Abstract
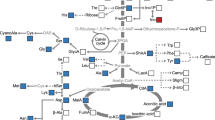
Iron is an essential element for plants growth and development. Biofortification has become an efficient way for iron accumulation in grains to enhance the nutritional quality of crops. Both iron and sucrose are transported via phloem during grain filling in rice. However, the relationship between iron and sucrose transport remains unclear. In this study, we aimed to elucidate the relationship between sucrose transport and iron accumulation in rice. We first found that the efficient iron accumulated stage and sucrose unloading stage overlapped at 5–10 DAF during grain filling. The close relationship was further validated by transgenic lines (Y2 and Y6) overexpressing yeast (Y) invertase (INV). The sucrose efflux rate in phloem decreased by 40.1% and 30.6%, and the iron efflux rate in phloem was also reduced by 73.7% and 51.0% in Y2 and Y6, respectively. Thus, the iron content in grains was decreased. The transcriptome analysis on grains of WT and Y2 at 10 DAF was used to compare the difference in carbohydrate and iron metabolism. Seven upregulated DEGs and twenty-four downregulated DEGs related to carbohydrate metabolism were identified in Y2. The expression levels of starch synthesis and sugar transport genes in Y2 were lower than those in WT. The expression levels of iron-related genes, including OsTOM1, OsNAS1, OsNAS2, OsIRT1, OsYSL9, and OsOPT7, were significantly downregulated in Y2. These results indicated that blocking photoassimilates allocation between source and sink reduces iron accumulation in grains.





Similar content being viewed by others
References
Aoyama T, Kobayashi T, Takahashi M, Nagasaka S, Usuda K, Kakei Y, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2009) OsYSL18 is a rice iron(III)–deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol 70:681–692
Ariga T, Hazama K, Yanagisawa S, Yoneyama T (2014) Chemical forms of iron in xylem sap from graminaceous and non-graminaceous plants. Soil Sci Plant Nutr 60:460–469
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281(43):32395–32402
Chen LQ (2014) SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol 201:1150–1155
Chung P, Hsiao HH, Chen HJ, Chang CW, Wang SJ (2014) Influence of temperature on the expression of the rice sucrose transporter 4 gene, OsSUT4, in germinating embryos and maturing pollen. Acta Physiol Plant 36:217–229
Clegg KM (1956) The application of the anthrone reagent to the estimation of starch in cereals. J Sci Food Agric 7:40–44
Dickinson CD, Altabella T, Chrispeels MJ (1991) Slow-growth phenotype of transgenic tomato expressing apoplastic Invertase. Plant Physiol 95:420–425
Fei HH, Yang ZP, Lu QT, Wen XG, Zhang Y, Zhang AH, Lu CM (2021) OsSWEET14 cooperates with OsSWEET11 to contribute to grain filling in rice. Plant Sci 306:110851
Garcia-Oliveira AL, Chander S, Ortiz R, Menkir A, Gedil M (2018) Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front Plant Sci 9:937
Gregorio GB (2002) Progress in breeding for trace minerals in staple crops. J Nutr 132:500S-502S
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK (2009) Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 284(6):3470–3479
Ishibashi Y, Okamura K, Miyazaki M, Phan T, Yuasa T, Iwaya-Inoue M (2014) Expression of rice sucrose transporter gene OsSUT1 in sink and source organs shaded during grain filling may affect grain yield and quality. Environ Exp Bot 97:49–54
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Rice plants take up iron as an Fe3+ -phytosiderophore and as Fe2+. Plant J 45:335–346
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese: OsYSL2 is required for Fe and Mn transport to the endosperm. Plant J 62:379–390
Jiang HW, Li MR, Liang NT, Yan HB, Wei YB, Xu XL, Liu J, Xu ZF, Chen F, Wu GJ (2007) Molecular cloning and function analysis of the stay green gene in rice. Plant J 52:197–209
Kakei Y, Ishimaru Y, Kobayashi T, Yamakawa T, Nakanishi H, Nishizawa NK (2012) OsYSL16 plays a role in the allocation of iron. Plant Mol Biol 79:583–594
Kawakami Y, Bhullar NK (2021) Delineating the future of iron biofortification studies in rice: challenges and future perspectives. J Exp Bot 72(6):2099–2113
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kobayashi T, Itai RN, Aung MS, Senoura T, Nakanishi H, Nishizawa NK (2012) The rice transcription factor IDEF1 directly binds to iron and other divalent metals for sensing cellular iron status. Plant J 69:81–91
Kobayashi T, Nagasaka S, Senoura T, Itai RN, Nakanishi H, Nishizawa NK (2013) Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat Commun 4:2792
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424
Kühn C, Grof CP (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13:287–297
Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G (2009) Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol 150:786–800
Lee S, Ryoo N, Jeon JS, Guerinot ML, An G (2012) Activation of rice yellow stripe1-like 16 (OsYSL16) enhances iron efficiency. Mol Cells 33:117–126
Liang CZ, Wang YQ, Zhu YN, Tang JY, Hu B, Liu LC, Ou SJ, Wu HK, Sun XH, Chu JF, Chu CC (2014) OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci USA 111(27):10013–10018
Ma QJ, Sun MH, Lu J, Liu YJ, Hu DG, Hao YJ (2017a) b) Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol 174:2348–2362
Ma L, Zhang DC, Miao QS, Yang J, Xuan YH, Hu YB (2017b) a) Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol 58:863–873
Maillard A, Diquélou S, Billard V, Laîné P, Garnica M, Prudent M, Garcia-Mina J-M, Yvin J-C, Ourry A (2015) Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci 6:317
Mori S, Nishizawa N, Kawai S, Sato Y, Takagi S (1987) Dynamic state of mugineic acid and analogous phytosiderophores in Fe-deficient barley. J Plant Nutr 10:1003–1011
Nishiyama R, Kato M, Nagata S, Yanagisawa S, Yoneyama T (2012) Identification of Zn - nicotianamine and Fe - 2’ -deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol 53:381–390
Nozoye T, Inoue H, Takahashi M, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2007) The expression of iron homeostasis-related genes during rice germination. Plant Mol Biol 64:35–47
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Yuki S, Yoko S, Uozumi N, Nakanishi H, Nishizawa NK (2011) Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 286(7):5446–5454
Pearson JN, Rengel Z, Jenner CF, Graham RD (1998) Dynamics of zinc and manganese movement in developing wheat grains. Functional Plant Biol 25:139
Richards FJ (1959) A flexible growth function for empirical use. J Exp Bot 10(29):290–301
Ruamrungsri S, Ruamrungsri S, Ikarashi T, Ohyama T (1999) Carbohydrate metabolism in Narcissus. J Hortic Sci Biotechnol 74:395–400
Saalbach I, Mora-Ramírez I, Weichert N, Andersch F, Guild G, Wieser H, Koehler P, Stangoulis J, Kumlehn J, Weschke W, Weber H (2014) Increased grain yield and micronutrient concentration in transgenic winter wheat by ectopic expression of a barley sucrose transporter. J Cereal Sci 60:75–81
Scofield GN, Hirose T, Gaudron JA, Furbank RT, Upadhyaya NM, Ohsugi R (2002) Antisense suppression of the rice transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Functional Plant Biol 29:815–826
Scofield GN, Hirose T, Aoki N, Furbank RT (2007) Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot 58(12):3155–3169
Senoura T, Sakashita E, Kobayashi T, Takahashi M, Aung MS, Masuda H, Nakanishi H, Nishizawa NK (2017) The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol Biol 95:375–387
Shi RL, Weber G, Köster J, Reza-Hajirezaei M, Zou CQ, Zhang FS, Von Wirén N (2012) Senescence-induced iron mobilization in source leaves of barley (Hordeum vulgare) plants. New Phytol 195:372–383
Sosso D, Luo DP, Li QB, Sasse J, Yang JL, Gendrot G, Suzuki M, Koch KE, McCarty DR, Chourey PS, Rogowsky PM, Ross-Ibarra J, Yang B, Frommer WB (2015) Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet 47:1489–1493
Takagi S, Nomoto K, Takemoto T (1984) Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr 7:469–477
Tang YY, Li MR, Chen YP, Wu PJ, Wu GJ, Jiang HW (2011) Knockdown of OsPAO and OsRCCR1 cause different plant death phenotypes in rice. J Plant Physiol 168:1952–1959
Tetyuk O, Benning UF, Hoffmann-Benning S (2013) Collection and analysis of Arabidopsis phloem exudates using the EDTA—facilitated method. J vis Exp 80:e51111
Tsukamoto T, Nakanishi H, Uchida H, Watanabe S, Matsuhashi S, Mori S, Nishizawa NK (2009) 52Fe translocation in Barley as monitored by a Positron-Emitting Tracer Imaging System (PETIS): evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol 50(1):48–57
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Vasconcelos MW, Gruissem W, Bhullar NK (2017) Iron biofortification in the 21st century: setting realistic targets, overcoming obstacles, and new strategies for healthy nutrition. Curr Opin Biotech 44:8–15
Von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9:3033–3044
Wang SD, Li L, Ying YH, Wang J, Shao JF, Yamaji N, Whelan J, Ma JF, Shou HX (2020) A transcription factor OsbHLH156 regulates Strategy II iron acquisition through localising IRO2 to the nucleus in rice. New Phytol 225:1247–1260
Wang GP, Wu Y, Ma L, Lin Y, Hu YX, Li MZ, Li WW, Ding YF, Chen L (2021) Phloem loading in rice leaves depends strongly on the apoplastic pathway. J Exp Bot 72(10):3723–3738
Yan W, Wu XY, Li YA, Liu GH, Cui ZF, Jiang TL, Ma QX, Luo LJ, Zhang P (2019) Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink. Front Plant Sci 10:541
Yang J, Luo DP, Yang B, Frommer WB, Eom J-S (2018) SWEET11 and 15 as key players in seed filling in rice. New Phytol 218:604–615
Yoneyama T, Gosho T, Kato M, Goto S, Hayashi H (2010) Xylem and phloem transport of Cd, Zn and Fe into the grains of rice plants (Oryza sativa L.) grown in continuously flooded Cd-contaminated soil. Soil Sci Plant Nutr 56:445–453
Yoneyama T, Ishikawa S, Fujimaki S (2015) Route and regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. Int J Mol Sci 16:19111–19129
Zentgraf U, Andrade AG, Doll J (2021) Editorial for special issue “leaf senescence” in plants. Plants 10:1490
Zhang YH, Zhang YP, Liu N, Su D, Xue QW, Stewart BA, Wang ZM (2012) Effect of source–sink manipulation on accumulation of micronutrients and protein in wheat grains. J Plant Nutr Soil Sci 175:622–629
Zhang CK, Han L, Slewinski TL, Sun JL, Zhang J, Wang ZY, Turgeon R (2014) Symplastic phloem loading in poplar. Plant Physiol 166:306–311
Funding
This work was supported by the National Natural Science Foundation of China (Grants No. 32272206), the Natural Science Foundation of Jiangsu Province for Distinguished Young Scientists (Grants No. BK20200079), and the Collaborative Innovation Center for Modern Crop Production co-sponsored by Province and Ministry (CIC-MCP).
Author information
Authors and Affiliations
Contributions
LC and YD designed the experiments. YL, YH, YW, and YQ performed the experiments. YL and YH analyzed the data and discussed the results. YL and LC wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interest
All authors have read and approved this version of the article and due care has been taken to ensure the integrity of this work. The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Monica Colombo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
344_2023_11200_MOESM1_ESM.tif
Supplementary Fig. S1 Relative expression of selected senescence-related genes in the flag leaves during the grain filling stage at 0–25 DAF. a Stay-green rice (OsSGR). b Red chlorophyll catabolite reductase 1 (OsRCCR1). c Pheophorbide a oxygenase (OsPAO). d Prematurely senile 1 (PS1). The relative expression of genes was measured by qRT-PCR. Each value in a-d presents the means ± SE (n = 3). Different letters (in a-d) indicate significant differences among different time points by Duncan’s test (P < 0.05).
Supplementary file1 (TIF 1056 KB)
344_2023_11200_MOESM2_ESM.tif
Supplementary Fig. S2 The expression patterns of genes related to carbohydrate transport and metabolism in grains of wild-type (WT) and transgenic line Y2 (35S::PI-INV) rice plants. a Heatmap showing the expression patterns of genes involved in carbohydrate metabolism obtained from RNA-seq. All of the genes shown in a belong to the 1094 DEGs. The genes with red boxes encode the same type of enzyme. b Heatmap showing the expression patterns of genes involved in starch synthesis and sugar transport obtained from RNA-seq. Genes with red fonts are included in the 1094 DEGs. Asterisks indicate significant differences in the expression of genes between WT and Y2 by Student’s t test (*P < 0.05, **P < 0.01). The relative values in a and b are read counts from RNA-seq analysis. Z-score was used for calculating the relative values of the heatmap. The sample names (WT_1, WT_2, WT_3, Y2_1, Y2_2 and Y2_3) in the x-axis represented independent biological replicates. c Starch content of grains and flag leaves at 10 days after flowering (DAF). Each value in c presents the means ± SE (n = 3). Asterisks (in c) indicate significant differences between WT and Y2 at each organ by Student’s t test (*P < 0.05).
Supplementary file2 (TIF 16028 KB)
344_2023_11200_MOESM3_ESM.tif
Supplementary Fig. S3 Expression analysis of genes involved in regulating iron homeostasis between wild-type (WT) and transgenic line Y2 (35S::PI-INV) rice plants. a Heatmap showing the expression patterns of genes involved in iron regulation obtained from RNA-seq. Z-score was used for calculating the relative values of the heatmap. Genes with red fonts are included in the 1094 DEGs. The relative values in a are read counts from RNA-seq analysis. b Relative expression of OsIRT2 and OsbHLH156 in grains was measured by qRT-PCR. Each value in b presents the means ± SE (n = 3). Asterisks indicate significant differences between WT and Y2 by Student’s t test (*P < 0.05).
Supplementary file3 (TIF 7604 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, Y., Hu, Y., Wu, Y. et al. Inhibition of Sucrose Source-to-Sink Transport Reduces Iron Accumulation in Rice. J Plant Growth Regul 43, 1496–1507 (2024). https://doi.org/10.1007/s00344-023-11200-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11200-y