Abstract
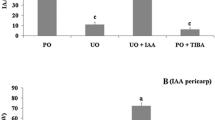
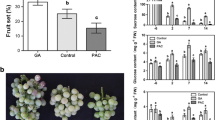
Gibberellins (GAs) have been widely used for many years to induce seedless grapevine and increase fruit set in production. However, the role of GAs and how they crosstalk with auxin and cytokinin during fruit set in grapevine remains unclarified. To investigate their role, GA3, 4-chlorophenoxyacetic acid (4-CPA), 6-benzylaminopurine (6-BA), and different hormone combinations were applied to unpollinated ovaries of the ‘Fenghou’ grape (Vitis vinifera × Vitis labrusca) at anthesis. The results showed that the application of 4-CPA, 6-BA, GA3, 4-CPA+GA3, 6-BA+GA3, and 4-CPA+6-BA induced parthenocarpy. Among them, 4-CPA-, 6-BA-, and 4-CPA+6-BA-induced fruits were smaller than those induced by GA3, 4-CPA+GA3, and 6-BA+GA3. Application of the GA biosynthesis inhibitor paclobutrazol reduced both the fresh weight and fruit set of 4-CPA- and 6-BA-induced fruits, but the effect of the inhibitor was reversed by the application of GA3. In contrast to unpollinated ovaries, the ovaries induced by 4-CPA and 6-BA showed enhanced accumulation of active GAs, but at a lower level than in pollinated ovaries between 7 and 21 days after anthesis (DAA). Gene analysis showed that the increase in the GA content in 4-CPA- and 6-BA-treated ovaries was the result of the upregulation of GA biosynthesis genes, such as VvGA3ox1, and suppression of GA catabolism genes, such as VvGA2ox3 and VvGA2ox4. In addition, the concentration of active IAA was enhanced in the ovaries induced by 6-BA, which was accompanied by the elevated expression of the IAA biosynthesis genes VvYUC2 and VvYUC6. These results suggested that 4-CPA- and 6-BA-induced fruit set in grapevine requires downstream gibberellin biosynthesis.








Similar content being viewed by others
References
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328. doi:10.1007/s00425-005-0088-9
Archbold DD, Dennis FG (1985) Strawberry receptacle growth and endogenous IAA content as affected by growth regulator application and achene removal. J Am Soc Hortic Sci 110:816–820
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61:3615–3625
Bünger-Kibler S, Bangerth F (1983) Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul 1:143–154
Coombe BG (1960) Relationship of growth and development to changes in sugars, auxins, and gibberellins in fruit of seeded and seedless varieties of Vitis vinifera. Plant Physiol 35:241
Coombe BG (1962) The effect of removing leaves, flowers and shoot tips on fruit-set in Vitis vinifera L. J Hortic Sci 37:1–15
Costantini E, Landi L, Silvestroni O, Pandolfini T, Spena A, Mezzetti B (2007) Auxin synthesis-encoding transgene enhances grape fecundity. Plant Physiol 143:1689–1694
Csukasi F, Osorio S, Gutierrez JR, Kitamura J, Giavalisco P, Nakajima M, Fernie AR, Rathjen JP, Botella MA, Valpuesta V, Medina-Escobar N (2011) Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol 191:376–390
Dauelsberg P, Matus JT, Poupin MJ, Leiva-Ampuero A, Godoy F, Vega A, Arce-Johnson P (2011) Effect of pollination and fertilization on the expression of genes related to floral transition, hormone synthesis and berry development in grapevine. J Plant Physiol 168:1667–1674
Ding J, Chen B, Xia X, Mao W, Shi K, Zhou Y, Yu J (2013) Cytokinin-induced parthenocarpic fruit development in tomato is partly dependent on enhanced gibberellin and auxin biosynthesis. PLoS One 8:e70080
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J Cell Mol Biol 58:318–332 (315)
El-Sharkawy I, Sherif S, Kayal WE, Mahboob A, Abubaker K, Ravindran P, Jyothi-Prakash PA, Kumar PP, Jayasankar S (2014) Characterization of gibberellin-signalling elements during plum fruit ontogeny defines the essentiality of gibberellin in fruit development. Plant Mol Biol 84:399–413
Fos M, Nuez F, García-Martínez JL (2000) The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–479
Fos M, Proaño K, Nuez F (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111:545–550
Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Ostergaard L (2012) Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 24:3982–3996. doi:10.1105/tpc.112.103192
Gallego-Giraldo C, Hu J, Urbez C, Gomez MD, Sun TP, Perez-Amador MA (2014) Role of the gibberellin receptors GID1 during fruit-set in Arabidopsis. Plant J 79:1020–1032
Giacomelli L, Rota-Stabelli O, Masuero D, Acheampong AK, Moretto M, Caputi L, Vrhovsek U, Moser C (2013) Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: functional characterization and evolution of grapevine gibberellin oxidases. J Exp Bot 64:4403–4419
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439
Gorguet B, Heusden AV, Lindhout P (2005) Parthenocarpic fruit development in tomato. Plant Biol 7:131–139
Guardiola JL, Barrés MT, Albert C, García-Louis A (1993) Effects of exogenous growth regulators on fruit development in Citrus unshiu. Ann Bot 71:169–176 (168)
Hedden P, Graebe JE (1985) Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J Plant Growth Regul 4:111–122
Hu JH, Mitchum MG, Neel B, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR, Swain SM, Yamaguchi S, Kamiya Y, Sun TP (2008) Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell Online 20:320–336
Jung CJ, Hur YY, Yu HJ, Noh JH, Park KS, Lee HJ (2014) Gibberellin application at pre-bloom in grapevines down-regulates the expressions of VvIAA9 and VvARF7, negative regulators of fruit set initiation, during parthenocarpic fruit development. PLoS One 9:e95634
Kühn N, Arce-Johnson P (2012) Pollination: a key event controlling the expression of genes related to phytohormone biosynthesis during grapevine berry formation. Plant Signal Behav 7:7–11. doi:10.4161/psb.7.1.18353
Lavee S (1960) Effect of gibberellic acid on seeded grapes. Nature 185:395
Lewis DH, Burge GK, Hopping ME, Jameson PE (1996) Cytokinins and fruit development in the kiwifruit (Actinidia deliciosa). II. Effects of reduced pollination and CPPU application. Physiol Plant 98:187–195
Lu J, Lamikanra O, Leong S (1997) Induction of seedlessness in ‘Triumph’ muscadine grape (Vitis rotundifolia Michx.) by applying gibberellic acid. HortScience 32:89–90
Mapelli S (1981) Changes in cytokinin in the fruits of parthenocarpic and normal tomatoes. Plant Sci Lett 22:227–233
Mariotti L, Picciarelli P, Lombardi L, Ceccarelli N (2011) Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J Plant Growth Regul 30:405–415. doi:10.1007/s00344-011-9204-1
Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A (2007) Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J 52:865–876
Martinelli F, Uratsu SL, Reagan RL, Chen Y, Tricoli D, Fiehn O, Rocke DM, Gasser CS, Dandekar AM (2009) Gene regulation in parthenocarpic tomato fruit. J Exp Bot 60:3873–3890 (3818)
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Ohara HKM, Matsui H, Hirata N, Takahashi E (1997) Comparison of the levels of endogenous plant growth substances in CPPU-treated and -untreated kiwi fruit. J Jpn Soc Hort Sci 65:692–705
Ojeda H, Deloire A, Carbonneau A, Ageorges A, Romieu C (1999) Berry development of grapevines: relations between the growth of berries and their DNA content indicate cell multiplication and enlargement. Vitis 38:145–150
Olimpieri I, Siligato F, Caccia R, Soressi GP, Mazzucato A, Mariotti L, Ceccarelli N (2007) Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 226:877–888
Ozga JA, Reinecke DM (1999) Interaction of 4-chloroindole-3-acetic acid and gibberellins in early pea fruit development. Plant Growth Regul 27:33–38
Ozga JA, Reinecke DM (2003) Hormonal interactions in fruit development. J Plant Growth Regul 22:73–81
Ozga JA, Reinecke DM, Ayele BT, Ngo P, Nadeau C, Wickramarathna AD (2009) Developmental and hormonal regulation of gibberellin biosynthesis and catabolism in pea fruit. Plant Physiol 150:448–462
Pattison RJ, Catalá C (2012) Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J 70:585–598
Pérez FJ, Viani C, Retamales J (2000) Bioactive gibberellins in seeded and seedless grapes: identification and changes in content during berry development. Am J Enol Vitic 51:315–318
Picken AJF (1984) A review of pollination and fruit set in the tomato (Lycopersicon esculentum Mill.). J Hortic Sci 59:1–13
Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips A, Hedden P (2008) The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53:488–504
Ruan Y-L, Patrick JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17:656–665. doi:10.1016/j.tplants.2012.06.005
Serrani JC, Sanjuán R, Ruiz-Rivero O, Fos M, García-Martínez JL (2007a) Gibberellin regulation of fruit set and growth in tomato. Plant Physiol 145:246–257
Serrani JC, Fos M, Atarés A, García-Martínez JL (2007b) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato. J Plant Growth Regul 26:211–221
Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Talon M, Zacarias L, Primo-Millo E (1992) Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol 99:1575–1581
Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 172:92–103
Tiwari A, Offringa R, Heuvelink E (2012) Auxin-induced fruit set in Capsicum annuum L. requires downstream gibberellin biosynthesis. J Plant Growth Regul 31:570–578
Van Huizen R, Ozga JA, Reinecke DM (1997) Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol 115:123–128
Vercher Y, Carbonell J (1991) Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence or fruit development induced by plant growth substances in Pisum sativum. Physiol Plant 81:518–526
Vivian-Smith A, Koltunow AM (1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121:437–452
Vivian-Smith A, Luo M, Chaudhury A, Koltunow A (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128:2321–2331
Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech JC, Fernie AR, Bouzayen M (2009) Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21:1428–1452
Weaver RJ, Mccune SB (1960) Further studies with gibberellin on Vitis vinifera grapes. Bot Gaz 121:155–162
Weaver R, Mccune S, Hale C (1962) Effect of plant regulators on set and berry development in certain seedless and seeded varieties of Vitis vinifera L. Vitis 3:84–96
Weaver RJ, van Overbeek J, Pool RM (1965) Induction of fruit set in Vitis vinifera L. by a kinin. Nature 206:952–953
Yu JQ (1999) Parthenocarpy induced by N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) prevents flower abortion in Chinese white-flowered gourd (Lagenaria leucantha). Environ Exp Bot 42:121–128 (128)
Zhang X, Luo G, Wang R, Wang J, Himelrick DG (2003) Growth and developmental responses of seeded and seedless grape berries to shoot girdling. J Am Soc Hortic Sci Am Soc Hortic Sci 128:316–323
Zhang C, Tateishi N, Tanabe K (2010) Pollen density on the stigma affects endogenous gibberellin metabolism, seed and fruit set, and fruit quality in Pyrus pyrifolia. J Exp Bot 61:4291–4302
Zhu YY, Takahito N, Xu YH, Zhang YY, Peng Y, Mao BZ, Atsushi H, Zhou HC, Wang RX, Li P, Zhu XD, Mander LN, Yuji K, Shinjiro Y, He ZH (2006) Elongated uppermost internode encodes a cytochrome p450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18:442–456
Acknowledgments
The National Natural Science Foundation of China (31471842) supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The sequences of the VvGA20oxs, VvGA3oxs, VvGA2oxs, and VvYUCs reported here are available in GenBank (http://www.ncbi.nlm.nih.gov/): VvGA20ox1 [GenBank accession no. KC898188]; VvGA20ox2 [GenBank accession no. KC898186]; VvGA20ox3 [GenBank accession no. KC KC898189]; VvGA3ox1 [GenBank accession no. KC898176]; VvGA3ox2 [GenBank accession no. KC898175]; VvGA3ox3 [GenBank accession no. KC898177]; VvGA2ox1 [GenBank accession no. KC898179]; VvGA2ox2 [GenBank accession no. KC898180]; VvGA2ox3 [GenBank accession no. KC898181]; VvGA2ox4 [GenBank accession no. KC898182]; VvGA2ox7 [GenBank accession no. KC898184]; VvYUC2 [GenBank accession no. XM_002280979]; VvYUC6 [GenBank accession no. XM_010650228].
Additional information
Communicated by M. Wirthensohn
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1
Primers used for the qRT-PCR performed in this work. (DOCX 18 kb)
Supplemental Table S2
The size of ovule from different treatment at 21 DAA. Values are the means of eight sections (±SE), one section per fruit, and eight fruits. Significant differences (p < 0.05) between treatments are indicated using different letters according to a Tukey’s test. (DOCX 16 kb)
Supplemental Fig. S1
Intact inflorescence (a) and emasculated ovaries (b) at 10 DBA (Days before anthesis). (JPG 3767 kb)
Supplemental Fig. S2
Ovule with degrading embryo sac from GA3-treated unpollinated ovaries at 14 (a) and 21 DAA (b). Scale bar=100 μm. (JPG 3776 kb)
Rights and permissions
About this article
Cite this article
Lu, L., Liang, J., Zhu, X. et al. Auxin- and cytokinin-induced berries set in grapevine partly rely on enhanced gibberellin biosynthesis. Tree Genetics & Genomes 12, 41 (2016). https://doi.org/10.1007/s11295-016-0980-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-0980-4