israel. Three sampling trees, located at an inner position of the row at various locations of the orchard were selected. Approximately 25 flowers/ fruitlets of a similar size were selected from the South-East side of each sampling tree, considered as one biological replicate.
Transcriptomic Analysis
Endocarp Tissue Samples
The two to three innermost cell layers of the ovary/fruitlet wall, adjacent to the locule, of closed flowers (CF), flowers at anthesis (A), and fruitlets 1 (A1W) and 2 (A2W) weeks after anthesis were collected using laser capture microdissection (Zeiss PALM MicroBeam laser microdissection system) as described previously (Martin et al. 2016). Three replicates of 4–5 ovaries/fruitlets, each containing 1000–4000 cells of endocarp tissue, were collected into the adhesive cap of a collection tube (Adhesive Cap 200 opaque, Zeiss, cat. no. 415190-9181-000). RNA was extracted using the RNeasy Micro Kit according to manufacturer’s instructions (Qiagen, Hilden, Germany). The samples were amplified and subjected to Illumina sequencing at the Nancy and Stephen Grand Israel National Center for Personalized Medicine, The Weizmann Institute of Science, Rehovot, Israel using the INCPM-mRNA-seq. Briefly, the poly A fraction (mRNA) was purified from 500 ng of total input RNA followed by fragmentation and the generation of double-stranded cDNA. After Agencourt Ampure XP beads cleanup (Beckman Coulter), end repair, A base addition, adapter ligation and PCR amplification steps were performed. Libraries were quantified by Qubit (Thermo fisher scientific) and TapeStation (Agilent). Sequencing was done on a NovaSeq 6000 SP 100 cycles kit, allocating 20 M reads per sample (Illumina; single read sequencing).
Whole Fruitlet Samples for Transcriptomic Analysis
When Calabria citron fruitlet sections of 0.5–1.0 cm in diameter are incubated on synthetic media, JSs become visible within 2 weeks after planting. Therefore, 0.5 cm fruitlets were sterilized by incubation for 10 min with diluted 2% sodium hypochlorite, followed by 95% technical ethanol and double distilled water (DDW) washing, each for 5 min. The fruitlets were cut into approximately 0.2 mm sections under aseptic conditions. The five to seven sections from the middle of the fruitlet were planted on MS medium without the exocarp and collected at 4 and 14 days after planting (DP). The RNA was extracted from the whole fruitlet section, excluding the peel (flavedo), seeds, locule epidermis and diaphragm (i.e., only the mesocarp and the endocarp were used) (Fig. 1a), and subjected to deep sequencing without amplification.
Raw reads were subjected to filtering and cleaning procedures. The Trimmomatic tool (Bolger et al. 2014) was used to remove Illumina adapters from the reads, and FASTX Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html, version 0.0.13.2) was used to trim read-end nucleotides with quality scores < 30 (FASTQ Quality Trimmer), and to remove reads with less than 70% base pairs with a quality score ≤ 30 (FASTQ Quality Filter). Clean reads of endocarp tissue samples were mapped to the reference genome of orange (Citrus sinensis v2.0_HZAU) and fruitlet samples were mapped to the reference genome of Citrus medica v1.0 (http://citrus.hzau.edu.cn/download.php) using STAR software (Dobin et al. 2013) with an average mapping rate of 93.7% and 96.1%, respectively. Gene abundance was estimated using Cufflinks (Trapnell et al. 2010) combined with gene annotations from the PLANTGARDEN database (https://plantgarden.jp/en/list/t2711/genome/t2711.G001) and Citrus Pan-Genome database. Gene-expression values were computed as fragments per kilobase of exon per million mapped fragments (FPKM). Differential expression analysis was completed using the DESeq2 R package (Love et al. 2014). Genes with an adjusted p-value of no more than 0.05, were considered differentially expressed.
The gene sequences were used as query terms for a search of the NCBI non-redundant (nr) protein database that was carried out with the DIAMOND program (Buchfink et al. 2014). The search results were imported into Blast2GO version 4.0 (Conesa et al. 2005) for gene ontology (GO) assignments. In accordance with Feng et al. (2021), BLAST searches with Arabidopsis protein sequences were used to identify hormone-related gene families.
Hormone Profiling
Three biological replicates of fresh ovaries/fruitlets at four developmental stages—CF, A, A1W and A2W—were collected and freeze dried using lyophilizer (Christ, Osterode am Harz, Germany). The analysis of hormones content was conducted following the method previously described by Albacete et al. (2008), with some modifications. In brief, following drying, the plant material was ground to powder using pastel and mortar, and 50–75 mg of it was shipped for analysis under ambient temperature. The content was combined with 1 ml of a cold extraction mixture (methanol: water, 80:20 v/v) maintained at -20 °C. To separate the solids, the mixture was centrifuged at 20,000 g for 15 min. Subsequently, the supernatant was collected, and the remaining plant material was re-extracted for 30 min at 4 ºC in an additional 1 ml of the same extraction solution. The collected supernatants were pooled and passed through a Sep-Pak Plus C18 cartridge (Sep-Pak Plus, Waters, Milford, MA, USA) to eliminate interfering lipids and some plant pigments. The pooled extract was then evaporated at 40 ºC under vacuum until it reached near dryness. To dissolve the residue, 1 ml of methanol: water (20:80 v/v) solution was used, aided by an ultrasonic bath. The dissolved samples were subsequently filtered through 13 mm diameter Millex filters with a 0.22 μm pore size nylon membrane (Millipore, Bedford, MA, USA). For the analysis of the extracted compounds, 10 µl of the filtered extract was injected into a U-HPLC-MS system, which consisted of an Accela Series U-HPLC (ThermoFisher Scientific, Waltham, MA, USA) coupled to an Exactive mass spectrometer (ThermoFisher Scientific) via a heated electrospray ionization interface. Mass spectra were recorded using Xcalibur software version 2.2 (ThermoFisher Scientific). To quantify plant hormones accurately, calibration curves were established for each analyzed component (1, 10, 50, and 100 µg l–1) and corrected using 10 µg/l–1 deuterated internal standards. The recovery percentages for the quantification process ranged from 92 to 95%. Hormone profiling data were analyzed using JMP 14 (SAS Institute Inc, Cary, NC, 1989–2023); factorial two-way ANOVA was used to test for significant differences.
Statistical Analysis
Statistical analysis of hormone levels was carried on using Tuckey Honestly Significant Difference (HSD) test using JMP14 (SAS Institute Inc, Cary, NC, 1989–2023), with a significance level of p ≤ 0.05. Multivariate analysis was used to measure the Pearson’s correlation coefficient between a hormone level and the expression of its related genes, using JMP14.
Application of PGRs
In-Vivo Hormone Experiment
Five homogenous 23-years-old Calabria trees were selected for the experiment, and about 10 ovaries of a similar size, located on the south-east side were marked on each tree for every treatment. These ovaries were sprayed twice a week for 3 weeks with a solution containing 0.1% Triton-X100 and 20 ppm of the following active compounds: CITRUS FIX®, a liquid formulation containing 45% (v/v) isopropyl 2,4-dichlorophenoxyacetate (AMVAC Chemical Corporation, Newport Beach, CA, USA); Giberllon, a liquid formulation containing 4% (v/v) GA3 (Fine Agrochemicals Limited, Whittington, UK); (±)-jasmonic acid (JA), a liquid bioreagent, containing JA (Sigma-Aldrich Israel Ltd., Tel Aviv, Israel); trans-zeatin riboside (tZR), a liquid bioreagent containing ~ 95% tZR (Sigma-Aldrich Israel Ltd. Tel Aviv); ProTone®, a soluble granule formation containing 20% active S-ABA (Valent BioSciences LLC, Libertyville, IL, USA). The solution was applied by 50 ml plastic spraying bottle. Approximately 1 ml of solution was applied to each ovary/fruitlet. Six to ten surviving ovaries/ fruitlets were collected from each treatment at 1, 2 and 3 weeks following anthesis, and their diameter and height were measured.
Tissue Embedding and Histological Studies
Ovary/fruitlet samples were collected 1, 2 and 3 weeks after anthesis. Samples were fixed in FAA (formaldehyde, acetic acid and ethanol), dehydrated in an ethanol–xylene series and embedded in paraplast (Paraplast Plus, Oxford Labware, USA) as described by Ruzin (1999). Tissue Sects. (15–20 μm) were stained with Safranin O/Fast green for examination of tissue morphology (Johansen 1940). Sections were observed under an optical microscope (Nikon Eclipse Ni, Tokyo, Japan) and images were displayed on a monitor through a microscope camera (Nikon DS-Ri2, Tokyo, Japan).
Scanning Electron Microscopy
Fruitlets of Yemenite and Calabria citrons were collected 3–4 weeks following anthesis, as described above. The fruitlets were hand- cut to thin slices followed by their fixation over night at room temperature in 70% ethanol, and their dehydration by a series of incubations, each for 1 h, in 90%, 95% and twice 100% ethanol. Samples were than dried in K850 critical point dryer (Quorum Technology Ltd., UK), followed by a coating with gold-palladium alloy using SC7620 mini sputter coater (Quorum Technology Ltd., UK), essentially as described (Tripathi et al. 2023).
Results and Discussion
Initiation and Development of JS Primordia in Citron
The source tissue for the JSs is the endocarp, defined as the two to three inner cell layers of the pericarp, which in citrus fruit is the albedo, the white inner peel. Developmental analysis of Calabria ovaries at the closed flower stage (CF), anthesis (A) and fruitlets 1 week (A1W) and 2 weeks (A2W) after anthesis showed JS primordia at A1W on the dorsal wall of the locule/section, and at A2W, they became clearly visible (Fig. 2). In contrast, Yemenite does not produce JSs (Fig. 1b, d). On occasion, a few structures, which might be primordia, could be detected on the section’s inner wall, but they never developed into JSs. The endocarp is composed of the inner side of the pericarp and a portion of the locular membrane, along with a layer of epidermis and several layers of adjacent parenchyma cells (Ford 1942; Schneider 1968). JS primordia initiate after anticlinal and periclinal division of the epidermis cells. The daughter cells retain characteristics of meristematic cells (Nii and Coombe 1988; Burns et al. 1992). The subepidermal cells also divide, but their daughter cells enlarge and lose their meristematic characteristics. Due to the oblique division of the epidermal cells and their subsequent enlargement, cone-shaped cells are produced within the newly formed dome (Ford 1942; Schneider 1968; Nii and Coombe 1988; Carrillo-López and Yahia 2019). As in most citrus fruit, such as Calabria, the primordial domes of the JSs appear on the surface cell layer of the endocarp, shortly after anthesis (Fig. 2). Yemenite does not initiate JSs, due to either a lack of anticlinal cell division in the endocarp epidermis cells or the inability of subepidermal cells to differentiate into enlarged JS cells. Detailed and high-resolution microscopic studies during the onset of JS initiation are required to determine this.
The Expression of Hormone-Related Genes and Hormone Profiling in Ovaries/Fruitlets of Yemenite and Calabria
Gene-expression analyses were conducted separately at the four analyzed developmental stages—CF, A, A1W and A2W—in the endocarp tissue, and in sections of 0.5 cm fruitlets 4 and 14 DP on MS medium. For the endocarp tissue, the two to three inner cell layers of the mesocarp adjacent to the locule were dissected using laser capture microdissection (Online Resource 1). Extracted RNA was amplified and subjected to deep sequencing. When the RNA was extracted from whole fruitlets, it was subjected to deep sequencing without amplification. Full results of the transcriptomic analyses will be presented elsewhere (Assili et al., in preparation). Here, we present data regarding hormone-associated genes (Online Resource 2). Considering the technical differences in the constructions of the two transcriptomes, expression levels of the same genes could not be compared between them. Hormone profiling could not be restricted to the endocarp tissue due to its small size—two to three cell layers. Therefore, it was performed on the entire ovary/fruitlet at the four developmental stages—CF, A, A1W and A2W—excluding the exocarp (flavedo), seeds, locule epidermis and diaphragm (i.e., only the mesocarp and the endocarp, defined in citrus fruit as the albedo, were used) (Fig. 1a). Using standards, the following compounds were identified and quantified: 1-aminocyclopropane-1-carboxylic acid (ACC), trans-zeatin ribosidet (tZR), trans-zeatin (tZ), N6-(Δ2-isopentenyl) adenine (iP), gibberellin A4 (GA4), indole-3-acetic acid (IAA), abscisic acid (ABA), jasmonic acid (JA) and salicylic acid (SA). Levels of most hormones—tZR, iP, GA4, IAA, ABA and JA—were overall higher in Yemenite than in Calabria. Levels of tZR and iP were induced in Calabria from CF to A2W; in Yemenite, they were more or less constant during CF, A and A1W, but were induced from A1W to A2W. IAA levels were also overall induced from CF to A1W in Calabria, but in Yemenite, they were induced from CF to A, and decreased thereafter. ABA showed an overall slight induction from CF to A2W in both cultivars. As opposed to tZR, iP, IAA and ABA, levels of GA4 and JA showed an overall reduction from CF to A1W in both cultivars; in Yemenite, GA4 showed a sharp reduction from A to A1W, and JA from A to A2W (Fig. 3). SA, ACC and tZ levels were generally constant throughout the analyzed developmental stages (Fig. 3). ACC and tZ were slightly higher in Calabria than in Yemenite at A2W, and SA was slightly higher in Calabria than in Yemenite at CF, but no significant difference was detected in the levels of these compounds between the two cultivars at any of the other analyzed stages. The overall higher levels of most of the hormones in Yemenite citron, without JSs, as compared to Calabria citron, with JSs, was somewhat surprising. However, JS formation is not the only difference between Calabria and Yemenite; Yemenite is characterized by a relatively large size and a thick albedo, the major part of the mesocarp, compared to Calabria (Nicolosi et al. 1963). Therefore, the following results are discussed in relation to both JS initiation and fruit size. The major aim was to investigate the possible involvement of hormones in the control of JS initiation taking place in the endocarp. However, as already noted, hormone profiling could not be performed in the endocarp itself, so it was performed in samples containing mostly mesocarp cells, where endocarp cells were ‘diluted’ due to the relatively large size difference between these two tissues (Fig. 1). Nevertheless, associating hormone homeostasis with their corresponding genes can indicate whether the hormone profiles, detected mostly in the mesocarp, are similar in the endocarp. Such an assumption is commonly used in transcriptomic works, where changes in metabolites are inferred from changes in their corresponding genes. Moreover, the whole fruitlet transcriptome, using 0.5 cm fruitlets approximately 14 days after anthesis, allowed generating more direct relationships between gene expression and hormone homeostasis in the same tissue. It should be noted that the gene annotations were performed using the citrus database based on the Arabidopsis gene annotations.
Hormonal profiling of ‘Calabria’ and ‘Yemenite’ ovaries and fruitlets. The indicated hormones were analyzed in ovaries and fruitlets of ‘Calabria’ (blue) and ‘Yemenite’ (red) at four developmental stages: closed flowers (CF), flowers at anthesis (A), and fruitlets 1 (A1W) and 2 (A2W) weeks after anthesis. Means of three replicates ± SE are shown. Asterisks denote significant difference between the two cultivars at a specific stage by Tukey–HSD test (P ≤ 0.05)
Changes in ABA Level and Its Related Genes in Ovaries/Fruitlets of Calabria and Yemenite
ABA Metabolism
In both cultivars, ABA levels increased from A to A2W, with Yemenite, without JSs, containing twice the levels in Calabria, with JSs, at all analyzed stages (Fig. 4a). ABA levels are considered to be controlled largely by the balance between its biosynthesis and catabolism (Endo et al. 2014). The biosynthesis initiates from carotenoids in the plastid, with NINE-CIS-EPOXYCAROTENOID DIOXYGENASE (NCED) catalyzing violaxanthin conversion to xanthoxin, as the key rate-limiting step (Kumar et al. 2022) (Fig. 4b). Xanthoxin is translocated to the cytosol, converted to abscisic aldehyde by ABA DEFICIENT 1 (ABA1), followed by ABA synthesis by ABSCISIC ALDEHYDE OXIDASE 3 (AAO3). Compared to the other biosynthesis genes, AAO3, ABA1, ABA2 and NCED1 were the most highly expressed in the endocarp transcriptome. Overall, these genes and NCED3 were expressed at higher levels in the Yemenite vs. Calabria endocarp, in agreement with the differences in hormone levels, which were higher in Yemenite than Calabria at all analyzed stages. Throughout the analyzed developmental stages, these genes’ transcription levels significantly correlated with hormone levels, with r = 0.71, 0.89, 0.79, and 0.77 for AAO3, ABA1, ABA2 and NCED1, respectively (Fig. 4b, Online Resource 3). However, no significant differences were found in their expression between the cultivars in the whole fruitlet sections (Fig. 4b). ABA catabolism takes place by hydroxylation with the cytochrome P450 (CYP) 707 A subfamily monooxygenase, believed to be the primary catabolism pathway (Ma et al. 2018). In addition, UDP-glycosyltransferases (UGT) modify ABA to ABA-glycosyls, while the reverse reaction (not shown in Fig. 4b) is catalyzed by glycosyl hydrolases (Xu et al. 2002). CYP707A1, CYP707A3, CYP707A4, their protein products catalyzing the conversion of ABA to 8’-hydroxy ABA, and AOG (ABA-GLYCOSYL TRANSFERASE), encoding for the enzyme catalyzing the conversion of ABA to ABA-glycosyl ester (ABA-GE), were the most highly expressed catabolism-associated genes (Online Resource 3), with CYP707A1 and CYP707A3 showing a reduction in their endocarp transcript levels along the tested developmental stages in association with the increase in ABA hormone levels (Fig. 4b), although the difference was not significant (Online Resource 3). Moreover, their expression was higher in Yemenite than in Calabria endocarp, in association with the higher hormone levels in Yemenite (Fig. 4b). In contrast, the catabolism-associated gene CYP707A4 increased markedly from CF to A2W in the Yemenite endocarp, and it was significantly associated with hormone levels (Online Resource 3). However, only the catabolism gene CYP707A1 showed significantly higher levels in Yemenite vs. Calabria fruitlet section transcriptomes (Fig. 4b).
ABA profiling and associated transcriptome data for ‘Calabria’ (blue line) and ‘Yemenite’ (red line). (a) Measured levels of ABA at developmental stages CF, A, A1W and A2W. (b) ABA-related metabolic pathways, showing FPKM of ABA synthesis and -catabolism genes in the endocarp (left) and in whole fruitlets (right) at CF, A, A1W, A2W, and at 4DP and 14DP, respectively. Enzymes and metabolites abbreviations are presented in the text
ABA Signaling
Considering the importance of ABA in enhancing plant performance under unfavorable conditions, its signaling involves three main components, responsible for transferring various exogenous and endogenous cues, such as drought and oxidative stress: the PYRABACTIN RESISTANCE (PYR)/PYRABACTIN RESISTANCE-LIKE (PYL) regulatory component of ABA receptor (RCAR), the PROTEIN PHOSPHATASE 2 C (PP2C), acting as a negative regulator, and the SUCROSE NON-FERMENTING 1 (SNF1) RELATED PROTEIN KINASE 2 (SnRK2), acting as a positive regulation mechanism (Kumar et al. 2022). Upon induced ABA levels, the PYR/PYL/RCAR receptor components bind the hormone, and associate with PPT2Cs, releasing SnRK2s, which are activated by autophosphorylation. SnRK2 activates the phosphorylation of downstream genes, including the transcription factors ABSCISIC ACID RESPONSIVE ELEMENT-BINDING FACTOR 1 (ABF1), G-BOX BINDING FACTOR 4 (GBF4.1) and HYPERSENSITIVE TO ABA1 (HAB1), which induce the expression of defense and other genes (Kumar et al. 2022). Compared to the other members of their gene families, the signaling genes AREB (ABA-RESPONSIVE ELEMENT-BINDING PROTEIN)3 − 1/2, PP2C 56-like, PYL2/8 and SnRK2.3 displayed the highest levels of expression in both cultivars during all analyzed developmental stages (Online Resource 3). Some of them—ABF1, GBF4.1, HAB1, PP2C8 and SnRK2.6—showed significant positive correlations with ABA levels (Online Resource 3). However, in the whole fruitlet transcriptome, only signaling gene PYL2.1 showed differential expression between cultivars, with higher expression in Calabria than in Yemenite at both tested stages (4 DP and 14 DP) (Online Resource 3).
ABA Transport
In its protonated form, ABA can diffuse through membranes, but the transport of the active hormone is mediated by various membranal proteins, including ATP-BINDING CASSETTE (ABC) transporter proteins, NITRATE DI/TRI-PEPTIDE TRANSPORTERS NRT1/PTR family proteins (NPF), MULTIDRUG AND TOXIC COMPOUND EXTRUSION (MATE)-TYPE/DTX TRANSPORTERS, and 19-KDA PLASMA MEMBRANE POLYPEPTIDE FAMILY PROTEINS (AWPM-19) (Kumar et al. 2022). However, in comparison to the metabolic and signaling genes, the expression of ABA-transport genes was relatively low, with ABCG40 showing decreasing expression along the analyzed developmental stages in the endocarp of both cultivars, whereas no differences between the cultivars were detected in the whole fruitlets (Online Resource 3).
Overall, these data showed that higher levels of ABA in Yemenite as compared to Calabria during all tested developmental stages were generally well-correlated with higher expression levels of the ABA-metabolism genes, including NCED1 and NCED3, ABA1 and CYP707A4. Similar correlations were detected for signaling genes belonging to the ABF, GBF, ABI (ABA INSENSITIVE) and PP2C gene families. This indicated that hormone homeostasis in the endocarp was similar to that in the whole ovary/fruitlet. ABA regulates many aspects of plant growth and development (Nambara 2016), including embryo maturation, seed dormancy, germination, cell division, elongation, and floral induction (Sharp 2002; Arc et al. 2013; Vergara et al. 2017; Shi et al. 2020). The data presented here showed higher levels of ABA in Yemenite than in Calabria at the four analyzed developmental stages. Similar to the initiation of JS primordia from the endocarp, lateral roots also initiate from asymmetric, anticlinal cell divisions of two adjoining cells (Fukaki and Tasaka 2009). In Arabidopsis, ABA has been shown to suppress lateral root meristem formation (De Smet et al. 2003) and repress the axillary buds during vegetative growth (Shimizu-Sato et al. 2001; Wang et al. 2020), by promoting meristem dormancy, and inhibiting cell division and cell differentiation (Zhang et al. 2010). Thus, our findings suggest that ABA inhibits JS initiation and development in Yemenite. However, in many processes, plant hormones act in concert—synergistically, antagonistically or additively (Sharp 2002; Steffens et al. 2006; Yang et al. 2016; Khan et al. 2020; Li et al. 2021). Therefore, other hormones are also likely to play a role in the process of JS initiation.
Changes in IAA Level and Its Related Genes in Ovaries/Fruitlets of Calabria and Yemenite
IAA Metabolism
IAA levels were gradually induced up to 4000-fold, from 0.1 to about 400 ng g− 1, across Calabria’s developmental stages, whereas in Yemenite, without JSs, they were induced about 4-fold from CF to A, and then gradually decreased to levels similar to those in Calabria at A2W (Fig. 5a). The precursor for IAA biosynthesis, which occurs in the plastid, is the amino acid tryptophan. Four tryptophan-dependent IAA-biosynthesis pathways are known: indole-3-acetamide (IAM), indole-3-pyruvic acid (IPA), tryptamine (TAM) and indole-3-acetaldoxime (IAOx), with the IPA pathway considered the major one in plants (Casanova-Sáez et al. 2021). Indeed, only the TRYPTOPHAN AMINOTRANSFERASE/YUCCA (TAA/YUC) genes, encoding for the enzymes converting IPA to IAA in the cytosol, were identified in the transcriptomes, suggesting that only the IPA pathway was active in the endocarp cells during the tested stages (Fig. 5b and Online Resource 4). The expression of the biosynthesis genes did not vary markedly throughout the analyzed developmental stages of either cultivar in either the endocarp or whole fruitlet transcriptomes. Although YUCCA10 expression in the endocarp was positively correlated (r = 0.66) with IAA levels, this correlation was not significant (Fig. 5 and Online Resource 4). Reversible or irreversible inactivation of IAA, essential for rapid maintenance of proper hormone homeostasis, occurs by conjugation, catabolism and compartmentalization. Three forms of conjugates are known: ester-linked, such as IAA-glucose and IAA-inositol, amide-linked, where IAA is conjugated with amino acids, peptides and proteins, and methyl-IAA (Wojtaczka et al. 2022). Amide linkage is catalyzed by GRETCHEN HAGEN 3 (GH3) enzymes, and genes belonging to this family—GH3.9, GH3.1, GH3.1.1 and DWARF IN LIGHT 2 (DFL2)—were the only catabolism genes identified in the transcriptome, reaching the highest expression in the endocarp (Online Resource 4). In the Calabria endocarp, the reduction of GH3.1 and GH3.1.1 expression across the analyzed developmental stages paralleled the induction of free IAA levels, with GH3.1.1 showing a negative but non-significant correlation of r = -0.57 (Fig. 5). In contrast, in Yemenite, induced hormone levels from CF to A followed by a reduction in hormone levels from A to A2W paralleled similar changes in GH3.1 expression, suggesting conjugation of free hormone (Fig. 5). The expression of GH3 family genes in the whole fruitlet transcriptome did not differ between the two cultivars (Fig. 5 and Online Resource 4).
IAA profiling and associated transcriptome data for ‘Calabria’ (blue line) and ‘Yemenite’ (red line). (a) Measured levels of IAA at developmental stages CF, A, A1W and A2W. (b) IAA-related metabolic pathways, showing FPKM of IAA-synthesis and -catabolism genes in the endocarp (left) and in whole fruitlets (right) at CF, A, A1W, A2W, and at 4DP and 14DP, respectively. Enzymes and metabolites abbreviations are presented in the text
IAA Signaling
IAA signaling is composed of three major components: the F-Box Containing Complex-TRANSPORT INHIBITOR RESPONSE/AUXIN SIGNALING F-BOX PROTEINS (SCFTIR1/AFB) receptor, AUXIN/INDOLE-3-ACETIC ACID RECEPTORS (AUX/IAA) acting as negative regulators of the auxin response, and AUXIN RESPONSE FACTORS (ARFs), transcription factors modulating the expression of IAA-responsive genes, such as SMALL AUXIN UPREGULATED (SAUR) (Wang and Estelle 2014; Yu et al. 2022). The expression of ARFs, AUX/IAA and SAUR in the endocarp showed a non-significant correlation with IAA levels. Compared to the other analyzed genes, only ARF19 showed a significant negative correlation with hormone levels, i.e., relatively high levels of expression at the CF stage in Yemenite and a reduction thereafter, while IAA displayed the lowest level at this stage. Three IAA-receptor genes were identified—TIR1, TIR1.1 and AFB3—with the second showing the highest expression of the three which seemed to remain constant throughout the analyzed developmental stages in the endocarp; AFB3 was the only gene showing a significant negative correlation with hormone levels (Online Resource 4). In Yemenite whole fruitlet samples, the signaling genes IAA9 and SAUR55 were more highly expressed than in the corresponding Calabria samples.
IAA Transport
Auxin polar transport across membranes is mediated by a few types of transporters: PINs (PIN-FORMED) and PILs (PIN-LIKE) acting as exporters, AUXIN RESISTANT1-LIKE-AUX1 (AUX1/LAX) acting as importers, and ATP-BINDING CASSETTE B (ABCB) acting as transporters (Hammes et al. 2022). Among them, PILS7 showed the highest expression in both cultivars’ endocarp tissues, but no association was found between the expression of these genes and IAA levels. In the whole fruitlet transcriptome, the IAA-transport-related gene LIKE AUX1 3 (LAX3) showed higher expression in Calabria than in Yemenite at 14 DP (Fig. 5 and Online Resource 4).
Overall, a lack of correlation between changes in IAA level and expression of its metabolism genes suggested that IAA homeostasis in the whole ovaries/fruitlets differed from that in the endocarp. However, based on the expression of IAA-metabolism genes, it was difficult to suggest its possible profile in Yemenite and Calabria. While YUCCA10 gene expression was not altered, the overall reduction in GH3.1 and GH3.1.1 expression throughout the analyzed developmental stages of both cultivars suggested an increase in free IAA levels. Following fertilization, an increase in IAA levels plays a significant role in fruit set and induction of pericarp growth in all fruit, including citrus (An et al. 2019; Bermejo et al. 2018). Therefore, altough the possibility that it regulates JS initiation could not be ruled out, the higher levels of IAA in Yemenite as compared to Calabria suggest that it is more likely to act in inducing Yemenite citron pericarp development.
Changes in GA Level and Its Related Genes in Ovaries/Fruitlets of Calabria and Yemenite
GA Metabolism
GA4 level showed a marked reduction from CF to A2W in both cultivars, with Yemenite, without JSs, showing 2- to 5-fold higher levels than Calabria, with JSs, at developmental stages CF and A (Fig. 6a). Ent-kaurene, the precursor for GA, is synthesized mostly from trans-geranylgeranyl diphosphate (GGPP), although mevalonate may also contribute to its biosynthesis (Hedden 2020). GGPP is converted to ent-copalyl diphosphate (ent-CDP), a reaction catalyzed by ENT-COPALYL DIPHOSPHATE SYNTHASE (CPS). Ent-CDP is converted to ent-kaurene by ENT-KAURENE SYNTHSE (KS) followed by the conversion of ent-kaurene to C20GA by two cytochrome P450 monooxygenases, ENT-KAURENE OXIDASE (KO) and ENT-KAURENOIC OXIDASE (KAO) (Fig. 6b). Further metabolic steps, catalyzed by oxidase (ox), GA13ox, GA20ox and GA3ox, lead to the biosynthesis of active GA forms, including GA1, GA4 and GA7. Numerous GA-metabolism-pathway genes were identified in the transcriptomes, with KAO2 and GA3ox8 showing the highest expression levels compared to other metabolic genes (Fig. 6b, Online Resource 5). The expression of CPS decreased from CF to A2W in Yemenite citron, in parallel to the reduction in GA4 levels. The biosynthesis genes GA3ox8 and GA3ox13 showed different expression levels in the endocarp of the two cultivars (Fig. 6b); GA3ox8 expression was significantly higher in Calabria endocarp compared to Yemenite endocarp at CF, A and A1W, whereas GA3ox13 expression was higher in Yemenite than in Calabria endocarp at A1W. GA is inactivated by a structural modification that reduces the affinity of the molecule to its receptor (Hedden 2020), with GA2 oxidases (GA2ox) modifying C19-GA or C20-GA. A significant difference was found in the expression of GA2ox4, deactivating C19-GA, and GA3ox8, deactivating C20-GA, in the whole fruitlet of Calabria vs. Yemenite at 4 DP and 14 DP, with the former displaying higher expression levels (Fig. 6b).
GA profiling and associated transcriptome data for ‘Calabria’ (blue line) and ‘Yemenite’ (red line). (a) Measured levels of GA4 at developmental stages CF, A, A1W and A2W. (b) GA-related metabolic pathways, showing FPKM of GA-synthesis and -catabolism genes in the endocarp (left) and in whole fruitlets (right) at CF, A, A1W, A2W, and at 4DP and 14DP, respectively. Enzymes and metabolites abbreviations are presented in the text
GA Signaling
The most significant component of GA signaling is the receptor GIBBERELLIN-INSENSITIVE DWARF1 (GID1) (Shah et al. 2023). DELLA proteins are negative regulators of the GA response, and when GA is present and binds the receptor, a GA–GID1–DELLA complex is formed leading to DELLA degradation. Several GA-signaling genes belonging to the DELLA and GID1 families were identified (Online Resource 5). In the Yemenite endocarp, GID1B and GID1C were expressed at higher levels than in Calabria endocarp at A2W, but they did not differ in expression level in the whole fruitlets (Online Resource 5).
Overall, with the exception of CPS in Yemenite citron, none of the GA-metabolism genes showed a significant correlation with hormone homeostasis, suggesting that that the GA pattern in the endocarp probably differed from that in the pericarp. Based on the transcriptomic data, it was also difficult to suggest changes in endocarp hormone profile. It is well-established that GA synthesis is induced by IAA following fertilization, and acts in concert with IAA to regulate pericarp development and growth in many plant species, including citrus (Dorcey et al. 2009; Achard et al. 2009; Mesejo et al. 2016; Bermejo et al. 2018). Therefore, as suggested for IAA, the relatively high levels of GA4 in Yemenite compared to Calabria might also be associated with enhanced cell division, leading to a larger mesocarp.
Changes in JA Level and Its Related Genes in Ovaries/Fruitlets of Calabria and Yemenite
JA Metabolism
JA levels showed a ca. 6-fold and 2-fold reduction between A and A2W in Yemenite, without JSs, and Calabria, with JSs, respectively. Overall, hormonal levels were 2.5- to 10-fold higher in Yemenite than in Calabria (Fig. 7a). Jasmonates are synthesized by oxygenation of α-linolenic acid (C18:3) or C16:3 fatty acids, originating from the chloroplast membrane, with 12-oxo-phytodienoic acid (OPDT) as the intermediate, in a series of reactions involving oxygenation and cyclization, catalyzed by LIPOXYGENASE (13-LOX), ALLENE OXIDE SYNTHASE (AOS) and ALLENE OXIDE CYCLASE (AOC) (Fig. 7a) (Wasternack and Strand 2018, Heitz et al. 2019). OPTD moves to the peroxisome where it is converted to JA via the intermediates 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid (OPC), OPC-coenzyme A (OPC-CoA) and JA-CoA in reactions catalyzed by 12-OXOPHYTODIENOATE REDUCTASE (OPCL), ACYL-COA OXIDASE (ACX), MULTIFUNCTIONAL PROTEIN (MFP) and PEROXISOMAL 3-KETOACYL-COA THIOLASE (KAT). Following biosynthesis, JA is conjugated with isoleucine (JA-Ile) by JASMONATE RESISTANT (JAR) and transported to the cytosol. Most of the biosynthesis genes of the JA pathway were identified in the transcriptomes, including JAR1. Among the three identified LOX genes, encoding the first step of JA biosynthesis—the conversion of linoleic acid to 13-hydroperoxylineloic acid—both LOX2 and LOX6 showed similar and constant expression levels throughout the analyzed developmental stages of the endocarp, as well as in the whole fruitlet of both cultivars (Fig. 7b and Online Resource 6). In contrast, LOX3, AOS and AOC3 expression decreased in the endocarp, generally paralleling the reduction in hormone levels. In Yemenite endocarp at the CF stage, JA-biosynthesis genes OPR1 and OPR2 had significantly higher expression than in the Calabria endocarp, with OPR1 showing a positive significant correlation (r = 0.8) with JA levels (Fig. 7b and Online Resource 6). Other downstream biosynthesis genes, OPC-8:0 COA LIGASE1 (OPCL1), ACX4, KAT3 and MFP2, were induced in Yemenite endocarp at A2W, while they showed constant expression in the Calabria endocarp (Fig. 7b). Biosynthesis genes identified in the fruitlet transcriptomes were similar in both citron cultivars (Fig. 7b and Online Resource 6).
JA profiling and associated transcriptome data for ‘Calabria’ (blue line) and ‘Yemenite’ (red line). (a) Measured levels of JA at developmental stages CF, A, A1W and A2W. (b) JA-related metabolic pathways, showing FPKM of JA-synthesis and -catabolism genes in the endocarp (left) and in whole fruitlets (right) at CF, A, A1W, A2W, and at 4DP and 14DP, respectively. Enzymes and metabolites abbreviations are presented in the text
JA Signaling
The JA-signaling pathway core components include the signal molecule JA-Ile, CORONATINE INSENSITIVE 1 (COI), JASMONATE-ZIM-DOMAIN PROTEIN (JAZ) and the transcription factor MYELOCYTOMATOSIS (MYC2) (Howe and Yoshida 2019; Schluttenhofer 2020). JAZ genes are members of a large gene family, TIFY, characterized by TIFY and JAZ domains. Although other components are also involved in the signaling, a simplified model assumes that in the absence of JA, JAZ interaction with MYC2 prevents its action. In the presence of JA, COL1 and JAZ interact to allow JAZ degradation, which releases MYB2 and other transcription factors, resulting in the activation of JA-responsive genes. As compared to other JA-signaling genes, relatively high expression levels were detected for the six identified TIFY genes in both cultivars. The endocarp expression of TIFY10A and TIFY10A.1 showed a positive and significant correlation with JA levels, r = 0.84 and 0.78, respectively, with reduced expression toward A2W (Online Resource 6). However, the expression of TIFY genes in the whole fruitlets did not differ between the two citron cultivars (Online Resource 6). There were three MYC2 homologs, and MYC2 was the most strongly expressed gene in the endocarp, with no significant difference between cultivars, whereas in the fruitlets, MYC2 and MYC2.1 showed higher expression in Calabria at 14 DP (Online Resource 6).
Overall, the data showing a good correlation between the reduced JA levels from CF to A2W and the reduced expression of the JA-biosynthesis genes LOX3 and OPR1, as well as the JA-signaling genes in the endocarp, suggest that JA level in the endocarp is similar to that in the entire ovary/fruitlet. JA metabolism is composed of relatively numerous biosynthetic and catabolic pathways, most likely to allow the necessary plasticity in the plant’s response to various stresses, such as wounding, herbivory and pathogen infection. It may well be that additional pathways exist, such as the more recently discovered one from 4,5-didehydro-JA, involving three β-oxidation steps catalyzed by OPR3 (not shown in Fig. 7) (Ghorbel et al. 2021). Although JA is usually synthesized in response to biotic or abiotic stress, it has also been suggested to play a role in fruit development, for instance, by promoting the biosynthesis of GA in the developing fruit (Wasternack 2015; Wasternack and Strnad 2016). The high levels of the hormone in Yemenite as compared to Calabria might, therefore, be associated with its larger pericarp, although an inhibitory role in JS initiation in Calabria citron cannot be ruled out.
Changes in CTK Level and Its Related Genes in Ovaries/Fruitlets of Calabria and Yemenite
CTK Metabolism
The levels of the two identified CTKs, tZR and iP, were positively correlated with each other (r = 0.85, p = 0.007). While tZR showed overall 10-fold higher levels than iP in Yemenite, without JSs, both displayed similar levels in Calabria, with JSs (Fig. 8a). tZR was gradually induced about 10-fold from A to A2W in Calabria, but it was two orders of magnitude higher in Yemenite, where it showed a slight decrease from A to A1W, and a ca. 3-fold increase from A1W to A2W. Levels of iP were also gradually induced about 10-fold, from 0.2 to about 115 ng g− 1, from CF to A2W in Calabria; they were remarkably higher in Yemenite, showing about 4-fold induction from A1W to A2W. Levels of the third identified CTK, tZ, were nearly the same in both cultivars and were constant throughout the analyzed developmental stages (Fig. 8). CTK biosynthesis initiates from two pathways; the major one is the methylerythritol phosphate (MEP) pathway, and the second begins with mevalonate (MVA), where dimethylallyl-pyrophosphate (DMAPP) is condensed with tRNA to form prenyl-tRNA, a reaction catalyzed by TRNA- ISOPENTENYL TRANSFERASE (IPT) (not shown in Fig. 8, which shows only pathways and compounds identified in the transcriptome and hormone analysis) (Sakakibara 2006; Kamada-Nobusada and Sakakibara 2009; Zürcer and Müller 2016). Neither tRNA-IPT nor other genes of the MVA pathway were identified in the transcriptome, suggesting that only the MEP pathway was operating. In the MEP pathway, DMAPP is formed from hydroxy-methylbutenyl diphosphate (HMBDP). The rate-limiting step in CTK biosynthesis is catalyzed by IPT, where DMAPP is condensed with either adenosine triphosphate (ATP), adenosine diphosphate (ADP) or adenosine monophosphate (AMP) to form iPRTP, IPRDP and iPRMP, respectively. The CTK-nucleotides are converted to their corresponding tZ-nucleotides by CYP735A, and the conversion of the CTK-nucleoside monophosphate to the active form is catalyzed by LONELY GUY (LOG), encoding adenosine kinase (Zürcer and Müller 2016). Four IPT genes and one CYP735A2 gene of the CTK-metabolism pathway were identified (Fig. 8b and Online Resource 7). Among the CTK-biosynthesis genes (IPT2, IPT5, IPT8 and IPT9), IPT2 showed the highest expression levels in the endocarp of both cultivars. The overall constant expression level of all IPT genes throughout the analyzed developmental stages, and in the whole fruitlets, did not correlate with the hormone levels (Fig. 8b and Online Resource 7). CYP735A2 expression decreased from C to A1W in Yemenite endocarp, and was relatively very low and constant in the Calabria endocarp, whereas in the whole fruitlet, it was induced at 14 DP in Calabria, reaching significantly higher levels than in Yemenite fruitlets (Fig. 8b and Online Resource 7). CTK catabolism involves N or O glycosylation, reactions catalyzed by glycosyltransferases (GT), or catabolism to adenosine/adenine, reactions catalyzed by cytokinin oxidase (CKX) (Zürcer and Müller 2016). CKX and CYTOKININ N-GLYCOSYLTRANSFERASE (UGT) expression was detected in the endocarp transcriptome, but only CKX was also identified in the whole fruitlet transcriptome. In Calabria fruitlets, CKX3 expression was induced from 4 DP to 14 DP, whereas it was constant in Yemenite fruitlets, and CKX5 expression was induced in both cultivars, while CKX1 was constant in both cultivars (Fig. 8b and Online Resource 7). In the endocarp, CKX1 expression was induced in Yemenite from A to A2W and was constant in Calabria, CKX3 showed a constant expression pattern in both cultivars, and CKX5 expression was induced from CF to A1W and decreased sharply from A1W to A2W in Calabria, while it was constant in Yemenite. The two UGT genes barely changed in both cultivars (Online Resource 7). DELAY OF GERMINATION 1 (DOG1), encoding a protein of unknown function, is considered a master regulator of seed dormancy. The Arabidopsis dog1 mutant shows remarkable induction in the expression of CTK-biosynthesis genes, suggesting that it might negatively regulate their biosynthesis (Li et al. 2022). The expression of this gene in Calabria was constant throughout the analyzed developmental stages, whereas in Yemenite, it was 2- to 3-fold higher at CF and A than at the other stages, and it showed a reduction from C to A1W in parallel with the induction in tZR and iP levels (Online Resource 8).
CTK profiling and associated transcriptome data for ‘Calabria’ (blue line) and ‘Yemenite’ (red line). (a) Measured levels of tZR and iP at developmental stages CF, A, A1W and A2W. (b) CTK-related metabolic pathways, showing FPKM of CTK-synthesis and -catabolism genes in the endocarp (left) and in whole fruitlets (right) at CF, A, A1W, A2W, and at 4DP and 14DP, respectively. Enzymes and metabolites abbreviations are presented in the text
CTK Signaling
CTK signaling is based on autophosphorylation and phosphorylation cascades (Kieber and Schaller 2018; Romanov et al. 2018; Wybouw and De Rybel 2018). The hormone binds to the extracellular domain of the receptor, HISTIDINE KINASE (HK) or WOODEN LEG (WOL) and the dimers undergo autophosphorylation. Next, HISTIDINE-CONTAINING PHOSPHOTRANSMITTER (AHP), acting as phosphor-transmitter, enters the nucleus and transfers a phosphate group to TYPE A RESPONSE REGULATORS (ARRs) and TYPE B REGULATORS (RRs), which are transcription activators or repressors of CTK-responsive genes. The genes encoding all of these components were identified in the endocarp transcriptome, but not in the whole fruitlet transcriptome. HK2, HK3 and WOL genes showed similar and relatively constant expression levels in both cultivars throughout the analyzed developmental stages. Among the identified AHP genes, AHP5 and AHP6 showed relatively high expression in the endocarp of both cultivars, with AHP6 displaying a gradual reduction in Calabria, and a reduction from A1W to A2W in Yemenite. The expression of AHP1 was significantly negatively correlated with tZR level (Online Resource 7). Among the response regulator genes, ARR21 showed relatively strong expression levels in both cultivars at all analyzed stages. Similarly, RR14 showed relatively high expression, which decreased across the analyzed developmental stages of both cultivars (Online Resource 7). RR11 showed a significant positive correlation with both tZR and iP levels, although its expression levels were relatively low (Online Resource 7). CTK efflux is mediated by ABC TRANSPORTERS, and two non-specific transporters are suggested to mediate their influx: PURINE PERMEASE (PUP) and EQUILIBRATIVE NUCLEOSIDE TRANSPORTER (ENT) (Kieber and Schaller 2018). PUP1 was identified in both the endocarp and whole fruitlet transcriptome of both cultivars, showing similar expression level patterns, with reduced levels at A1W.
In summary, the overall lack of correlation between CTK levels in the mesocarp and its metabolism genes in the endocarp suggested that hormone homeostasis varied between the two tissues. Like IAA and GA, it was difficult to infer the changes in CTK homeostasis from the transcriptomic data. However, similar to IAA and GA, CTKs have also been suggested to play a role in fruit set and early developmental stages, by regulating cell division and differentiation (Francisca et al. 1990; Agustí and Primo-Millo 2020). We suggest that the induction pattern of CTK levels in both citron cultivars from CF to A2W is related to the development and enlargement of the ovaries/fruitlets in accordance with their size differences.
Effect of Exogenous Application of PGRs on Early Development of Calabria
Higher mesocarpal ABA levels in Yemenite as compared to Calabria at all analyzed stages were in a good agreement with the differences in endocarpal expression of ABA-metabolism genes in the two cultivars, suggesting that the hormone plays an inhibitory role in JS initiation. However, considering that most detected hormone levels were higher in Yemenite than in Calabria, we examined whether their application to Calabria would affect JS initiation and development. Various PGRs—2,4-D, tZR, ABA, GA and JA—were applied on closed flowers, and their effect was tested for 3 weeks after anthesis. Axial histological analyses of treated fruitlets showed initial formation of JS primordia at A1W (Fig. 9), suggesting that the applied PGRs, including ABA, were incapable of inhibiting JS initiation in Calabria. Although these results question the hypothesis that these PGRs play a role in the control of JS development, additional experiments which include various application times and different PGR concentrations, as well as their co-application, might be required to achieve the desired result.
Figure 9. Juice sacs initiation is not affected by exogenous PGRs application. Flowers of Calabria citron were treated with ABA, JA and 2,4-D, as described in materials and methods. Fruitlets were collected two (a, c, e, g) and three weeks (b, d, f, h) following anthesis, and their equatorial sections were subjected to light microscopy analysis. a and b, control treatment; c and d, ABA; e and f, JA, g and h, 2,4-D
Summary
The major aim of this work was to investigate whether hormones play a role in the control of JS initiation in the endocarp of citrus fruit. Thorough analyses of hormone levels in the mesocarp along with transcriptomic analyses of their corresponding genes in the endocarp and mesocarp at relevant developmental stages were performed. Correlation analysis between hormone levels and their corresponding genes suggested that ABA homeostasis was similar in the pericarp and endocarp. This also seemed to be the case for JA, but not for IAA, GA4 or CTKs. However, it is not argued that these hormones do not play a role in the relevant process. For instance, CTKs and IAA, which are well-known to control cell proliferation and growth, might well play a role in JS initiation. Considering the pattern of ABA accumulation, the relatively high level it displayed in Yemenite, without JSs, compared to Calabria, with JSs, and its inhibitory role in other investigated systems, it is suggested to inhibit JS initiation in Yemenite citron. However, in a primary PGR-application experiment, ABA, as well as the other PGRs, failed to affect JS initiation. Regardless, further investigation is required to determine the involvement of hormones in this process, including more comprehensive PGR-application experiments, and knockdown of key hormone-biosynthesis genes.
References
Achard P, Gusti A, Cheminant S et al (2009) Gibberellin Signaling Controls Cell Proliferation Rate in Arabidopsis. Curr Biol 19:1188–1193. https://doi.org/10.1016/J.CUB.2009.05.059
Agustí M, Primo-Millo E (2020) Flowering and fruit set. Genus Citrus 219–244. https://doi.org/10.1016/B978-0-12-812163-4.00011-5
Albacete A, Ghanem ME et al (2008) Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 59(15):4119–4131. https://doi.org/10.1093/jxb/ern251PMID: 19036841; PMCID: PMC2639025
Ali-Dinar HM, Wheaton TA, Krezdorn AH (1988) The sexual-hormonal relation in citrus during fruit set. Acta Hortic 159–174. https://doi.org/10.17660/ACTAHORTIC.1988.218.21
Altman A, Gülsen Y, Goren R (1982) Growth and metabolic activity of lemon juice vesicle explants in vitro. Plant Physiol 69:1–6. https://doi.org/10.1104/pp.69.1.1
An J, Almasaud RA, Bouzayen M, Zouine M, Chervin C (2020) Auxin and ethylene regulation of fruit set. Plant Sci 292:110381. https://doi.org/10.1016/j.plantsci.2019.110381
Arc E, Sechet J, Corbineau F et al (2013) ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4. https://doi.org/10.3389/FPLS.2013.00063
Bermejo A, Primo-Millo E, Agustí M et al (2015) Hormonal Profile in ovaries of Mandarin varieties with Differing Reproductive Behaviour. J Plant Growth Regul 34:584–594. https://doi.org/10.1007/S00344-015-9492-Y/TABLES/5
Bermejo A, Granero B, Mesejo C et al (2018) Auxin and Gibberellin Interact in Citrus Fruit Set. J Plant Growth Regul 37:491–501. https://doi.org/10.1007/S00344-017-9748-9
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114. https://doi.org/10.1093/BIOINFORMATICS/BTU170
Bons H, Kaur N, Agriculture HR-IJ, of (2015) U (2015) Quality and quantity improvement of citrus: role of plant growth regulators. Int J Agric Environ Biotechnol 8:433–447. https://doi.org/10.5958/2230-732X.2015.00051.0
Buchfink B, Xie C, Huson DH (2014) Fast and sensitive protein alignment using DIAMOND. Nat Methods 2014(121):12–59. https://doi.org/10.1038/nmeth.3176
Burns JK, Achor DS, Echeverria E (1992) Ultrastructural studies on the ontogeny of grapefruit juice vesicles (Citrus Paradisi Macf. CV Star Ruby). Int J Plant Sci 153:14–25. https://doi.org/10.1086/297002
Burns JK, Achor DS, Echeverria E (1994) Carpellary Outgrowth Development in the endocarp of Grapefruit, Citrus paradisi (Rutaceae). Am J Bot 81:760. https://doi.org/10.2307/2445656
Carrillo-López A, Yahia EM (2019) Morphology and anatomy. Postharvest Physiol Biochem Fruits Veg 113–130. https://doi.org/10.1016/B978-0-12-813278-4.00006-3
Casanova-Sáez R, Mateo-Bonmatí E, Ljung K (2021) Auxin metabolism in plants. Cold Spring Harb Perspect Biol 13:a039867. https://doi.org/10.1101/cshperspect.a039867
Conesa A, Götz S, García-Gómez JM et al (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. https://doi.org/10.1093/BIOINFORMATICS/BTI610
De Smet I, Signora L, Beeckman T et al (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33:543–555. https://doi.org/10.1046/J.1365-313X.2003.01652.X
Dobin A, Davis CA, Schlesinger F et al (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. https://doi.org/10.1093/BIOINFORMATICS/BTS635
Dorcey E, Urbez C, Blázquez MA et al (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332. https://doi.org/10.1111/J.1365-313X.2008.03781.X
Einset JW (1978) Citrus tissue culture. Plant Physiol 62:885–888. https://doi.org/10.1104/pp.62.6.885
Endo A, Okamoto M, Koshiba T (2014) ABA Biosynthetic and catabolic pathways. In: Zhang DP (ed) Abscisic acid: metabolism, transport and signaling. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9424-4_2
Erner Y, Reuveni O, Goldschmidt EE (1975) Partial purification of a growth factor from Orange Juice which Affects Citrus Tissue Culture and its replacement by citric acid. Plant Physiol 56:279–282. https://doi.org/10.1104/pp.56.2.279
Esau K (1965) Plant Anatomy, 2nd edn. New York
Fahn A (1990) Plant anatomy. Pergamon Press, Oxford [England];;New York
Feng G, Wu J, Xu Y et al (2021) High-spatiotemporal-resolution transcriptomes provide insights into fruit development and ripening in Citrus sinensis. Plant Biotechnol J. https://doi.org/10.1111/PBI.13549
Ford ES (1942) Anatomy and histology of the Eureka Lemon. Univ Chic 104:288–305
Francisca M, MiÑana H, Primo-Millo E (1990) Studies on endogenous cytokinins in Citrus. J Hortic Sci 65:595–601. https://doi.org/10.1080/00221589.1990.11516098
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69:437–449. https://doi.org/10.1007/S11103-008-9417-2
Garcia-Papi MA, Garcia-Martinez JL (1984) Endogenous plant growth substances content in young fruits of seeded and seedless clementine mandarin as related to fruit set and development. Sci Hortic (Amsterdam) 22:265–274. https://doi.org/10.1016/0304-4238(84)90060-8
Ghorbel M, Brini F, Sharma A, Landi M (2021) Role of jasmonic acid in plants: the molecular point of view. Plant Cell Rep 40:1471–1494. https://doi.org/10.1007/s00299-021-02687-4
Guardiola JL, Almela V, Barrés MT (1988) Dual effect of auxins on fruit growth in Satsuma mandarin. Sci Hortic (Amsterdam) 34:229–237. https://doi.org/10.1016/0304-4238(88)90096-9
Guardiola JL, Barrés MT, Albert C, Garcia-Luis A (1993) Effects of Exogenous Growth regulators on Fruit Development in Citrus Unshiu. Ann Bot 71:169–176. https://doi.org/10.1006/ANBO.1993.1021
Gülsen Y, Altman A, Goren R (1981) Growth and development of Citrus pistils and fruit explants in vitro. Physiol Plant 53:295–300. https://doi.org/10.1111/j.1399-3054.1981.tb04503.x
Hammes UZ, Murphy AS, Schwechheimer C (2022) Auxin transporters—a biochemical view. Cold Spring Harb Perspect Biol 14:a039875. https://doi.org/10.1101/cshperspect.a039875
Harada H, Mukai H, Takagi T (2001) Effects of explant age, growth regulators and carbohydrates on sugar accumulation in Citrus juice vesicles cultured in vitro. Sci Hortic (Amsterdam) 90:109–119. https://doi.org/10.1016/S0304-4238(00)00263-6
Hedden P (2020) The current status of research on gibberellin biosynthesis. Plant Cell Physiol 61:1832–1849. https://doi.org/10.1093/pcp/pcaa092
Heitz T, Smirnova E, Marquis V, Poirier L (2019) Metabolic control within the jasmonate biochemical pathway. Plant Cell Physiol 60:2621–2628. https://doi.org/10.1093/pcp/pcz172
Howe GA, Yoshida Y (2019) Evolutionary origin of JAZ proteins and jasmonate signaling. Mol Plant 12:153–155. https://doi.org/10.1016/j.molp.2019.01.015
Johansen D (1940) Plant microtechnique. McGraw-Hill, New York, p 523
Kamada-Nobusada T, Sakakibara H (2009) Molecular basis for cytokinin biosynthesis. Phytochemistry 70:444–449. https://doi.org/10.1016/j.phytochem.2009.02.007
Khan A, Bilal S, Khan AL et al (2020) Silicon and gibberellins: synergistic function in harnessing aba signaling and heat stress tolerance in date palm (phoenix dactylifera l). Plants 9. https://doi.org/10.3390/plants9050620
Kieber JJ, Schaller GE (2018) Cytokinin signaling in plant development. Development 145. dev149344. https://doi.org/10.1242/dev.149344
Koch KE, Avigne WT (1990) Postphloem, nonvascular transfer in citrus. Plant Physiol 93:1405–1416. https://doi.org/10.1104/pp.93.4.1405
Kordan HA (1974) Mitotic activity in lemon fruit explants (Citrus limon L.) incubated on a calcium-potassium-sucrose medium. Experientia 30:749–751. https://doi.org/10.1007/bf01924163
Kumar S, Shah SH, Vimala Y et al (2022) Abscisic acid: metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front Plant Sci 13:972856. https://doi.org/10.3389/fpls.2022.972856
Li Q, Xu F, Chen Z et al (2021) Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat Plants 7:1108–1118. https://doi.org/10.1038/s41477-021-00959-1
Li Q, Chen X, Zhang S, Shan S, Xiang Y (2022) DELAY OF GERMINATION 1, the master regulator of seed dormancy, integrates the regulatory network of phytohormones at the transcriptional level to control seed dormancy. Curr Issues Mol Biol 44:6205–6217. https://doi.org/10.3390/cimb44120423
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Ma Y, Cao J, He J et al (2018) Molecular mechanism for the regulation of ABA homeostasis during Plant Development and stress responses. Int J Mol Sci 19:3643. https://doi.org/10.3390/ijms19113643
Martin LBB, Nicolas P, Matas AJ et al (2016) Laser microdissection of tomato fruit cell and tissue types for transcriptome profiling. Nat Protoc 11:2376–2388. https://doi.org/10.1038/nprot.2016.146
Mesejo C, Yuste R, Reig C et al (2016) Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci 247:13–24. https://doi.org/10.1016/J.PLANTSCI.2016.02.018
Nambara E (2016) Abscisic acid. Encycl Appl Plant Sci 1:361–366. https://doi.org/10.1016/B978-0-12-394807-6.00098-8
Nicolosi E, Malfa S, La, El-Otmani M et al (1963) The search for the authentic Citron. Historic and Genetic Analysis, Citrus medica L.)
Nii N, Coombe BG (1988) Anatomical aspects of juice sacs of Satsuma mandarin in relation to translocation. J Japan Soc Hort Sci 56:375–381
Romanov GA, Lomin SN, Schmülling T (2018) Cytokinin signaling: from the ER or from the PM? That is the question! New Phytol 218:41–53. https://doi.org/10.1111/nph.14991
Ruan Y-L, Patrick JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17:656–665
Ruzin SE (1999) Plant microtechnique and microscopy. 322
Sadka A, Shlizerman L, Kamara I, Blumwald E (2019) Primary metabolism in Citrus Fruit as affected by its unique structure. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01167
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449. https://doi.org/10.1146/annurev.arplant.57.032905.105231
Schluttenhofer C (2020) Origin and evolution of jasmonate signaling. Plant Sci 298:110542. https://doi.org/10.1016/j.plantsci.2020.110542
Schneider H (1968) The anatomy of citrus. In: Walter R, Leon D, Batchelor W, Herbert J (eds) Citrus industry Volum II, reuther. University of California Press, Riverside, California, pp 2–23
Shah SH, Islam S, Mohammad F et al (2023) Gibberellic acid: a versatile regulator of plant growth, development and stress responses. J Plant Growth Regul. https://doi.org/10.1007/s00344-023-11035-7
Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25:211–222. https://doi.org/10.1046/J.1365-3040.2002.00798.X
Shi M, Liu X, Zhang H et al (2020) The IAA- and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic Res 7:139. https://doi.org/10.1038/S41438-020-00360-7
Shimizu-Sato S, Physiology HM-P (2001) undefined (2001) Control of outgrowth and dormancy in axillary buds. academic.oup.com. https://doi.org/10.1104/pp.010841
Spreen TH, Gao Z, Fernandes W, Zansler ML (2020) Global economics and marketing of citrus products. The Genus Citrus. Elsevier Inc., pp 471–493
Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223:604–612. https://doi.org/10.1007/S00425-005-0111-1
Tadeo FR, Terol J, Rodrigo MJ et al (2020) Fruit growth and development. The Genus Citrus. Elsevier Inc., pp 245–269
Talón M, Hedden P, Primo-Millo E (1990a) Gibberellins inCitrus sinensis: a comparison between seeded and seedless varieties. J Plant Growth Regul 1990 91 9:201–206. https://doi.org/10.1007/BF02041963
Talón M, Zacarias L, Primo-Millo E (1990b) Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol Plant 79:400–406. https://doi.org/10.1111/J.1399-3054.1990.TB06759.X
Tisserat B, Galletta PD (1987) In vitro culture of lemon juice vesicles. Plant Cell Tissue Organ Cult 11:81–95. https://doi.org/10.1007/BF00041842
Tisserat B, Jones D, Galletta P (1988) Adventitious juice vesicle initiation in lemon (Citrus limon L.), mandarin (Citrus reticulata Blanco), sour orange (Citrus aurantium L.), and sweet orange (Citrus sinensis (L.) Osb.) Fruit explants. Experientia 44:722–724. https://doi.org/10.1007/BF01941044
Tisserat B, Jones D, Galletta PD (1990) Juice vesicle populations in Citrus Fruit. Bot Gaz 151:64–72. https://doi.org/10.1086/337806
Trapnell C, Williams BA, Pertea G et al (2010) Transcript assembly and quantification by rNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511. https://doi.org/10.1038/NBT.1621
Tripathi PK, Ayzenshtat D, Kumar M et al (2023) An efficient and reproducible Agrobacterium-mediated genetic transformation method for the ornamental monocotyledonous plant Ornithogalum Dubium Houtt. Plant Growth Regul 101:201–214. https://doi.org/10.1007/s10725-023-01013-0
Unger JW, Feng KA (1978) Growth and differentiation of Juice vesicles of Orange grown in Vitro. Am J Bot 65:511. https://doi.org/10.2307/2442583
Vergara R, Noriega X, Aravena K et al (2017) ABA represses the expression of cell cycle genes and may modulate the development of endodormancy in grapevine buds. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00812
Wang R, Estelle M (2014) Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr Opin Plant Biol 21:51–58. https://doi.org/10.1016/j.pbi.2014.06.006
Wang L, Wang B, Yu H et al (2020) Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 583:277–281. https://doi.org/10.1038/s41586-020-2382-x
Wasternack C (2015) How jasmonates earned their laurels: past and Present. J Plant Growth Regul 2015(344):34–761. https://doi.org/10.1007/S00344-015-9526-5
Wasternack C, Strnad M (2016) Jasmonate signaling in plant stress responses and development – active and inactive compounds. N Biotechnol 33:604–613. https://doi.org/10.1016/J.NBT.2015.11.001
Wasternack C, Strnad M (2018) Jasmonates: news on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int J Mol Sci 19:2539. https://doi.org/10.3390/ijms19092539
Wojtaczka P, Ciarkowska A, Starzynska E, Ostrowsk (2022) The GH3 amidosynthetases family and their role in metabolic crosstalk modulation of plant signaling compounds. Phytochemistry 194:113039. https://doi.org/10.1016/j.phytochem.2021.113039
Wybouw B, De Rybel B (2018) Cytokinin - a developing story. Trends Plant Sci 24:177–185. https://doi.org/10.1016/j.tplants.2018.10.012
Xu ZJ, Nakajima M, Suzuki Y, Yamaguchi I (2002) Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol 129:1285–1295. https://doi.org/10.1104/pp.001784
Yang X, Bai Y, Shang J et al (2016) The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant Cell Environ 39:1994–2003. https://doi.org/10.1111/PCE.12763
Yu Z, Zhang F, Friml J, Ding Z (2022) Auxin signaling: research advances over the past 30 years. J Integr Plant Biol 64:371–392. https://doi.org/10.1111/jipb.13225
Zhang H, Han W, De Smet I et al (2010) ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J 64:764–774. https://doi.org/10.1111/J.1365-313X.2010.04367.X
Zürcher E, Müller B (2016) Cytokinin synthesis, signaling, and function—advances and new insights. Internl Rev Cell Mol Biol 324:1–38. https://doi.org/10.1016/bs.ircmb.2016.01.001
Acknowledgements
We would like to express our sincere gratitude to Dr. Joshua D. Klein and Hanita Zemach for their guidance and support throughout the course of this research project.
Funding
Open access funding provided by Hebrew University of Jerusalem.
Author information
Authors and Affiliations
Contributions
Avi Sadka and Siwar Assili contributed to the study conception and design. Material preparation, data collection and analysis were performed by Siwar Assili, Adi Doron-Faigenboim, Alfonso AA Moreno and Rosa M. Rivero and Avi Sadka. The first draft of the manuscript was written by Siwar Assili and Avi Sadka commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
This research was conducted without any commercial or financial relationships that could be construed as conflicts of interest.
Additional information
Communicated by Charitha Jayasinghege.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assili, S., Doron-Faigenboim, A., Moreno, A.A.A. et al. Changes in Hormonal Profiles and Corresponding Gene Expressions During the Initiation and Development of Juice Sac Primordia in Citrus Ovaries and Fruitlets. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11320-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11320-z