Abstract
Bioactive ingredients derived from brown algae have been extensively used in the food, medicine, and cosmetic industries. In this study, fucoidans of low and high molecular fractions (LMF and HMF) extracted and isolated from brown alga Turbinaria decurrens were analyzed for their efficacy on seed germination, seedling growth, callus induction, direct organogenesis, and adventitious root formation in eggplant and finger millet. The yield and sugar content of LMF were higher than HMF. FTIR confirmed that the isolated fractions containing fucoidan has more sulfate groups in HMF than in LMF. The results showed an enhanced seed germination and seedling growth in both crops. In eggplant, 1 mg/L LMF treatment showed the maximum germination (91.6%), whereas, in finger millet, 0.1 and 0.5 mg/L LMF recorded a substantial increase in germination percentage (41.6 and 46%). Maximum fresh weight (FW) was noted with 1.0 mg/L LMF, and 1.0 mg/L LMF and 0.5 mg/L HMF showed maximum dry weight (DW) in eggplant. In finger millet, maximum DW was observed in 0.5 mg/L LMF and 1.0 mg/L HMF. Maximum biomass was noted in the 0.1 mg/L LMF treated group in the case of callus growth in eggplant. Similarly, the shoot tip initiation, proliferation, and plantlet regeneration were significantly improved with fucoidan LMF (0.1 mg/L). In conclusion, fucoidan extracted from T. decurrens exhibiting natural growth promoter property is reported for the first time in this study. These fucoidan fractions, LMF and HMF, can be utilized as cost-effective supplements in plant tissue culture media replacing the commercial PGRs for micropropagation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive usage and applications of chemical fertilizers have paved the way for pollution, leading to harmful socio-economic and environmental impacts such as global warming, climate change, and eventually sabotaging agribusiness. To combat this excessive use of chemical fertilizers, as a practical, eco-friendly, and sustainable alternative, natural biostimulant/bio effectors came into practice (Claros Cuadrado et al. 2019). Seaweed liquid fertilizers are commonly used as growth enhancers/biostimulants to positively influence plant growth and development. Seaweeds generally contain polysaccharides/hydrocolloids, pigments, lipids (less quantity), phenolics, and some mineral nutrients (iodine, iron, etc.) (Collins et al. 2016; El-Shenody et al. 2019; Sharawy et al. 2020). They are rich in nutrients as plant growth stimulators, which support the biochemical process of plants, and positively regulate stress signaling, thereby favoring plant growth and biomass production. Seaweed elicitors can also be used for in vitro growth of higher plants (Gurusarava et al. 2017) as they are abundant in nature with enormous bioactive stimulatory compounds and major and minor elements (Esserti et al. 2017; Circuncisão et al. 2018). Biotic stress and abiotic stress tolerance in plants can be enhanced using seaweed extracts, and the treatment can also help to control pests and diseases (Sarkar et al. 2018; Karthik et al. 2020). Seaweed extracts showing biostimulatory effects are suitable for sustainable agriculture because they could reduce the dependency on chemical fertilizers to improve yield and help the plants tackle environmental stresses (Collins et al. 2016; Circuncisão et al. 2018).
Turbinaria sp. can grow up to 30-m depth in reef habitats along the tropical and subtropical regions of the seawater. Turbinaria decurrens is a source of biomolecules such as polyphenols, sulfated polymers (alginate and fucoidans), uronic acids, fucoxanthin pigment, amino acids, essential lipids. These biomolecules are valuable for food and biomedical applications (Chattopadhyay et al. 2010; El-Shenody et al. 2019; Sanniyasi et al. 2019; Sami et al. 2019; Zubia et al. 2020).
The eggplant (Solanum melongena L.) belonging to the Solanaceae family has great interest globally due to its high-value bioactive compounds, especially antioxidant polyphenols. The finger millet (Eleusine coracana Gaertn), a famine crop, family Poaceae, is rich in amino acids, particularly methionine and lysine (Mbithi-Mwikya et al. 2000; Mitharwal et al. 2021). The bioactive compounds include nutritional fiber, polyphenols, and minerals with high calcium (Devi et al. 2014). In vitro propagation of these crops involves expensive plant growth regulators (PGRs). On the other hand, applying organic stimulants, especially from seaweeds has proven to show stimulatory effects, which is cost-effective but less studied. Seaweeds are the source for producing sulfated polysaccharides such as agar, carrageenan, alginate, and fucoidans. They have been utilized in various food, pharmaceutical, and biomedical industries for multiple product developments (Shanthi et al. 2021). Studies proved seaweed polysaccharides of low molecular fractions has an excellent inhibitory effect against cancer cell lines (Arunkumar et al. 2021). There are studies demonstrating the effect of carrageenan and alginate polysaccharides as biostimulants (García-Fortea et al. 2020; Shanthi et al. 2021). But, the effect of seaweed polysaccharides particularly role of low molecular fucoidan fractions as biostimulant has not been studied (García-Fortea et al. 2020). Hence, this study is carried out to understand the effect of high and low molecular fucoidans fractions extracted from brown seaweed T. decurrens as biostimulant for seed germination (in vitro), seedling growth of eggplant and finger millet, and callus induction, shoot tip culture, organogenesis, and adventitious root formation in eggplant.
Materials and Methods
Seaweed Collection and Processing
Healthy specimens of 1.5 kg brown seaweed T. decurrens (Bory de Saint-Vincent) J. Agardh (Sargassaceae) remain immersed in the seawater during low tide were collected along the coast of Gulf of Mannar (Pamban, 9° 17′ N 79° 18′ E/9.28° N, 79.3° E, India). The specimens were cleaned by washing thoroughly in seawater, followed by tap water for 20 min and distilled water for 10 min to remove unwanted dirt and debris. After air-drying in the dark for 3 days, specimens were pulverized into fine powder.
Pretreatment to Remove Pigments and Protein
Seaweed powder weighing 500 g was extracted initially by 250 mL of 1:1 (v/v) chloroform:methanol solvent for three days at 30 °C in a dark room to remove non-fucoidan portions such as pigments and lipophilic substances. The extract was then filtered (Whatman No. 1 paper). The residue was extracted further using 200 mL of 85% ethanol at 23 °C (twice at 12 h) and 70 °C (twice at 5 h), and the protein was removed (Rioux et al. 2007).
Extraction of Fucoidan
After removing the pigments and protein, the residue was extracted thrice in 2% CaCl2 in distilled H2O (3 × 500 mL) at pH 8.5 for 3 h at 70 °C, and extracts were collected. Then the residue was extracted thrice in 0.01 N HCl (3 × 500 mL) at 70 °C temperature for 3 h, and extracts were collected. Both extracts obtained in 2% CaCl2 and 0.01 N HCl were pooled and combined as crude fucoidan. Then the combined extract was filtered (Whatman No. 1 filter paper) and kept for 20 min on a centrifuge at 10,000 rpm. The supernatant was discarded, and the pellet was considered crude fucoidan (Rioux et al. 2007; Shanthi et al. 2021).
Isolation and Purification of Fucoidan
Crude fucoidan 500 mg dissolved in 5 mL of distilled H2O was loaded in DEAE-cellulose 52 column (SRL, India) for elution at 25 °C. The column was eluted stepwise by increasing the concentration of 300 mL 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 M NaCl in distilled H2O. The flow rate was maintained at 1.0 mL min−1, and the eluents of 50 mL separated by 3.0 M NaCl of 300 mL containing fucoidan were collected and freeze-dried (Shanthi et al. 2021).
Fucoidan Fractionation
Fractions eluted in 3 M NaCl were combined, freeze-dried, and dialyzed (MWCO3500, #21–152-9 flat width 46 mm vol/cm 6.74 mL, wall thickness 28 µm dry cylinder diameter 29.3 mm, Fisher, India) for 48 h in distilled water. The fucoidan substances retained in the membrane bag were considered high molecular fractions (HMF < 3.5 kDa) and passed across as low molecular fractions (LMF > 3.5 kDa) were collected. The dialyzed both fractions were lyophilized and stored at 4 °C till further study. The yield in alga dry wt. was recorded by taking the freeze-dried fucoidan fractions' weight, and their sugar content (Dubois et al. 1956) and sulfate (Dodgson and Price 1962) were recorded.
FTIR Characterization of Fucoidan Fractions
The FTIR spectrum of LMF, HMF, and standard fucoidans was recorded using the Perkin-Elmer FT-IR spectroscopy (RXI model). 2 mg of each sample mixed with 200 mg of KBr and the spectrum was recorded in between 400 and 4000 cm−1 range in 4 cm−1 resolution. Both the samples were characterized by comparing with the spectral properties of the fucoidan standard.
Collection of Seeds and Germination In Vitro
The seeds of eggplant (S. melongena L. var. Mattu Gulla) (MG) and finger millet (E. coracana Gaertn) were obtained from Mattu Village, Udupi District, Karnataka, and the Tamil Nadu Agricultural University, India, respectively. The uniform size seeds were selected and soaked in distilled water for 3 h, and the extra moisture content of the seeds was removed by filter paper (Muthusamy et al. 2012). As mentioned in our previous reports, the seeds of both plants were inoculated on MS medium after surface sterilization. Seeds were soaked in soap solution for 5 min, followed by 70% ethanol for 1 min, then rinsed with 0.1% (w/v) mercuric chloride for 5 min and finally with sterile distilled water (Swathy et al. 2017). Three different concentrations (0.1, 0.5, and 1.0 mg/L) of LMF and HMF of fucoidans were added to the Murashige and Skoog (MS) basal medium; then, pH 5.8 was adjusted and sterilized at 121 °C for 15 min. Similarly, the MS basal medium was used for control groups. The seeds were maintained at 25 ± 2 °C with 40 μmol m−2 s−1-white cool-fluorescent tubes in a 16 h photoperiod. The eggplant and finger millet germination patterns were observed every week and calculated after the fourth week. The growth characteristics of seedlings, such as total length and fresh weight (FW) and dry weight (DW), were measured from five replicates, and the values were recorded as per our earlier reports (Muthusamy et al. 2012).
Callus Induction and Adventitious Root Formation
The leaf and stem explants were expunged from 30 days old in vitro seedlings aseptically and transferred to supplemented MS medium with 0.1 mg/L 2,4-D and benzyl amino purine (BAP; Muthusamy et al. 2014), 0.1, 0.5, and 1.0 mg/L fucoidans of LMF/HMF. Further, the leaf and stem callus were sub-cultured every fortnight in the same medium for further development. The developing callus was shifted to a fortified MS medium with five concentrations of BAP (1.0, 1.5, 2.0, 2.5, and 3.0 mg/L) and 0.1 mg/L kinetin (KIN) for proliferation and sub-cultured thrice. After 30 days of proliferation, the leaf and stem callus were carefully removed from the culture bottle. The FW of the developed leaf and stem callus was weighed and recorded. Later, the developed callus was kept for drying in a hot air oven at 40 °C for 3 days, and finally, the DW of the callus was measured. During the experiment, adventitious root initiation was noted in MS basal medium fortified with 0.1 mg/L LMF after 30 days of culture, and the results are explained in the discussion section. Further, a separate experiment was conducted in fortified MS basal medium with and without 0.1 mg/L LMF for adventitious root formation as this concentration has shown better in vitro callus response. The percentage of response, the number of roots per explant, FW, and the total length of the adventitious root were measured.
Shoot Tip Culture and Plantlet Regeneration
The shoot tip explants (~ 0.5 cm) were carefully removed from in vitro seedlings using a sterile scalpel and inoculated on MS medium augmented with 3.0 mg/L and 0.1 mg/L KIN; and 0.1, 0.5, and 1.0 mg/L LMF fucoidans, separately as two sets of experiments. The shoot cultures were subcultured twice for proper growth of the shoot tip; later, they were transferred onto MS medium with the combinations mentioned above of plant growth hormones along with 0.3 mg/L gibberellic acids (GA3) for elongation of the shoot. The elongated shoots (approx. 3.0 cm) were transplanted on a fortified MS medium with 0.5 mg/L IBA (Muthusamy et al. 2014) for root initiation.
Statistical Analysis
Five replicates were maintained for each treatment and repeated twice, and the data were presented as mean ± SD. The statistical significance (P < 0.05) of the control and the treatment group (LMF/HMF) was determined using one-way ANOVA and Bonferroni's test for multiple comparisons using Graph Pad Prism 8 (Graph-Pad Software, Inc.).
Results and Discussion
Various studies demonstrated the potential of seaweeds and their extracts for improving the growth, stress tolerance, disease, and pest resistance in plants (Khedia et al. 2020; Mukherjee and Patel 2020; Nanda et al. 2022) and some extended the application in plant micropropagation (Sharma et al. 2015; Anbazhakan et al. 2022). This plant growth-promoting effect of seaweed extracts has been ascertained by increased uptake of nutrients, lipid synthesis, and plant defense mechanism (Vasantharaja et al. 2019; Mutale-Joan et al. 2020; Yang et al. 2021). Several bioreagents developed from seaweed extracts for crop production constitute bioactive components such as fatty acids, minerals, polysaccharides, and vitamins (Zhao et al. 2018; Vasantharaja et al. 2019; Dobrinčić et al. 2020; Mutale-Joan et al. 2020; Yang et al. 2021). Extracts prepared from brown algae such as Durvillaea spp., Ecklonia spp., Fucus spp., Laminaria spp., Sargassum spp., Ascophyllum spp., Macrocystis spp., and Turbinaria spp., are widely used as biostimulants in agricultural practices (Khan et al. 2009; Craigie 2011; Sharma et al. 2014; Bulgari et al. 2015; Collins et al. 2016). Carrasco-Gil et al. (2021) studied the effect of seaweed extract of Ascophyllum nodosum components to tolerate iron deficiency in tomato plants. Marine algae extracts can be selectively utilized as sulfated polysaccharides for crop improvement and micropropagation.
Seaweed sulfated polysaccharides are known for their wider range of biological activities, and alginate and fucoidans polymers are extracted only from the brown algae source. Studies demonstrated the potential of fucoidan in the animal system in showing antitumor, antioxidant, and anticoagulant (Yuan and Macquarrie 2015; Li et al. 2017; Wang et al. 2019). Also, few reports about fucoidans enhancing the plant defense against stress conditions (Bouissil et al. 2020). Fucoidans can be broken down into l-fructose by acidic treatment or enzymatic degradation methods. This l-fructose helps increase the chlorophyll content in plants and is important in plant metabolism and immunity (Soto et al. 2019; Zhang et al. 2019). In another study, fucoidans fraction from Macrocystis pyrifera (L.) was observed to enhance salt stress resistance in wheat plants (Zou et al. 2021). In this study, results obtained on the effect of different concentrations (0.1, 0.5, and 1.0 mg/L) of LMF and HMF of fucoidans extracted from T. decurrens for in vitro seed germination of a dicot eggplant and a monocot finger millet are elaborately discussed. Further, a study on fucoidans LMF and HMF on callus induction, growth rate, and direct organogenetic potential in eggplant is presented.
Constituents of Fucoidan and FTIR Characterization
In this study, fucoidan extracted from the brown seaweed T. decurrens was separated into two fractions LMF (˃3.5 kDa) and HMF (˂ 3.5 kDa). The LMF recorded more yield (2.17% algal dry wt.) and its total sugar (662.33 mg g−1 fucoidan) than HMF, which recorded higher sulfate (53.82 mg g−1 fucoidan) (Table 1). The LMF and HMF of fucoidans extracted from the T. decurrens were characterized based on FTIR spectral properties by comparing the standard fucoidan spectrum (Fig. 1) and fucoidan isolated from other brown seaweeds (Zayed et al. 2016; Rajeshkumar 2017; Ptak et al. 2019; Shanthi et al. 2021; Yu et al. 2021). Fucoidans of LMF and HMF and the standard was recorded bands at 3459 cm−1 assigned for the OH group of fucose and galactose, and at 2933 cm−1 for aliphatic C–H vibration of C6 fucose pyrenoid ring (Zou et al. 2021). Both fucoidan samples were found at 1636 cm−1 for O–C–O stretching vibration on par with standard, confirming the existence of O-acetyl groups of sugar monomers (Zou et al. 2021). A strong peak observed at 1406 cm−1 in the LMF and HMF fucoidan that is not prominent in the standard indicates the presence of scissoring vibration of CH2 (galactose, xylose) and asymmetric bending vibration of CH3 (fucose, O-acetyls)/sugar moiety particularly COO of uronic acid (Synytsya et al. 2010). This observation suggested that fucoidans isolated from the T. decurrens constitute high galactose and xylose (Shanthi et al. 2021). Even though a peak at 830 cm−1 indicates the presence of C–S–O of fucose sugar with sulfate attached at the C-4 position observed in both samples and standard, a strong band at 1261 cm−1 for asymmetric O-S–O stretching vibration of sulfate esters observed in HMF and standard not in LMF show that latter has less sulfate (Synytsya et al. 2010; Zou et al. 2021) that reflected less estimated sulfate (Table 1). The peak at 1189 cm−1 in LMF shows the presence of S = O stretching of alkyl sulfoxide, which did not find in HMF and standard fucoidan (Zou et al. 2021). Further, a small peak at 1038 cm−1 shows the C–O stretching of carboxylic and alcohol groups of fucoidan mono sugars, recorded in both the samples and standard (Rajeshkumar 2017). A band at 830 cm−1 observed in the HMF and LMF fractions as like standard confirm the existence of sulfate at C2 of galactose in the fucoidan (Jing et al. 2021). The band observed at 622 cm−1 means for asymmetric and symmetric O–S–O deformation of sulfates was observed in both samples and standard (Synytsya et al. 2010). The FTIR spectral properties observed in the present study suggest that both isolated fractions were confirmed as fucoidans and HMF constitute more sulfate groups than the LMF.
Effect of Fucoidans LMF and HMF on Seed Germination
Germination percentage was recorded as high in fucoidans treatments observed for three consecutive weeks. In eggplant, the germination percentage doubled at the end of the first week. 10–20-fold higher germination was recorded during the second week (Figs. S1, S2). Reached a maximum during the third week by 1.0 mg/L fucoidan of LMF and HMF with 91.6 and 94.4%, respectively, followed by control (77.7%). In finger millet, high germination percentage was observed by 0.1 mg/L LMF (41.6%) followed by 0.5 (40%), 1.0 (36%) mg/L, and control (30%), and 0.5 mg/L (46.0%) HMF followed by 0.5 (46%), 1.0 (45.3%) mg/L and control (30%) (Fig. 2). Even though all the treatment groups showed a higher germination percentage than the control groups, no significant difference was observed, as variations in phenotypic character and time of seed’s maturity.
This observation shows that low fucoidans concentrations of 1.0 mg/L LMF and HMF induced seed germination of more than 90% germination over control in eggplant, whereas in finger millet, 41 and 46%, respectively. Similarly, a low concentration of seaweed liquid extract affecting increased seed germination in fruits and vegetable crops was observed (Kumar and Sahoo 2011; Vinoth et al. 2012; Latique et al. 2013; Rupawalla et al. 2022). Seaweeds contain precursors of elicitor compounds and polysaccharides, which can facilitate seed germination, its growth, and plant health maintenance (Nabti et al. 2017). Navarro et al. studied the effect of biomass, as well as the aqueous extract of algae (Desmodesmus subspicatus), on in vitro germination and the emergence of seedlings in orchids (Cattle yawarneri) and found well-developed seedlings with enhanced germination and shoot formation, in the treated group (Navarro et al. 2021).
Effect of Fucoidans LMF and HMF on Seedling Growth Characters
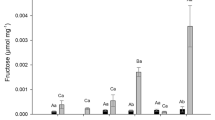
The growth characteristics of the seedlings, such as seedling length and biomass, were recorded in the fourth week. The fucoidans (LMF and HMF) significantly increased the seedling length and other growth characteristics over control groups. In eggplant, the high seedling length was recorded at 1.0 mg/L LMF (17.67 cm) followed by 0.1 (16.63 cm), 0.5 (15.55 cm) mg/L, and control (15.48 cm) groups. However, in HMF 0.1 mg/L (18.58 cm) showed high seedling length followed by 0.5 (18.57 cm), 1.0 (17.92 cm) mg/L, and control (15.48 cm) groups (Figs. 3, 4, 5). The maximum FW was witnessed at 1.0 mg/L LMF with 5.46 g and 0.5 mg/L HMF with 6.66 g. Similarly, maximum DW was recorded at 1.0 mg/L LMF with 0.39 g and 0.5 mg/L HMF with 0.511 g in eggplant seedlings (Fig. 6). In finger millet, the maximum seedling length was recorded at 1.0 mg/L LMF and HMF fucoidans with 13.28 and 11.79 cm, respectively (Figs. 5, 7, 8). The maximum FW was noted at 1.0 mg/L LMF with 0.533 g and 0.1 mg/L HMF with 0.476 g. The maximum DW was observed at 0.5 mg/L LMF with 0.033 g and 1.0 mg/L HMF with 0.050 g in finger millet seedlings (Fig. 6). From the above observations on the effect of fucoidan fractions on growth characteristics eggplant alone was taken up for assessing the callus induction and direct organogenesis study.
Similar to this extracted fucoidans effect observed in this study, extracts of seaweeds Gracilaria edulis and Sargassum wightii promoted 20% seedling growth in tomatoes (Kumar and Sahoo 2011). An increase in germination, seedling growth and some metabolic processes of Vicia faba L. were recorded by the seaweed extract of Cladophora dalmatica. It was found that calcium ions (Ca2+) are present in the algal extracts and regulate important metabolic and other vital cell functions, thereby contributing to an increase in plant growth and development (Shariatmadari et al. 2015). Plant growth-promoting effect of seaweeds, Padina durvillaei, and Ulva lactuca was reported in Arabidopsis (Battacharyya et al. 2015; Li et al. 2017; Benítez García et al. 2020; Ghaderiardakani et al. 2019). Better growth in the number of leaves, leaf size, and length and shoot growth was noted in shallot (Allium wakegi) when supplemented with 20% seaweed extract (Yusuf et al. 2021). This suggests that mono sugars, uronic acid, and sulfate constituents of fucoidans extracted from T. decurrens would elicit increased germination, growth patterns, and total biomass of finger millet and eggplant.
Effect of Fucoidans LMF and HMF on the Callus Growth Rate of Eggplant
Leaf and stem explants of eggplant were inoculated on fortified MS medium with 1.0 mg/L 2,4-D and 1.0 mg/L BAP in combination with varying concentrations of LMF fucoidan (0, 0.1, 0.5, and 1.0 mg/L) had shown differential response on callus FW and DW (Table 3). The highest FW and DW of leaf and stem callus were noted at 0.1 mg/L LMF fucoidan with 4.4 g of FW and 0.27 g of DW in leaf callus, whereas 6.52 g of FW and 0.72 g of DW in stem callus, followed by 1.0 and 0.5 mg/L LMF fucoidan and control groups (Fig. 9; Fig. S3).
In another study, leaf and stem explants were inoculated on the fortified MS medium with variable concentrations of 2, 4-D (1.0–3.0 mg/L), and BAP (0.1, 1.0 mg/L) with 0.1 mg/L HMF fucoidan. They showed differential responses on callus FW and DW. The maximum FW and DW of leaf and stem callus were noted on MS media with 1.0 mg/L 2, 4-D, and 1.0 mg/L BAP with 0.1 mg/L HMF as 9.05 g of FW and 0.58 g of DW in leaf explant, and 12.46 g of FW and 0.78 g of DW in stem callus, followed by other treatments and control groups (Fig. 10). Further, the effect of LMF and HMF fucoidans extracted from T. decurrens on callus induction, direct organogenesis, and adventitious root formation has evaluated in this study. Indeed, all the concentrations of LMF fucoidan showed a significant increase in the FW and DW of eggplant callus. The best effect was observed at 1.0 mg/L whereas for HMF fucoidan was at 0.5 mg/L (Fig. 10). Similarly, 20% extract prepared from brown seaweed S. wightii showed an increased DW of Vigna sinensis at Sivasankari et al. (2006) and 3.0 mg/L seaweed extract induced maximum callus response in Withania somnifera (Kannan et al. 2014). Besides seaweed extracts, this study showed that selective seaweed compounds like fucoidans are more effective for increased callus induction, and a better effect was by HMF over LMF that would be due to increased sulfate constituent in HMF than the latter.
Effect of Fucoidan LMF on Shoot Initiation, Multiplication, and Plantlet Regeneration
This study used fucoidan LMF supplementation for direct organogenesis since no substantial difference was noted between control and HMF-treated shoot tip cultures. The in vitro responses of shoot tip explants shifted to supplemented MS medium with BAP (3.0 mg/L) and KIN (0.1 mg/L), and three concentrations of fucoidan LMF (0.1–1.0 mg/L) are shown in Table 2. The substantial growth of the shoot tip was observed at 0.1 mg/L LMF with 6.02 cm, followed by 0.5 and 1.0 mg/L LMF with 4.87 cm and 3.50 cm, respectively. Further, the control groups measured the least growth with a 2.24 cm shoot tip. Shoot tip explants inoculated on fortified MS medium with 3.0 mg/L and 0.1 mg/L KIN and 0.1 mg/L fucoidan LMF showed varying growth responses (Tables 2, 3). The maximum percentage of shoot response (44%), number of shoots (3), FW (2.54 g), and total length (18.6. cm) were recorded at 0.1 mg/L fucoidan LMF compared to control and other treated groups (Fig. S4a–d). The proliferated shoots were further sub-cultured on MS with 0.1 mg/L of fucoidan LMF and GA3 (0.3 mg/L) for shoot elongation. The elongated shoots were further transplanted on an augmented MS medium with IBA (0.5 mg/L) for root initiation (Fig. S4e–h). The well-developed root plantlets were transplanted into plastic cups with autoclaved soil, watered with sterile distilled water, and maintained at 25 ± 2 °C (Fig. S5). Later, the plantlets were transplanted in pots with natural soil and acclimatized in the greenhouse at 30 ± 5 °C with 60% moisture.
Based on our previous observations (Muthusamy et al. 2014; Swathy et al. 2017), the shoot tip was inoculated on MS medium fortified with BAP (3.0 mg/L), KIN (0.1 mg/L), and three concentrations of fucoidans LMF (0.1, 0.5, and 1.0 mg/L). Among three concentrations, 0.1 mg/L showed a significant increase in shoot tip proliferation growth in 30 days over other treatments. Shoot initiation and multiplications were observed in shoot tip explants treated with fucoidan LMF (0.1 mg/L), and no response was recorded in the control group. Similarly, the effect of 0.1 mg/L fucoidan LMF of seaweed, T. decurrens for shoot initiation and multiplications in eggplants observed from this study, liquid extract of seaweed G. edulis and Padina boergesenii showed a significant increase in shoot and root growth of four finger millet genotypes (Satish et al. 2016). Further, a substantial number of shoots per callus and shoot G. edulis reported elongation, and S. wightii extracts (Vinoth et al. 2019). Improved root growth, esterase activity, nutritional and sugar content was reported in Zea mays by A. nodosum extract (Ertani et al. 2018). Acadian marine plant extract enhanced the shoot and root growth of Arabidopsis thaliana (Rayorath et al. 2008). MS media supplemented with 20% blue-green algae (Oscillatoria tenuis) extract showed a significant increase in proliferation and rooting percentage in dates palm. Also, traits like plant height, the number of leaves, and compatibility of acclimatization were observed to be better in treated plants than in control (Ibrahim et al. 2018). Ertani et al. (2018) and Vinoth et al. (2011) proved IAA- and GA3-like activity found in algal extracts of Laminaria and A. nodosum, G. edulis and S. wightii for improved in vitro growth and plantlet development in Z. mays and tomato (Vinoth et al. 2012; Ertani et al. 2018). Predominantly aspartic and glutamic acids are found in Turbinaria, which supports the in vitro cultivation of eggplant (Zubia et al. 2020). Seaweed liquid fertilizer of A. nodosum was an effective alternative to PGR for in vitro regeneration of Capparis decidua explants showing increased germination, shooting, and rooting at various concentrations suggested as a substitute for PGRs for the micropropagation plantlet development (Ahlawat et al. 2022). With the strength of previous studies, our results suggest that seaweed extracts, particularly compounds like fucoidans, are cost-effective (as they produce larger biomass, less operational cost, and comparatively simple production procedure) analog substances to commercial PGRs that can be utilized not only for plant growth-promoting activity but also as a suitable substitute for in vitro micropropagation.
In vitro culture of Cattleya labiata's culture was supplemented with microalgal extract (Messastrum gracile and Chlorella vulgaris) instead of PGRs. By replacing PGRs (6-benzyl amino purine; thidiazuron; zeatin) with algal biomass and extract in the media, efficient micropropagation protocol for orchid, C. labiata was optimized, and high-strength plant tissue culture media (MS media) decreased germination of Comanthera mucugensis. In contrast, a twofold increase in bud formation was noted. Seaweed extracts of P. durvillaei, U. lactuca, and Ulva intestinalis were reported to possess PGR-like activities similar to plant growth hormones like auxin, cytokinin, and gibberellin (Ghaderiardakani et al. 2019; Benítez García et al. 2020). Furthermore, the Hypnea pseudomusciformis extract enhanced the plant's above-ground biomass and root growth (Carmo et al. 2020).
Effect of Fucoidan LMF on Adventitious Root Formation
Leaf explants were inoculated on supplemented MS with 0.1 mg/L fucoidan LMF for callus initiation. Interestingly, the adventitious root formation was noted after 30 days of inoculation and showed varying root responses. The highest percentage of root response (14.5%), number of roots (3–6), FW (0.38 g), and total length (3.76 cm) were recorded at 0.1 mg/L LMF compared to control groups (Fig. 11; Table 4). Among 40 leaf explants that were inoculated, only 14.5% of adventitious roots were noted.
Algal extracts contain abscisic acid, which promotes rooting and positively affects rooting in the treated group in this study. A molecular with modulated metabolic pathways study found that crops treated with extract prepared from the alga A. nodosum show significant growth and resilience effects when facing different stresses (De Saeger et al. 2020). The present study demonstrated an interesting result: leaf explant (eggplant) inoculated on MS media supplemented with fucoidan LMF showed adventitious root initiation (17%), whereas control groups had no root formation. The biostimulant properties of seaweeds are well-known for plant growth and development through enhanced seed germination, photosynthetic pigments, and stress tolerance (Battacharyya et al. 2015; Mansori et al. 2016). Recently the biostimulant activity of glycosides along with zeatin from biomass suspension and aqueous algal extract of Desmodesmus subspicatus was reported for enhanced hypocotyl volume and length, growth of roots, and leaf area (Mazepa et al. 2021).
Conclusion and Future Perspectives
In conclusion, fucoidan fractions extracted and isolated from brown alga T. decurrens show that the yield and sugar content of LMF were higher than HMF, and the sulfate content was high in the latter. The identity of the fractions was confirmed from FTIR spectral properties as fucoidan with more sulfate groups in HMF than the LMF. This study demonstrated the effect of fucoidans LMF and HMF extracted from T. decurrens as a supplement in MS media for seed germination and growth of seedlings in eggplant and finger millet micropropagation as well as plantlet regeneration in eggplant to facilitate an efficient micropropagation system. Fucoidan fractions are suitable substances for inducing germination in finger millet and eggplants. Also, it enhanced the plants FW and DW and callus induction.
Furthermore, both fucoidans LMF and HMF showed a significant effect on shoot initiation, elongation, and root induction were observed from shoot tip explants, and the effect more pronounced by HMF. This present study demonstrates the in vitro growth-promoting effects of a fucoidan of T. decurrens in both monocot and dicot plants for the first time. Thus, fucoidan fractions can supplement tissue culture media to facilitate faster growth responses as a cost-effective alternative to commercial PGRs for plant micropropagation application.
References
Ahlawat J, Sehrawat AR, Chaudhary R, Pandey D (2022) Ascophyllum nodosum: a potential substitute for synthetic hormones for tissue culture propagation of Capparis decidua (Forsk) Edgew. Regen Eng Transl Med 8:145–151. https://doi.org/10.1007/S40883-021-00215-0
Anbazhakan R, Parthibhan S, Senthil Kumar T (2022) Effect of seaweeds extract and plant growth regulators on high-frequency in vitro regeneration and ex vitro rooting of Ceropegia maculata Bedd.: an endemic species of Southern Western Ghats. PCTOC 151:293–306. https://doi.org/10.1007/s11240-022-02352-y
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48. https://doi.org/10.1016/J.SCIENTA.2015.09.012
Benítez García I, Dueñas Ledezma AK, Martínez Montaño E, Salazar Leyva JA, Carrera E, Osuna Ruiz I (2020) Identification and quantification of plant growth regulators and antioxidant compounds in aqueous extracts of Padina durvillaei and Ulva lactuca. Agronomy 10:866. https://doi.org/10.3390/AGRONOMY10060866
Bouissil S, Alaoui-Talibi ZE, Pierre G, Rchid H, Michaud P, Delattre C, El Modafar C (2020) Fucoidans of Moroccan brown seaweed as elicitors of natural defenses in date palm roots. Mar Drugs 18:596. https://doi.org/10.3390/MD18120596
Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A (2015) Biostimulants and crop responses: a review. Biol Agric Hortic 31:1–17. https://doi.org/10.1080/01448765.2014.964649
Carmo LP, Moura CWN, Lima-Brito A (2020) Red macroalgae extracts affect in vitro growth and bud formation in Comanthera mucugensis (Giul.) LR Parra & Giul., an endemic dry flower species from the Chapada Diamantina (Brazil). S Afr J Bot 135:29–34. https://doi.org/10.1016/J.SAJB.2020.07.033
Carrasco-Gil S, Allende-Montalbán R, Hernández-Apaolaza L, Lucena JJ (2021) Application of seaweed organic components increases tolerance to Fe deficiency in tomato plants. Agronomy 11:507. https://doi.org/10.3390/AGRONOMY11030507
Chattopadhyay N, Ghosh T, Sinha S, Chattopadhyay K, Karmakar P, Ray B (2010) Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chem 118:823–829. https://doi.org/10.1016/j.foodchem.2009.05.069
Circuncisão AR, Catarino MD, Cardoso SM, Silva AM (2018) Minerals from macroalgae origin: health benefits and risks for consumers. Mar Drugs 16:400. https://doi.org/10.3390/md16110400
Claros Cuadrado JL, Pinillos EO, Tito R, Mirones CS, Gamarra Mendoza NN (2019) Insecticidal properties of capsaicinoids and glucosinolates extracted from Capsicum chinense and Tropaeolum tuberosum. Insects 10:132
Collins KG, Fitzgerald GF, Stanton C, Ross RP (2016) Looking beyond the terrestrial: the potential of seaweed derived bioactives to treat non-communicable diseases. Mar Drugs 14:60. https://doi.org/10.3390/md14030060
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393. https://doi.org/10.1007/S10811-010-9560-4
De Saeger J, Van Praet S, Vereecke D, Park J, Jacques S, Han T, Depuydt S (2020) Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J Appl Phycol 32:573–597. https://doi.org/10.1007/S10811-019-01903-9/TABLES/1
Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB (2014) Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol 51:1021–1040. https://doi.org/10.1007/S13197-011-0584-9
Dobrinčić A, Balbino S, Zorić Z, Pedisić S, Bursać Kovačević D, Elez Garofulić I, Dragović-Uzelac V (2020) Advanced technologies for the extraction of marine brown algal polysaccharides. Mar Drugs 18:168. https://doi.org/10.3390/md18030168
Dodgson KS, Price RG (1962) A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J 84:106. https://doi.org/10.1042/bj0840106
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
El-Shenody RA, Ashour M, Ghobara MME (2019) Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz J Food Technol. https://doi.org/10.1590/1981-6723.20318
Ertani A, Francioso O, Tinti A, Schiavon M, Pizzeghello D, Nardi S (2018) Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front Plant Sci 9:428. https://doi.org/10.3389/FPLS.2018.00428/BIBTEX.
Esserti S, Faize M, Rifai LA, Smaili A, Belfaiza M, Faize L, Alburquerque N, Burgos L, Koussa T, Makroum K (2017) Media derived from brown seaweeds Cystoseira myriophylloides and Fucus spiralis for in vitro plant tissue culture. PCTOC 128:437–446. https://doi.org/10.1007/s11240-016-1121-3
García-Fortea E, Lluch-Ruiz A, Pineda-Chaza BJ, García-Pérez A, Bracho-Gil JP, Plazas M, Gramazio P, Vilanova S, Moreno V, Prohens J (2020) A highly efficient organogenesis protocol based on zeatin riboside for in vitro regeneration of eggplant. BMC Plant Biol 20:1–16. https://doi.org/10.1186/s12870-019-2215-y
Ghaderiardakani F, Collas E, Damiano DK, Tagg K, Graham NS, Coates JC (2019) Effects of green seaweed extract on Arabidopsis early development suggest roles for hormone signalling in plant responses to algal fertilisers. Sci Rep 9:1–13. https://doi.org/10.1038/s41598-018-38093-2
Gurusarava P, Vinoth S, Prem Kumar G, Pandiselvi, (2017) Seaweed extract promotes high-frequency in vitro regeneration of Solanum surattense Burm. f: a valuable medicinal plant. Res J Med Plants 11:134–141. https://doi.org/10.3923/rjmp.2017.134.141
Ibrahim MA, Ali AH, Hashem MS (2018) The use of blue-green algae in increasing the efficiency of the tissue culture system in date palm Phoenix dactylifera L. cv. “Barhee.” AAB Bioflux 10:97–103
Jing Y, Li Q, Wu J, Yang X, Yang S, Zhu W, Liu Y, Tang W, Nie S, Hassouna A, White WL, Zhao Y, Lu J (2021) Fucoidan extracted from sporophyll of Undaria pinnatifida grown in Weihai, China—chemical composition and comparison of antioxidant activity of different molecular weight fractions. Front Nutr 8:134. https://doi.org/10.3389/fnut.2021.636930
Kannan S, Sownthariya S, Anbazhakan S (2014) In vitro mass propagation of Withania somnifera Dunal using seaweed extract. Int Lett Nat Sci. https://doi.org/10.18052/www.scipress.com/ilns.24.8.
Karthik T, Sarkar G, Babu S, Amalraj LD, Jayasri MA (2020) Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva reticulata. Biocatal Agric Biotechnol 28:101712. https://doi.org/10.1016/j.bcab.2020.101712.
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399. https://doi.org/10.1007/S00344-009-9103-X
Khedia J, Dangariya M, Nakum AK, Agarwal P, Panda A, Parida AK, Gangapur DR, Meena R, Agarwal PK (2020) Sargassum seaweed extract enhances Macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity. J Appl Phycol 32:4373–4384. https://doi.org/10.1007/S10811-020-02263-5
Kumar G, Sahoo D (2011) Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold J Appl Phycol 23:251–255. https://doi.org/10.1007/S10811-011-9660-9
Latique S, Chernane H, Mansori M, El Kaoua M (2013) Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris variety Paulista) under hydroponic system. Eur Sci J https://doi.org/10.19044/ESJ.2013.V9N30P%25P.
Li S, Gao A, Dong S, Chen Y, Sun S, Lei Z, Zhang Z (2017) Purification, antitumor and immunomodulatory activity of polysaccharides from soybean residue fermented with Morchella esculenta. Int J Biol Macromol 96:26–34. https://doi.org/10.1016/j.ijbiomac.2016.12.007
Mansori M, Chernane H, Latique S, Benaliat A, Hsissou D, El Kaoua M (2016) Effect of seaweed extract (Ulva rigida) on the water deficit tolerance of Salvia officinalis L. J Appl Phycol 28:1363–1370. https://doi.org/10.1007/S10811-015-0671-9
Mazepa E, Malburg BV, Mógor G, de Oliveira AC, Amatussi JO, Corrêa DO, Lemos JS, Ducatti DR, Duarte MER, Mógor ÁF, Noseda MD (2021) Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res 59:102434. https://doi.org/10.1016/J.ALGAL.2021.102434.
Mbithi-Mwikya S, Ooghe W, Van Camp J, Ngundi D, Huyghebaert A (2000) Amino acid profiles after sprouting, autoclaving, and lactic acid fermentation of finger millet (Eleusine coracan) and kidney beans (Phaseolus vulgaris L.). J Agric Food Chem 48:3081–3085. https://doi.org/10.1021/JF0002140
Mitharwal S, Kumar S, Chauhan K (2021) Nutritional, polyphenolic composition and in vitro digestibility of finger millet (Eleusine coracana L.) with its potential food applications: a review. Food Biosci 44:101382. https://doi.org/10.1016/J.FBIO.2021.101382.
Mukherjee A, Patel JS (2020) Seaweed extract: biostimulator of plant defense and plant productivity. Int J Environ Sci Technol 17:553–558. https://doi.org/10.1007/s13762-019-02442-z
Mutale-Joan C, Redouane B, Najib E, Yassine K, Lyamlouli K, Laila S, Zeroual Y, Hicham EA (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-59840-4
Muthusamy A, Kudwa PP, Prabhu V, Mahato KK, Babu VS, Rao MR, Rao MR, Gopinath PM, Satyamoorthy K (2012) Influence of Helium-Neon laser irradiation on seed germination in vitro and physico-biochemical characters in seedlings of Brinjal (Solanum melongena L.) var. Mattu Gulla. Photochem Photobiol 88:1227–1235. https://doi.org/10.1111/J.1751-1097.2012.01162.X
Muthusamy A, Vidya KS, Pratibha PK, Rao MR, Vidhu SB, Guruprasad KP, Raghavendra U, Gopinath PM, Satyamoorthy K (2014) Establishment of an in vitro plantlet regeneration protocol for unique varieties of brinjal (Solanum melongena L.) var. Mattu Gulla and Perampalli Gulla. Indian J Exp Biol 52:80–88
Nabti E, Jha B, Hartmann A (2017) Impact of seaweeds on agricultural crop production as biofertilizer. Int J Environ Sci Technol 14:1119–1134. https://doi.org/10.1007/s13762-016-1202-1
Nanda S, Kumar G, Hussain S (2022) Utilization of seaweed-based biostimulants in improving plant and soil health: current updates and future prospective. Int J Environ Sci Technol 19:12839–12852. https://doi.org/10.1007/s13762-021-03568-9
Navarro QR, de Oliveira CD, Behling A, Noseda MD, Amano É, Suzuki RM, Ribas LLF (2021) Efficient use of biomass and extract of the microalga Desmodesmus subspicatus (Scenedesmaceae) in asymbiotic seed germination and seedling development of the orchid Cattleya warneri. J Appl Phycol 33:2189–2207. https://doi.org/10.1007/S10811-021-02442-Y
Ptak SH, Christensen KV, Meichßner R, Fretté X (2019) Improving fucoidan yield from Fucus brown algae by microwave extraction. Chem Eng 74:109–114. https://doi.org/10.3303/CET1974019
Rajeshkumar S (2017) Phytochemical constituents of fucoidan (Padina tetrastromatica) and its assisted AgNPs for enhanced antibacterial activity. IET Nanobiotechnol 11:292–299. https://doi.org/10.1049/iet-nbt.2016.0099
Rayorath P, Jithesh MN, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429. https://doi.org/10.1007/S10811-007-9280-6
Rioux LE, Turgeon SL, Beaulieu M (2007) Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym 69:530–537. https://doi.org/10.1016/j.carbpol.2007.01.009
Rupawalla Z, Shaw L, Ross IL, Schmidt S, Hankamer B, Wolf J (2022) Germination screen for microalgae-generated plant growth biostimulants. Algal Res 66:102784. https://doi.org/10.1016/j.algal.2022.102784.
Sami FJ, Soekamto NH, Latip J (2019) Total phenolic, antioxidant activity and toxicity effect of Turbinaria decurrens extracts from South Sulawesi. J Phys Conf Ser 1341(3):032008. https://doi.org/10.1088/1742-6596/1341/3/032008.
Sanniyasi E, Venkatasubramanian G, Anbalagan MM, Raj PP, Gopal RK (2019) In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana JV Lamouroux and Turbinaria decurrens Bory). Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-47917-8
Sarkar G, Jatar N, Goswami P, Cyriac R, Suthindhiran K, Jayasri MA (2018) Combination of different marine algal extracts as biostimulant and biofungicide. J Plant Nutr 41:1163–1171. https://doi.org/10.1080/01904167.2018.1434201
Satish L, Rathinapriya P, Rency AS, Ceasar SA, Pandian S, Rameshkumar R, Ramesh M (2016) Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana). J Appl Phycol 28:2083–2098. https://doi.org/10.1007/S10811-015-0696-0
Shanthi N, Arumugam P, Murugan M, Sudhakar MP, Arunkumar K (2021) Extraction of fucoidan from Turbinaria decurrens and the synthesis of fucoidan-coated AgNPs for anticoagulant application. ACS Omega 6:30998–31008. https://doi.org/10.1021/ACSOMEGA.1C03776/SUPPL_FILE/AO1C03776_SI_001.PDF
Sharawy ZZ, Ashour M, Abbas E, Ashry O, Helal M, Nazmi H, Kelany M, Kamel A, Hassaan M, Rossi W Jr, El-Haroun E, Goda A, (2020) Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquacult Res 51:2216–2228. https://doi.org/10.1111/ARE.14566
Shariatmadari Z, Riahi H, Abdi M, Hashtroudi MS, Ghassempour AR (2015) Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. J Appl Phycol 27:2279–2287. https://doi.org/10.1007/S10811-014-0512-2
Sharma HS, Fleming C, Selby C, Rao JR, Martin T (2014) Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol 26:465–490. https://doi.org/10.1007/S10811-013-0101-9
Sharma N, Chauhan RS, Sood H (2015) Seaweed extract as a novel elicitor and medium for mass propagation and picroside-I production in an endangered medicinal herb Picrorhiza kurroa. PCTOC 122:57–65. https://doi.org/10.1007/S11240-015-0749-8
Sivasankari S, Venkatesalu V, Anantharaj M, Chandrasekaran M (2006) Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour Technol 97:1745–1751. https://doi.org/10.1016/J.BIORTECH.2005.06.016
Soto MJ, Urbanowicz BR, Hahn MG (2019) Plant fucosyltransferases and the emerging biological importance of fucosylated plant structures. Crit Rev Plant Sci 38:327–338. https://doi.org/10.1080/07352689.2019.1673968
Swathy PS, Rupal G, Prabhu V, Mahato KK, Muthusamy A (2017) In vitro culture responses, callus growth and organogenetic potential of brinjal (Solanum melongena L.) to He–Ne laser irradiation. J Photochem Photobiol B 174:333–341. https://doi.org/10.1016/j.jphotobiol.2017.08.017
Synytsya A, Kim WJ, Kim SM, Pohl R, Synytsya A, Kvasnička F, Čopíková J, Park YI (2010) Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr Polym 81:41–48. https://doi.org/10.1016/j.carbpol.2010.01.052
Vasantharaja R, Abraham LS, Inbakandan D, Thirugnanasambandam R, Senthilvelan T, Jabeen SA, Prakash P (2019) Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal Agric Biotechnol 17:589–594. https://doi.org/10.1016/J.BCAB.2019.01.021
Vinoth S, Gurusaravanan P, Jayabalan N (2012) Effect of seaweed extracts and plant growth regulators on high-frequency in vitro mass propagation of Lycopersicon esculentum L (tomato) through double cotyledonary nodal explant. J Appl Phycol 24:1329–1337. https://doi.org/10.1007/S10811-011-9717-9
Vinoth S, Gurusaravanan P, Sivakumar S, Jayabalan N (2019) Influence of seaweed extracts and plant growth regulators on in vitro regeneration of Lycopersicon esculentum from leaf explant. J Appl Phycol 31:2039–2052. https://doi.org/10.1007/s10811-018-1703-z
Wang Y, Xing M, Cao Q, Ji A, Liang H, Song S (2019) Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar Drugs 17:183. https://doi.org/10.3390/MD17030183
Yang SH, Seo J, Koo Y (2021) Alginate and fucoidan changes the bacterial community in different directions and the alginate or fucoidan degrading bacteria isolated from paddy soil promotes the plant growth. Arch Microbiol 203(8):5183–5192. https://doi.org/10.1007/S00203-021-02480-7
Yu J, Li Q, Wu J, Yang X, Yang S, Zhu W, Liu Y, Tang W, Nie S, Hassouna A, White WL, Zhao Y, Lu, J (2021) Fucoidan extracted from sporophyll of Undaria pinnatifida grown in Weihai, China—chemical composition and comparison of antioxidant activity of different molecular weight fractions. Front Nutr 8: 636930.https://doi.org/10.3389/FNUT.2021.636930/BIBTEX.
Yuan Y, Macquarrie D (2015) Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr Polym 129:101–107. https://doi.org/10.1016/J.CARBPOL.2015.04.057
Yusuf R, Made U, Syakur A, Kalaba Y, Pasigai MA (2021) The effect of seaweed extract on the growth of shoot of shallot (Allium wakegi Araki) Lembah Palu variety on in vitro. IOP Conf Ser Earth Environ Sci 750:012024. https://doi.org/10.1088/1755-1315/750/1/012024.
Zayed A, Muffler K, Hahn T, Rupp S, Finkelmeier D, Burger-Kentischer A, Ulber R (2016) Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar Drugs 14:79. https://doi.org/10.3390/md14040079
Zhang L, Paasch BC, Chen J, Day B, He SY (2019) An important role of l-fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. N Phytol 222:981–994. https://doi.org/10.1111/NPH.15639
Zhao C, Yang C, Liu B, Lin L, Sarker SD, Nahar L, Yu H, Cao H, Xiao J (2018) Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci Technol 72:1–12. https://doi.org/10.1016/J.TIFS.2017.12.001
Zou P, Yang X, Yuan Y, Jing C, Cao J, Wang Y, Zhang L, Zhang C, Li Y (2021) Purification and characterization of a fucoidan from the brown algae Macrocystis pyrifera and the activity of enhancing salt-stress tolerance of wheat seedlings. Int J Biol Macromol 180:547–558. https://doi.org/10.1016/j.ijbiomac.2021.03.039
Zubia M, Stiger-Pouvreau V, Mattio L, Payri CE, Stewart HL (2020) A comprehensive review of the brown macroalgal genus Turbinaria JV Lamouroux (Fucales, Sargassaceae). J Appl Phycol 32:2743–2760. https://doi.org/10.1007/s10811-020-02188-z
Acknowledgements
We thank Manipal Academy of Higher Education (MAHE), Manipal, Karnataka, India, TIFAC-CORE and FIST, DST New Delhi, DBT BUILDER grant (BT/INF/22/SP43065/2021), DBT, New Delhi, and K-FIST, VGST, Govt. of Karnataka for the facilities. We are grateful to Manipal Academy of Higher Education (MAHE) for the Dr. T.M.A. Pai Ph.D. Scholarship to Arya K. and Sachin AT. Arya K. is thankful to Indian Council of Medical Research (ICMR), Govt. of India for financial assistance as Project Associate-I through research grant (No. 59/08/2022-TRM/BMS). We are indebted to Professor B.S. Satish Rao, Director, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, India, for his encouragement.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal, Karnataka, India.
Author information
Authors and Affiliations
Contributions
AM and KA: Conceptualization, Visualization, Investigation. AM, ST, KA, and NS: Methodology, Supervision. AK, SAT, AM, KA, NS, and MPS: Data curation, Writing—Original draft preparation, Software. AK, SAT, KA, and AM: Software, Validation. SAT, AK, AM, KA, MPS: Writing—Reviewing and Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have none to declare.
Additional information
Handling Editor: Stefaan Werbrouck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaniyassery, A., Thorat, S.A., Shanthi, N. et al. In Vitro Plant Growth Promoting Effect of Fucoidan Fractions of Turbinaria decurrens for Seed Germination, Organogenesis, and Adventitious Root Formation in Finger Millet and Eggplant. J Plant Growth Regul 43, 283–298 (2024). https://doi.org/10.1007/s00344-023-11084-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11084-y