Abstract
Waterlogging significantly impacts plant growth and development by altering nutrient uptake and antioxidant enzyme functions, resulting in reduced yield. Plants need sulfur (S) to produce reduced glutathione (GSH), a thiol compound that combats abiotic stresses. It is hypothesized that supplying S to high S-demanding oilseed rape plants beyond its growth requirements can reduce the adverse effect of waterlogging stress. Therefore, this study evaluated the comparative effect of different S doses (mg kg− 1 soil), i.e., no-S (S0), low-S (S1, 35), medium-S (S2, 70), and high-S (S3, 140) on growth, yield and antioxidant defense systems of normal growing and waterlogged oilseed rape plants. Waterlogging was imposed at the inflorescence emergence stage for 7 days by retaining a 3-cm layer of water above the soil surface. Waterlogged plants supplemented with high-S showed improved growth and higher yield than those supplemented with lower S levels, and this response was associated with improved activity/contents of antioxidants, including ascorbate (AsA), GSH, ascorbate peroxidase, catalase, glutathione reductase, glutathione peroxidase, glutathione S-transferase, monodehydroascorbate reductase, and dehydroascorbate reductase, with concomitant lowering of hydrogen peroxide, dehydroascorbate and malondialdehyde content. Furthermore, leaf S concentration was enhanced in waterlogged plants treated with high-S, while anions were regulated whereby Cl− uptake was decreased. However, under control conditions, high-S did not provide additional benefits of improved plant growth, yield, and antioxidant activities compared to the medium-S application. Thus, it is concluded that additional S supplementation mitigates the adverse effects of waterlogging stress on oilseed rape, and needs to be employed as a potential strategy to alleviate the negative effects of this abiotic stress.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities in the past and present are responsible for the ever-increasing global warming, resulting in a substantial increase in dry periods worldwide. However, not only drought but the frequency and intensity of extreme climate events, such as heavy rainfalls, have also increased (Alifu et al. 2022; Tian et al. 2021). Together with excessive irrigation and river overflow, high precipitation can increase the probability of long periods of waterlogged soil conditions (Tabari et al. 2020). The recent trend analyses of excessive rain across Europe revealed an increasing tendency in the magnitudes of extreme rainfall, mainly concentrated in winter (4–10%) (Madsen et al. 2014). Although trends vary regionally and seasonally, heavy rains in Germany have become more erosive and significantly higher in the past 20 years (Deumlich et al. 2020; Maraun 2013).
The primary effect of waterlogging is the impediment of gas diffusion through saturated soil pores (10,000 slower diffusion of gases in water than in air), which causes hypoxia and then dysfunction in the roots (Jackson and Ricard 2003). Waterlogging can potentially affect physiological processes, such as suppressing the activities of antioxidant enzymes and reducing the photosynthetic capability of crop plants (Simova-Stoilova et al. 2012). Moreover, it can inhibit plant growth and development by inducing nutrient deficiencies/toxicities and promoting the production of reactive oxygen species (ROS), mainly hydrogen peroxide (H2O2) (Teoh et al. 2022; Zhang et al. 2015). High levels of ROS cause membrane lipid peroxidation, protein oxidation, and enzyme inhibition, resulting in cell death (Tanou et al. 2009). Thus, the antioxidant defense mechanism is of prime importance for plants’ survival under waterlogging through scavenging ROS and protecting cells from oxidative stress (Doupis et al. 2017).
An adaptation to waterlogging stress involves morphological changes in the root system, the development of aerenchyma, and adventitious roots that improve the exchange of gases inside the roots (Sauter 2013). Due to the lack of aerenchyma, oilseed rape (Brassica napus L.) is highly prone to waterlogging (Voesenek et al. 1999). Thus, globally, including in Europe and North America, one of the most extreme abiotic stresses affecting rapeseed production is waterlogging (Pachauri et al. 2014). This is even more alarming, as oilseed rape, after soybeans, is the world’s second-largest oilseed crop (FAO 2021). In addition to edible oil, rapeseed has many other uses, e.g., cattle feed, a source of biodiesel, or bioethanol (Kdidi et al. 2019). Over recent decades, rapeseed production expanded across most major production regions, including Canada, Europe, China, India, and Australia (FAO 2021). Still, rapeseed is commonly grown in Asia as a rotation crop on rice fields (Zou et al. 2015). Consequently, rapeseed naturally experiences waterlogging stress during growth and development. In the past decades, rapeseed growth was severly hampered due to waterlogging, resulting in more than 50% yield reductions (Wollmer et al. 2018; Herzog et al. 2016). Moreover, waterlogging affected the oil quality by increasing erucic acid and glucosinolate content as well as altering lipid biosynthesis metabolic pathways (Xu et al. 2015).
It is well known that sulfur (S) alone or its derivatives can positively regulate antioxidant defense under abiotic stress conditions and thus mitigates oxidative stress (Bashir et al. 2015). In this context, for example, GSH acts as a cell redox regulator which scavenges reactive oxygen species. Keeping a favorable redox state for the detoxification of H2O2 depends on the balance between glutathione (GSH) and oxidized glutathione (GSSG) (Arbona et al. 2008). Although many researches have focused on the effects of waterlogging on growth, yield, ion homeostasis, antioxidant system, and some other physiological as well as molecular traits of the rapeseed (Ding et al. 2022; Ambro et al. 2022; Lee et al. 2014), the attenuation of the adverse effects of abiotic stresses by S supplementation has been inadequately studied. Increased elemental S in soil decreased salt toxicity in B. juncea (Fatma et al. 2014; Tong et al. 2014), the adverse effects of freezing (4 °C) (Lou et al. 2017), heavy metal (Cd) exposure (Min et al. 2016) and heat stress in plants (Shi et al. 2015). To the best of our knowledge, there is rare evidence of whether soil supplementation with S can enhance waterlogging stress tolerance in rapeseed. Therefore, this study aimed to investigate whether waterlogged plants supplemented with excess S can improve S-assisted antioxidant defense and offset waterlogging induced adverse effects of oxidative stress on the growth and yield of rapeseed. We hypothesized that supplementing plants with high-S augments GSH levels which alleviates waterlogging stress by upregulating the antioxidant system.
Materials and Methods
Plant Material and Plant Cultivation
Four overnight-soaked seeds of oilseed rape (Brassica napus L. cv. campino; Norddeutsche Pflanzenzucht Lembke, Hohenlieth, Germany) were sown in Mitscherlich pots, filled with 6 kg of a loamy sand soil (85% sand, 10.9% silt, and 4.1% clay, 6.5 mg S kg− 1 soil, pHCaCl2 6.5) (Table 1). Based on the standard field fertilizer application, the following four S-levels (as MgSO4 ∙ 7H2O) were applied to the soil 5 days prior to sowing: 0 mg S kg− 1 soil, no-S (S0); 35 mg S kg− 1 soil, low-S (S1); 70 mg S kg− 1 soil, medium-S (S2); and 140 mg S kg− 1 soil, high-S (S3). Basal fertilization was carried out as follows (in g kg− 1 soil): 0.47 NH4NO3, 0.74 KH2PO4, 0.46 MgCl2 ∙ 6H2O, 0.3 CaCl2 ∙ 2H2O, 0.015 Fe-EDTA, 0.006 CuSO4 ∙ 5H2O, 0.011 ZnSO4 ∙ 7H2O, 0.015 MnSO4 ∙ H2O, 0.009 H3BO3, 0.0006 (NH4)6Mo7O24 ∙ 4H2O. After germination,
only two plants were retained in each pot. The soil moisture level in all the pots was equally maintained at 60% water holding capacity (WHC) for six weeks after sowing. Thereafter, waterlogging treatment was imposed at the inflorescence emergence stage by keeping approximately a 3 cm layer of water above the soil surface in the pots for seven consecutive days. The pots were placed in 15 L plastic buckets which were pre-filled with water to achive a water depth of 3 cm above the soil in pots. Throughout the experiment, a control was maintained at 60% WHC for each S level. Plants were grown under controlled conditions in the greenhouse with the following conditions: Day/night temperature 22/18°C, photoperiod 14/10 h (day/night), relative humidity 62–70%, light intensity 350 µM photon m− 2 s− 1 (recorded by a light meter, Li-198, Lincoln, NE, USA). The experiment consisted of two sets of treatments, each having four replications, which were arranged according to completely randomized design. One set was used for growth and yield data recording as well as analysis of nutrients, while the other set was used for biochemical analyses.
Morphological Assessment and Plant Harvest
Plants from the first set of the experiments (four replications) were harvested 7 days after the onset of waterlogging treatment. Before the harvest, the number of senesced leaves was recorded in each treatment based on visual observation. The fresh shoot samples were frozen in liquid N2, and stored at − 80 °C until used for biochemical analysis. The waterlogging treatment of the second set of plants was finished after seven days, and further were grown under normal soil water (60% WHC) for the rest of the growth period; plants were harvested at agronomic maturity. Plant height was recorded, and the plants were cut at the base. The number of siliqua, thousand seed weight, and seed yields were recorded. The plant samples were dried at 65 °C for 72 h, and their dry matter was recorded. The dried samples were ground to a fine powder with a ball mill (MM 200, Retsch GmbH, Haan, Germany).
Determination of Inorganic Anions
The extraction of inorganic anions was done according to Cataldi et al. (2000) with a few modifications. Briefly, approximately 20 mg of dried and powdered leaf samples were dissolved and boiled in 1.5 mL deionized water for 5 min. The samples were then thoroughly mixed and subsequently placed in an ice-water bath for 30 min. The supernatants were collected after centrifugation at 17,500 x g at 4 °C for 10 min. For the precipitation of protein from the supernatant, 200 µL chloroform was added and centrifuged at 17,500 x g at 4 °C for 5 min. To purify the supernatant, it was passed through a strata C-18 column (Phenomenex, Torrance). Afterward, the anions were determined by isocratic ion chromatography (IC-5000 Capillary Reagent- Free IC System, Thermo Scientific).
Determination of Total Sulfur
The total sulfur of the leaf was determined following the method of Hussain et al. (2022). Briefly, 5–10 mg of finely ground samples were weighed into tin capsules along with 10 mg wolfram-(IV)-oxide. Subsequently, total S concentration was determined by the Dumas combustion method with CNS elemental analyzer (Flash EA 1112 NCS, Thermo Fisher Scientific, Waltham, MA, USA).
Quantification of Malondialdehyde Content
Malondialdehyde (MDA) content was measured following the method of Heath and Packer (1968). In brief, frozen leaf tissues were homogenized in 5% trichloroacetic acid (TCA) using mortar and pestle. After centrifuging at 11,500 × g and 4 °C for 15 min, 1 mL of the aliquot and 4 mL of 20% TCA containing 0.5% TBA were mixed. After heating at 95 °C for 30 min, the mixture was cooled promptly on an ice bath and centrifuged at 10,000 × g for 10 min. To correct for nonspecific turbidity, the absorbance of the supernatant was estimated at 532 nm and subtracted from the absorbance of the same at 600 nm. The contents of MDA were calculated based on the extinction coefficient (155 mM− 1 cm− 1), and the concentration was expressed as nmol g− 1 fresh weight.
Determination of H2O2 Production Level
Leaves H2O2 concentration was determined following the method described by Velikova et al. (2000). Leaf samples (0.2 g) were homogenized in 1.6 mL of 0.1% trichloroacetic acid (TCA) in an ice bath for 30 min and centrifuged at 12,000 × g at 4 °C for 20 min. Next, 0.5 mL of 0.1 M potassium phosphate buffer (K-P buffer; pH 7.8) and 1 mL of 1 M potassium iodide (KI) were added to 0.5 mL of supernatant and kept in the dark for 1 h. The absorbance of the mixture was measured at 390 nm. Quantification of H2O2 was done using a standard curve, and the concentration was presented as µmol g− 1 fresh weight.
Extraction and Measurement of Ascorbate and Glutathione
For the analysis of ascorbate and glutathione, frozen leaves (0.5 g) were homogenized in 5% meta-phosphoric acid containing 1 mM ethylene diamine tetra acetic acid (EDTA) and centrifuged at 11,500 × g and 4 °C for 15 min, and the supernatant was collected. Ascorbate content was determined following Anee et al. (2019). In brief, 0.3 mL of 0.5 M K-P buffer (pH 7.0) and 0.1 mL of 0.1 M dithiothreitol were added to reduce the oxidized fraction. Finally, the reaction mixture was formulated using K-P buffer (pH 6.5) and ascorbate oxidase with a neutralized solution. In the presence of 0.5 units of ascorbate oxidase (AO), a change in absorbance was measured at 265 nm in 100 mM K-P buffer (pH 7.0). The standard curve used to determine total ascorbate and AsA was subtracted from total ascorbate to calculate dehydroascorbate (DHA). The ascorbate (AsA) content was expressed as nmol g− 1 fresh weight.
Reduced glutathione (GSH) and oxidized glutathione (GSSG) were assayed following Yu et al. (2003). Shortly, the extract solution was neutralized by adding 0.5 M K-P buffer (pH 7) and dH2O for total glutathione. In the meantime, the GSH was oxidized by DTNB [5,5-dithiol-bis (2-nitrobenzoic acid)] and reduced by nicotinamide adenine dinucleotide phosphate (NADPH) with GR. Changes in absorbance were photometrically measured at 412 nm. Standard curves were made with the known concentrations ranging from 5 to 40 µM of reduced glutathione (GSH) and oxidized glutathione (GSSG). The content of GSH was calculated by subtracting GSSG from the total GSH and expressed as µmol g− 1 fresh weight.
Antioxidant Enzymes Assay
The steps involved in preparing crude plant extract were carried out at 4 °C. Frozen leaf tissue (0.3 g) was homogenized with a 2 mL extraction mixture containing 0.5 M K-P buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10% (w/v) glycerol. The mixture was then centrifuged at 12,000 × g for 20 min. The crude extract was stored at -20 °C until further analysis. Spectrophotometric measurements were performed to determine antioxidant enzymes with a UV visible spectrophotometer (Thermo Helios Gamma, 9423 UVG 1000E, United Kingdom).
Protein Quantification
The protein concentration of the samples was measured as described by Bradford (1976). In this case, bovine serum albumin was used as a standard, and absorbance was measured at 595 nm.
Catalase (CAT; EC: 1.11.1.6) activity was measured according to Aebi (1984) with slight modifications. Initiation of the reaction was accomplished by adding 15 mM H2O2 with mixture of 50 mM K-P buffer (pH 7.0) and plant extract. The drop-in absorbance was monitored for up to 1 min at 240 nm. The calculation was performed using an extinction coefficient of 39.4 M − 1 cm − 1. One unit of catalase was expressed as 1 µM of substrate reacted per minute per mg protein.
Ascorbate peroxidase (APX; EC: 1.11.1.11) was observed by the decrease in absorbance at 290 nm following of H2O2 dependent oxidation of ascorbate (Nakano and Asada 1981). The assay mixture contained 50 mM K-P buffer (pH 7.0), 0.1 mM EDTA and 0.1 mM H2O2. The activity of APX was calculated using the extinction coefficient of 2.8 mM− 1 cm − 1, and one unit of APX was defined as µmol H2O2 min− 1 mg− 1 protein.
Glutathione reductase (GR; EC: 1.6.4.2) activity was measured following the method described by Mohsin et al. (2019). The oxidation of glutathione-dependent NADPH was monitored at 340 nm. There were five components in the assay mixture: phosphate buffer (0.5 M, pH 7), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH, and crude plant extract. For calculating GR activity, 6.2 mM − 1 cm − 1 was used as the extinction coefficient. The activity of GR was defined as 1 nM of substrate reacted per minute per mg of protein (nmol min− 1 mg− 1 protein).
Monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) activity was determined using the method described by Hossain et al. (1985). The decrease in absorbance due to NADH oxidation was monitored at 340 nm. The reaction assay contained 50 mM Tris-HCl buffer (pH 7.5), 0.2 mM NADPH, 2.5 mM AsA, 0.5 units of ascorbate oxidase (AO) and crude plant extract. The activity of MDHAR was calculated using the extinction co-efficient of 6.2 mM− 1 cm− 1 and expressed as nmol min− 1 mg− 1 protein.
The activity of dehydroascorbate reductase (DHAR, EC 1.8.5.1) was measured following the method of Anee et al. (2019). The enhanced absorbance due to GSH-dependent AsA generation was measured spectrophotometrically. To prepare reaction mixtures, 50 mM K-P buffer (pH 7.0), 2.5 mM GSH, 0.1 mM EDTA, 0.1 mM dehydroascorbate (DHA), and enzyme extract were used. The change in absorbance was measured at 265 nm for 1 min, and DHAR activity was expressed as nmol min− 1 mg− 1 protein.
The activity of glutathione peroxidase (GPX, EC: 1.11.1.9) was measured following Elia et al. (2003). The oxidation of NADPH to NADP + was marked by a reduction in absorbance at 340 nm that presented a spectrophotometric means for monitoring GPX activity. The assay mixture contained 100 mM of K-P buffer (pH 7.0), 1 mM EDTA, 1 mM sodium azide (NaN3), 0.12 mM NADPH, 2 mM GSH, 1 unit GR, 0.6 mM H2O2 (as a substrate), and plant crude extract. The absorbance of the mixture was monitored for 1 min, and the extinction coefficient used for the calculation was 6.62 mM− 1 cm− 1. The activity of GPX was defined as 1 nM of substrate reacted per minute per mg of protein.
Glutathione S-transferase (GST, EC: 2.5.1.18) activity was determined using the method described by Hasanuzzaman et al. (2019). The conjugation of the thiol group of glutathione (GSH) to the CDNB substrate was detected with the increased absorbance at 340 nm. The reaction mixture contained 2 mM GSH, 0.25 mM K-P buffer (pH 6.5), 30 mM CDNB, and crude plant extract. The extinction coefficient 9.6 mM–1 cm–1 was utilized to estimate GST activity, where the unit of GST activity was expressed as nmol min− 1 mg− 1 protein.
Statistical Analysis
The data were statistically analyzed following two-way ANOVA using R (version 4.0.4) software. The differences among the treatments were compared with Tukey’s HSD test at 5% probability level.
Results
Sulfur Enhances Growth Performance Under Waterlogging Stress
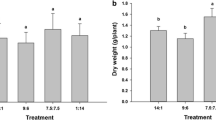
Waterlogging stress significantly inhibited the growth performance and biomass production of Brassica napus plants (Fig. 1). The waterlogged plants applied with no-S (S0 + WL), low-S (S1 + WL), and medium-S (S2 + WL) showed reduced height and dry matter production as compared to respective controls (Fig. 2A and B). On the other hand, waterlogging-induced reduction in dry matter was not observed in high-S supplemented plants (S3 + WL) compared to its corresponding control (Fig. 2B). Conversely, S application significantly mitigated the waterlogging-induced decline in dry matter production: S1, S2, and S3 treated waterlogged plants exhibited dry matter enhancement by 1.5, 4.8 and 5.6-fold, respectively, compared to S0 treatment (Fig. 2B). With the same trend, waterlogged plants supplemented with low-S (S1 + WL), medium-S (S2 + WL), and high-S (S3 + WL) had 6%, 60% and 71% lesser senesced leaves compared with no-S (S0 + WL) plants (Fig. 2C).
Effect of different levels of soil-applied S (S0, No-S application; S1, 35 mg S kg− 1 soil; S2, 70 mg S kg− 1 soil; S3, 140 mg S kg− 1 soil) on plant height (A), dry matter (B), number of senescent leaves (C), number of siliqua (D), thousand seed weight (E) and seed yield (F) of rapeseed (Brassica napus L.) grown under control (normal irrigated) and waterlogged condition. The data are means ± SE of four independent pot replicates. Different letters indicate significant differences between the treatment groups (Tukey’s test; p ≤ 0.05)
Although compared with controls the number of siliqua in no-S (S0 + WL) and low-S (S1 + WL) waterlogged plants did not differ statistically, medium-S (S2 + WL) and high-S (S3 + WL) supplemented waterlogged plants exhibited a significant decline (Fig. 2D). However, the thousand-seed weight and seed yield of S0, S1, S2 and S3 treated waterlogged plants markedly decreased compared to respective controls (Fig. 2E F). Although high-S (S3 + WL) treated waterlogged plants showed a slight increase in the number of siliqua, but displayed a significant increase of 20% and 11% in thousand seed weight and seed yield respectively, compared to medium-S (S2 + WL) supplied waterlogged plants (Fig. 2E). Surprisingly, number of siliqua, 1000-seed weight and seed yield of high-S (S3) and medium-S (S2) supplied control plants were similar (Fig. 2D F).
Sulfur Balances Anions Contents Under Waterlogging Stress
Total S concentrations in leaves of waterlogged plants supplied with high-S (S3 + WL) increased by 3.7-, 5.2- and 1.3-fold compared to no-S (S0 + WL), low-S (S1 + WL), and medium-S (S2 + WL) supplemented plants, respectively (Table 2). The application of high-S (S3) proved ineffective in increasing S concentration compared to medium-S (S2) under control conditions. Similar to total S contents, waterlogging decreased water-soluble sulfate (SO42−) concentration in Brassica napus leaves by 70%, 60%, and 21% in no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) treatment, compared to their corresponding controls, respectively (Table 2). Conversely, waterlogging did not affect SO42− concentration in the leaves of high-S (S3 + WL) supplied plants compared with its control (S3) (Table 2). Moreover, in contrast to medium S supplied waterlogged plants (S2 + WL), high S supplied waterlogged plants (S3 + WL) had 15% higher SO42− concentration in leaves (Table 2).
High-S (S3 + WL) supplemented plants increased nitrate (NO3−) concentration by 17-, 10-, and 1.1-fold compared with no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) treatments under waterlogging stress, respectively (Table 2). In contrast, NO3− concentration of control plants treated with medium-S (S2) and high-S (S3) was statistically similar (Table 2). Application of high-S (S3 + WL) improved phosphate (PO43−) concentration by 16% compared to the medium-S (S2 + WL) supplied waterlogged plants (Table 2). Additionally, waterlogging declined PO43− concentration by 22% in high-S (S3 + WL) and 35% in medium-S (S2 + WL) supplied plants compared to the respective controls (Table 2).
Leaf chloride (Cl−) concentration of high-S (S3 + WL) treated waterlogged plants was significantly declined by 63% and 53% compared with no-S (S0 + WL) and low-S (S1 + WL) treated waterlogged plants respectively, but slightly (by 13%) reduced compared with medium-S (S2 + WL) treated waterlogged plants (Table 2). Furthermore, compared with control counterparts Cl− concentrations in low-S (S1 + WL) and no-S (S0 + WL) plants were notably elevated by 43% and 44% respectively, whereas medium-S (S2 + WL) and high-S (S3 + WL) treated plants did not differ statistically under waterlogging condition (Table 2).
Sulfur Mitigates Waterlogging stress-mediated hike in H2O2 and MDA Content
The waterlogging stress resulted in significantly higher H2O2 contents than the respective controls. Compared to no-S (S0 + WL) treated plants under waterlogging condition, low-S (S1 + WL), medium-S (S2 + WL), and high-S (S3 + WL) supplementation notably decreased H2O2 content by 10%, 49% and 61%, respectively (Fig. 3A). Although, H2O2 content did not differ between high-S (S3) and medium-S (S2) supplemented plants under control condition, whereas high-S (S3 + WL) supplemented plants H2O2 contents declined significantly by 23% than medium-S (S2 + WL) supplemented plants under waterlogged conditions (Fig. 3A). Under waterlogged conditions, MDA content of all treatments exhibited a similar trend as that of H2O2 content (Fig. 3B). Across all S treatments, the waterlogged plants had significantly higher MDA contents than the respective control plants (Fig. 3B). Under waterlogging, low-S (S1 + WL), medium S (S2 + WL), and high-S (S3 + WL) supplementation decreased MDA content markedly by 19%, 65% and 73% respectively, compared to no-S (S0 + WL) treated plants (Fig. 3B).
Effect of different levels of soil-applied S (S0, No-S application; S1, 35 mg S kg− 1 soil; S2, 70 mg S kg− 1 soil; S3, 140 mg S kg− 1 soil) on H2O2 content (A) and malondialdehyde content (B) in leaves of rapeseed (Brassica napus L.) grown under control and waterlogged soils. Data are means ± SE of four independent pot replicates. Bars followed by different letters indicate significant differences between the treatment groups (Tukey’s test; p ≤ 0.05)
Sulfur Supplementation Enhances the Activities of Enzymatic and non-enzymatic Antioxidants Under Waterlogging
In comparison with no-S treated waterlogged plants (S0 + WL), low-S (S1 + WL), medium-S (S2 + WL), and high-S (S3 + WL) treated plants had 1.3-, 2.5- and 3-fold higher AsA content, respectively (Fig. 4A). Moreover, AsA contents in no-S (S0 + WL) treated waterlogged plants was similar to control, while that of low-S (S1 + WL), medium-S (S2 + WL) and high-S (S3 + WL) supplemented waterlogged plants were 0.3, 1.5- and 1.7-fold higher than the respective controls (Fig. 4A). Although DHA content notably decreased in no-S (S0 + WL) waterlogged plants, it remained unchanged in low-S (S1 + WL), medium-S (S2 + WL) and high-S (S3 + WL) supplemented waterlogged plants compared to their control group (Fig. 4B). In addition, the DHA content of high- S (S3 + WL) supplemented plants dropped by 66% and 60% compared with no-S (S0 + WL) and low-S (S1 + WL) treated plants, while exhibiting statistically similar values with medium-S (S2 + WL) treated plants under waterlogging condition, respectively (Fig. 4B). Accordingly, in high-S (S3 + WL) treated waterlogged plants, AsA/DHA ratio was 67% higher than respective control (S3) and 27% higher than medium-S (S2 + WL) treated waterlogged plants (Fig. 4C).
Effect of different levels of soil-applied S (S0, No-S application; S1, 35 mg S kg− 1 soil; S2, 70 mg S kg− 1 soil; S3, 140 mg S kg− 1 soil) on ascorbate (AsA) content (A), dehydroascorbate (DHA) content (B), AsA/DHA ratio (C), glutathione (GSH) content (D), oxidized glutathione (GSSG) content (E), and GSH/GSSG ratio (F) in leaves of rapeseed (Brassica napus L.) grown under control and waterlogged soils. Data are means ± SE of four independent pot replicates. Bars followed by different letters indicate significant differences between the treatment groups (Tukey’s test; p ≤ 0.05)
The glutathione (GSH) content of both control and waterlogged plants linearly increased with S supplementation, with the exception of a non-significant difference between high-S (S3) and medium-S (S2) treated control plants (Fig. 4D). On the other hand, a non-significant increase (only by 5%) of glutathione content (GSH) was observed in high-S (S3 + WL) supplemented waterlogged plants compared to control (Fig. 4D). Although the oxidized glutathione (GSSG) contents of no-S (S0 + WL) waterlogged plants increased by 23% over control, there was no difference in GSSG contents between control and waterlogged plants of low-S (S1), medium S (S2) and high-S (S3) supplemented plants (Fig. 4E). The GSH/GSSG ratio of high-S supplemented (S3 + WL) waterlogged plants was 18% higher than the respective control (S3), and 35% higher than medium-S treated (S2 + WL) waterlogged plants (Fig. 4F).
The activity of APX in high-S (S3 + WL) plants was considerably higher by 124% and 161% than low-S (S1 + WL) and no-S (S0 + WL) treated plants, while compared with medium-S (S2 + WL) treated plants the APX activity increased non-significantly by 30% under waterlogging condition (Fig. 5A). Conversely, under waterlogging conditions the activity of APX in medium-S (S2 + WL) supplemented plants declined notably by 26%, while it declined slightly ( by 8%) in high-S (S3 + WL) supplemented plants compared to the control group (Fig. 5A). Catalase (CAT) activity in medium-S (S2 + WL) and high-S (S3 + WL) supplemented waterlogged plants was 15% and 30% higher compared to respective controls (Fig. 5B). Furthermore, CAT activity of high-S (S3 + WL) waterlogged plants increased significantly by 157%, 115%, and 12% compared with no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) supplemented waterlogged plants, respectively (Fig. 5B).
Effect of different levels of soil-applied S (S0, No-S application; S1, 35 mg S kg− 1 soil; S2, 70 mg S kg− 1 soil; S3, 140 mg S kg− 1 soil) on the activity of APX (A), CAT (B), GR (C), MDHAR (D) and DHAR (E) in leaves of rapeseed (Brassica napus L.) grown under control and waterlogged soils. Data are means ± SE of four independent pot replicates. Bars followed by different letters indicate significant differences between the treatment groups (Tukey’s test; p ≤ 0.05)
Glutathione reductase (GR) activity of waterlogged plants supplemented with high-S (S3 + WL) increased by 17%, while of no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) waterlogged plants did not significantly increased in comparison with the control counterparts (Fig. 5C). However, glutathione reductase (GR) activity of high-S (S3 + WL) treated waterlogged plants increased was 17% higher than that of medium-S (S2 + WL) waterlogged plants (Fig. 5C). By contrast, GR activity of high-S (S3) supplemented control plants did not differ statistically compared with medium-S (S2) supplemented control plants (Fig. 5C). Furthermore, MDHAR activity of no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) treated waterlogged plants decreased non-significantly by 25%, 6% and 12% respectively, whereas in high-S (S3 + WL) treated plants it increased slightly by 8% compared with respective controls (Fig. 5D). Compared with medium-S (S2 + WL) waterlogged plants, high-S (S3 + WL) treated waterlogged plants had 20% higher MDHAR activity (Fig. 5D). On the other hand, the MDHAR activity of high-S (S3) supplemented control plants had no significant difference from that of medium (S2) supplemented control plants (Fig. 5D).
The DHAR activity of no-S (S0 + WL) and low-S (S1 + WL) waterlogged plants slightly reduced by 21% and 14% compared with respective controls (Fig. 5E). Conversely, the DHAR activity of high S (S3 + WL) treated waterlogged plants increased considerably by 43%, whereas medium S (S2 + WL) supplemented waterlogged plants increased non-significantly (by 6%) compared to respective controls (Fig. 5E). Moreover, high-S waterlogged plants (S3 + WL) exhibited 34% increase in DHAR activity compared to medium S (S2 + WL) supplemented waterlogged plants (Fig. 5E). However, the DHAR activity of control plants that received medium- S (S2) and high- S (S3) showed was statistically similar (Fig. 5E).
Under waterlogging conditions, GPX activity of high-S (S3 + WL) treated waterlogged plants increased by 18%, while that of no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) treated waterlogged plants was statistically similar to respective controls (Fig. 6A). Strikingly, high-S supplemented waterlogged plants (S3 + WL) showed 25% higher GPX activity compared to the medium-S supplemented waterlogged plants (S2 + WL) (Fig. 6A). On the other hand, control plants receiving medium- S (S2) and high S (S3) had statistically similar GPX activities (Fig. 6A).
Effect of different levels of soil-applied S (S0, No-S application; S1, 35 mg S kg− 1 soil; S2, 70 mg S kg− 1 soil; S3, 140 mg S kg− 1 soil) on the activity of GPX (A) and GST (B) in leaves of rapeseed (Brassica napus L.) grown under control and waterlogged soils. Data are means ± SE of four independent pot replicates. Bars followed by different letters indicate significant differences between the treatment groups (Tukey’s test; p ≤ 0.05)
GST activity of no-S (S0 + WL), low-S (S1 + WL) and medium-S (S2 + WL) and high-S (S3 + WL) was statistically similar to respective non-waterlogged controls (Fig. 6B). However, GST activity of high-S treated waterlogged plants (S3 + WL) increased considerably by 34% compared to medium-S (S2 + WL) supplemented waterlogged plants, while medium S (S2) and high-S (S3) treated control plants had statistically similar GST activity (Fig. 6B).
Discussion
In plants, metabolism is a prerequisite for better productivity and higher yields, but it generally gets disturbed under abiotic stress conditions, mainly due to the excess production of reactive oxygen species (ROS). Therefore, redox balance is one of the key features by which oxidized products are reduced, and harmful effects such as lipid peroxidation are mitigated (Anee et al. 2019). As such, waterlogging exerts a detrimental effect due to the formation of hypoxic or even anoxic conditions, subsequently being followed by an impairment of the photosynthetic machinery that can promote the overproduction of ROS. To alleviate such stress responses, S supplementation is reported to be an effective strategy for crop plants (Lou et al. 2017; Boldrin et al. 2016). Thus, in the current study, the influence of different levels of S supplementation (no-S, low-S, medium-S, and high-S) on the growth, recovery, and antioxidant defense mechanisms of rapeseed plants grown under waterlogged conditions was evaluated.
Supplementation of S to Waterlogged Plants Stimulates Growth, Yield, and Nutrient Status
The results showed that rapeseed is highly susceptible to waterlogging, depicting hampered plant performance under only seven days of waterlogging stress (Figs. 1 and 2). Besides yellowing and chlorosis of older leaves, younger leaves also showed discoloration (Fig. 1). Waterlogged leaves showed symptoms of a serious S deficiency and low NO3−, SO42− and PO43− accumulation (Table 2). Such reduction in nutrient uptake resulted in reduced growth (Fig. 2A), seed yield, and yield-contributing traits (Fig. 2B C) of waterlogged plants (Hussain et al. 2022; Wollmer et al. 2018). On the other hand, S supplementation increased S content together with increased NO3−, SO42− and PO43− uptake (Table 2), possibly contributing to retrieval of waterlogging-induced loss in growth, yield, and yield contributing traits (Fig. 2A F). It can be reasoned that under abiotic stresses, the reduction of S in the plant certainly increases the demand for its supplementation for foiling photosynthetic apparatus and cellular metabolism, which finally leads to increased growth and development (Narayan et al. 2022; Fatma et al. 2014). Additional S, as supplemented here under waterlogging, was used as an alternative electron donor, as oxygen is depleted and soil microbes switch from using O2 as an electron acceptor to SO42− (Madigan et al. 2003). In this study, it was found that under control (normal water) conditions, high application of S beyond medium-S level did not benefit S accumulation, growth, yield and antioxidant metabolites (Figs. 2, 4 and 5). This finding is consistent with the early reports that high-S doses did not benefit non-stressed control plants (Rehman et al. 2013; Ahmad et al. 2011). It is assumed that plants do not accumulate S beyond an adequate S application level, and further increases in S application would not benefit the growth and yield. On the other hand, waterlogged plants need excess S application due to reduced S availability in soil and the increased demand for S to synthesize compounds containing reduced S (Fatma et al. 2014).
In the present investigation, it was also observed that chloride (Cl−) concentration under waterlogging stress was much higher in the Brassica napus leaf (Table 2), possibly due to its increased transport rates in plants (Barrett-Lennard 2003). As a result of higher Cl− concentrations and subsequent S deprivation under waterlogging stress, NO3−, SO42− and PO43− uptake was strongly negatively affected (Table 2). Supplemental S, especially high-S level, decreased Cl− concentrations in waterlogged plants (Table 2), which might be attributed to the efficient Cl− exclusion because Cl− efflux has a positive correlation with stress tolerance (Sagervanshi et al. 2020; Li et al. 2016). Antagonistic interaction between Cl− and SO42− could also explain the reduction in Cl− by increased S supplementation (Geilfus 2018).
Waterlogged Plants Reduced H2O2 and MDA with Supplemented S
Waterlogging treatment increased H2O2 content, which resulted in an elevation of MDA content in rapeseed leaves, indicating a high degree of lipid peroxidation (Fig. 3A and B). It is logical to infer that the antioxidant defense system could not mitigate the ROS surplus generated as a result of waterlogging stress (Wang et al. 2021). The antioxidant systems in plants possibly suffer from a lack of adequate S supply required for synthesizing antioxidant enzymes and other molecules, such as GSH, to suppress ROS production (Bashir et al. 2015). In contrast, S supplementation to waterlogged plants significantly decreased H2O2 and malondialdehyde (MDA) contents, possibly by enhanced antioxidant activities (Fig. 3A and B). In the S-assimilatory pathway, GSH synthesis is controlled and linked to ascorbate-glutathione (AsA-GSH) enzymes. As a result, the S assimilation-regulated antioxidant system helps eliminate H2O2 and repair cellular damage (Han et al. 2013).
Supplementation of S Enhances Antioxidant System in Waterlogged Plants
In this study, a considerable linear increase in the content of AsA was observed in plants supplemented with increasing S doses, which is most likely due to the increase in GSH content of S-supplemented waterlogged plants (Fig. 4D). This mechanism can be explained by the Mehler-peroxidase reaction where GSH plays a vital role in the regeneration of AsA from dehydroascorbate (DHA) via the enzyme dehydroascorbate reductase (DHAR), resulting in oxidation of GSH to glutathione disulfide (GSSG) (Li et al. 2010; Noctor and Foyer 1998). It is clear from the findings that sulfur supplementation was associated with higher waterlogging tolerance, as AsA can directly scavenge ROS (Gill and Tuteja 2010). Again, in this study, the increasing trend of DHAR and MDHAR, together with APX and GR activity of the S-supplemented waterlogged plants (Fig. 5), highly supports this reasoning. As found in this study, compared with low- and medium-S, high-S applied waterlogged plants had higher CAT and APX activities (Fig. 5A and B), being involved in the reduction of H2O2 into H2O (Teoh et al. 2022). The increase in APX and CAT activities could be attributed to maintaining the balance of the GSH–AsA cycle with the augmentation of GSH (Gomes-Junior et al. 2006).
An essential indicator of the amount of oxidative stress experienced by the cell is the ratio of AsA and DHA; an increase in DHA and a decrease in AsA indicate the inhibition of ascorbate synthesis. In this study, a reduction in DHA content was recorded with increasing S application to the waterlogged plants (Fig. 4B). Therefore, relatively higher waterlogging tolerance of high-S supplemented plants could also be related to higher AsA/DHA and GSH/GSSG ratios, which maintain the redox state in plant cells under ominous stress conditions (Mohsin et al. 2019). It is assumed that the addition of S beyond plant needs tends to restore GSH levels and maintain the redox state of the cells under waterlogging conditions. Higher GSH/GSSG ratio, as well as GR activity (Fig. 5C) in S-supplemented plants, led to the inference that the former attribute is directly related to the latter one. This interpretation is supported by Mohsin et al. (2019), who observed a similar effect of increasing GR activity on balancing the redox state while elevating salt stress in wheat. Furthermore, these findings are consistent with the observation of Bano et al. (2022), indicating that higher S supplementation reduced arsenic (As) toxicity by boosting the antioxidant system and thus mitigated oxidative stress in Brassica napus plants. Aside from that, the higher tolerance of high-S supplemented waterlogged plants over medium-S waterlogged plants is attributed to higher GPX and GST activity (Fig. 6A-B), which are responsible for the detoxification and reduction of H2O2. The underlying mechanism is oxygen radical scavenging; GPX reduces H2O2 via glutathione (GSH) to create water and glutathione disulfide (Li et al. 2020). Thus, the increased production of GSH in S-supplemented waterlogged plants accelerates GPX and GST activities to inhibit H2O2 accumulation (Dixon 2010). The results of this study showed that an efficient antioxidant system is correlated with increased resistance to waterlogging stress and the removal of ROS (Fig. 7). Waterlogged plants supplemented with lower amounts of S were found to have a reduced synthesis of GSH and lower antioxidant capacity, which led to a lower ability to counteract waterlogging stress. On the other hand, a higher S accumulation in high-S-supplemented plants resulted in an upsurge in GSH production, allowing the plants to balance ion uptake and cope with waterlogging (Fig. 7).
Schematic illustration for possible mechanism of S-assist tolerance of rapeseed (Brassica napus L.) under control and waterlogged soils.Here, ASA, ascorbate; GSH, glutathione; ROS, Reactive oxygen species; MDA, malondialdehyde; APX, ascorbate peroxidase; CAT, catalase; GPX, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase
Conclusion
In Brassica napus, waterlogging stress negatively affected plant growth and yield, possibly sparking oxidative stress due to the overproduction of reactive oxygen species. In contrast, supplementing waterlogged plants with high levels of S proved beneficial for the growth and development of plants, due to a remarkable increase in ROS scavenging capabilities, especially at the highest rate of application. Waterlogging-induced oxidative stress was ameliorated by high-S supplementation through increasing antioxidant metabolites (AsA and GSH) and enzyme activities (APX, CAT, GR, MDHR, DHAR). Moreover, it was found that high-S induced waterlogging resistance in rapeseed plants was also linked with the regulation of anion uptake, i.e., reduction in Cl- uptake and increase in the uptake of sulfate, nitrate, and phosphate. Overall, this study provided valuable insight about the S-assisted waterlogging tolerance in Brassica napus L.
References
Aebi HE (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad G, Jan A, Arif M, Jan M, Shah H (2011) Effect of nitrogen and sulfur fertilization on yield components, seed and oil yields of canola. J Plant Nutr 34(14):2069–2082. https://doi.org/10.1080/01904167.2011.618569
Alifu H, Hirabayashi Y, Imada Y et al (2022) Enhancement of river flooding due to global warming. Sci Rep 12:20687. https://doi.org/10.1038/s41598-022-25182-6
Ambro S, Kotewitsch M, Wittig PR, Bammer B, Mustroph A (2022) Transcriptional response of two Brassica napus Cultivars to short-term hypoxia in the Root Zone. Front Plant Sci 13:897673. https://doi.org/10.3389/fpls.2022.897673
Anee TI, Nahar K, Rahman A, Mahmud JA, Bhuiyan TF, Alam MU, Fujita M, Hasanuzzaman M (2019) Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 8:196. https://doi.org/10.3390/plants8070196
Arbona V, Hossain Z, López-Climent M, Pérez-Clemente R, Gómez-Cadenas A (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132(4):452–466. https://doi.org/10.1111/j.1399-3054.2007.01029.x
Bano K, Kumar B, Alyemeni MN, Ahmad P (2022) Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol Biochem 188:1–11. https://doi.org/10.1016/j.plaphy.2022.07.026
Barrett-Lennard EG (2003) The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant Soil 253(1):35–54. https://doi.org/10.1023/A:1024574622669
Bashir H, Ibrahim MM, Bagheri R, Ahmad J, Arif IA, Baig MA, Qureshi MI (2015) Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in indian mustard. AoB PLANTS 7:plv001. https://doi.org/10.1093/aobpla/plv001
Boldrin PF, De Figueiredo MA, Yang Y, Luo H, Giri S, Hart JJ, Faquin V, Guilherme LR, Thannhauser TW, Li L (2016) Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum L). Physiol Plant 158:80–91. https://doi.org/10.1111/ppl.12465
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cataldi TR, Margiotta G, Iasi L, Di Chio B, Xiloyannis C, Bufo SA (2000) Determination of sugar compounds in olive plant extracts by anion-exchange chromatography with pulsed amperometric detection. Anal Chem 72(16):3902–3907. https://doi.org/10.1021/ac000266o
Deumlich D, Gericke A (2020) Frequency trend analysis of heavy rainfall days for Germany. Water 12:1950. https://doi.org/10.3390/w12071950
Ding LN et al (2022) Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin-more mutant. Biotechnol Biofuels 15(1):1–19. https://doi.org/10.1186/s13068-022-02155-5
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71(4):338–350. https://doi.org/10.1016/j.phytochem.2009.12.012
Doupis G, Kavroulakis N, Psarras G, Papadakis I (2017) Growth, photosynthetic performance and antioxidative response of ‘Hass’ and ‘Fuerte’avocado (Persea americana Mill.) Plants grown under high soil moisture. Photosynthetica 55:655–663. https://doi.org/10.1007/s11099-016-0679-7
Elia A, Galarini R, Taticchi M, Dörr A, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55(2):162–167. https://doi.org/10.1016/S0147-6513(02)00123-9
Fatma M, Asgher M, Masood A, Khan NA (2014) High sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63. https://doi.org/10.1016/j.envexpbot.2014.05.008
Geilfus CM (2018) Chloride: from nutrient to toxicant. Plant Cell Physiol 59(5):877–886. https://doi.org/10.1093/pcp/pcy071
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gomes-Junior RA, Moldes CA, Delite FS, Pompeu GB, Grata˜o PL, Mazzafera P, Lea PJ, Azevedo RA (2006) Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65:1330–1337. https://doi.org/10.1016/j.chemosphere.2006.04.056
Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G (2013) Functional analysis of Arabidopsis mutants’ points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signa 18:2106–2121. https://doi.org/10.1089/ars.2012.5052
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8(9):384. https://doi.org/10.3390/antiox8090384
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Herzog M, Striker GG, Colmer TD, Pedersen O (2016) Mechanisms of waterlogging tolerance in wheat – a review of root and shoot physiology. Plant Cell Environ 39:1068–1086. https://doi.org/10.1111/pce.12676
Hossain M, Asada K (1985) Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem 260(24):12920–12926. https://doi.org/10.1016/S0021-9258(17)38813-0
Hussain MA, Naeem A, Sulieman S, Pitann B, Mühling K (2022) Sulfur uptake and distribution, grain yield, and quality of hybrid and inbred winter wheat (Triticum aestivum L.) varieties under early and late waterlogging. J Plant Nutr Soil Sci 185(5):622–631. https://doi.org/10.1002/jpln.202200149
Jackson MB, Ricard B (2003) Physiology, biochemistry and molecular biology of plant root systems subjected to flooding of the soil. In: de Kroon H, de Visser EJW (eds) Root ecology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-09784-7_8
Kdidi S, Vaca-Medina G, Peydecastaing J, Oukarroum A, Fayoud N, Barakat A (2019) Electrostatic separation for sustainable production of rapeseed oil cake protein concentrate: effect of mechanical disruption on protein and lignocellulosic fiber separation. Powder Technol 344:10–16. https://doi.org/10.1016/j.powtec.2018.11.107
Lee YH, Kim KS, Jang YS, Hwang JH, Lee DH, Choi ICH (2014) Global gene expression responses to waterlogging in leaves of rape seedlings. Plant Cell Rep 33:289–299. https://doi.org/10.1007/s00299-013-1529-8
Li Y, Liu Y, Zhang J (2010) Advances in the research on the AsA–GSH cycle in horticultural crops. Front Agric China 4:84–90. https://doi.org/10.1007/s11703-009-0089-8
Li B, Qiu J, Jayakannan M, Xu B, Li Y, Mayo GM et al (2016) AtNPF2.5 modulates chloride (Cl–) efflux from roots of Arabidopsis thaliana. Front Plant Sci 7:2013. https://doi.org/10.3389/fpls.2016.02013
Li H, Wang H, Wen W, Yang G (2020) The antioxidant system in suaeda salsa under salt stress. Plant Signal Behav 15(7):1771939. https://doi.org/10.1080/15592324.2020.1771939
Lou L, Kang J, Pang H, Li Q, Du X, Wu W, Chen J, Lv J (2017) Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int J Mol Sci 18:1628. https://doi.org/10.3390/ijms18081628
Madigan MT, Martinko JM, Parker J (2003) In: Brock (ed) Biology of microorganisms. Prentice Hall, London, UK
Madsen H, Lawrence D, Lang M, Martinkova M, Kjeldsen TR (2014) Review of trend analysis and climate change projections of extreme precipitation and floods in Europe. J Hydrol 519:3634–3650. https://doi.org/10.1016/j.jhydrol.2014.11.003
Maraun D (2013) When will trends in european mean and heavy daily precipitation emerge? Enviro Res Let 8(1):014004. https://doi.org/10.1088/1748-9326/8/1/014004
Min Y, Ping QB, Xue-li M, Ping W, Mei-ling L, Lu-lu C, Lei-tai C, Ai-qing S, Zhen-lin W, Yanping Y (2016) Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L). J Integr Agric 15:2745–2758. https://doi.org/10.1016/S2095-3119(16)61358-8
Mohsin SM, Hasanuzzaman M, Bhuyan MHMB, Parvin K, Fujita M (2019) Exogenous tebuconazole and trifloxystrobin regulates reactive oxygen species metabolism toward mitigating salt-induced damages in cucumber seedling. Plants 8:428. https://doi.org/10.3390/plants8100428
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Narayan OP, Kumar P, Yadav B, Dua M, Johri AK (2022) Sulfur Nutrition and its role in plant growth and development. Plant Signal Behav. https://doi.org/10.1080/15592324.2022.2030082
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279. https://doi.org/10.1146/annurev.arplant.49.1.249
Pachauri RK et al (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.In: Pachauri RK and Meyer LA (ed) IPCC 2014. Geneva, Switzerland pp 151
Rehman H, Iqbal Q, Farooq M, Wahid A, Afzal I, Basra SMA (2013) Sulfur application improves the growth, seed yield and oil quality of canola. Acta Physiol Plant 35(10):2999–3006. https://doi.org/10.1007/s11738-013-1331-9
Sagervanshi A, Naeem A, Geilfus C, Kaiser H, Mühling KH (2020) One-time abscisic acid priming induces long‐term salinity resistance in Vicia faba: changes in key transcripts, metabolites, and ionic relations. Physiol Plant 172(1):146–161. https://doi.org/10.1111/ppl.13315
Sauter M (2013) Root responses to flooding. Curr Opin Plant Bio 16(3):282–286. https://doi.org/10.1016/j.pbi.2013.03.013
Shi H, Ye T, Han N, Bian H, Liu X, Chan Z (2015) Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol 57:628–664
Simova-Stoilova L, Demirevska K, Kingston-Smith A, Feller U (2012) Involvement of the leaf antioxidant system in the response to soil flooding in two Trifolium genotypes differing in their tolerance to waterlogging. Plant Sci 183:43–49. https://doi.org/10.1016/j.plantsci.2011.11.006
Tabari H (2020) Climate change impact on flood and extreme precipitation increases with water availability. Sci Rep 10:13768. https://doi.org/10.1038/s41598-020-70816-2
Tanou G, Molassiotis A, Diamantidis G (2009) Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ Exp Bot 65:270–281. https://doi.org/10.1016/j.envexpbot.2008.09.005
Teoh EY, Teo CH, Baharum NA, Pua TL, Tan BC (2022) Waterlogging stress induces antioxidant defense responses, Aerenchyma formation and alters Metabolisms of Banana plants. https://doi.org/10.3390/plants11152052. Plants 11:2052
Tian L, Zhang Y, Chen P, Zhang F, Li J, Yan F, Dong Y, Feng B (2021) How does the Waterlogging Regime affect crop yield? A Global Meta-Analysis. Front Plant Sci 12:634898. https://doi.org/10.3389/fpls.2021.634898
Tong Y, Gabriel-Neumann E, Ngwene B, Krumbein A, George E, Platz S, Rohn S, Schreiner M (2014) Topsoil drying combined with increased sulfur supply leads to enhanced aliphatic glucosinolates in Brassica juncea leaves and roots. Food Chem 152:190–196. https://doi.org/10.1016/j.foodchem.2013.11.099
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Voesenek LACJ, Armstrong W, Bogemann GM, McDonald MP, Colmer TD (1999) A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Aust J Plant Physiol 26:87–93. https://doi.org/10.1071/PP98086
Wang J, Shi SH, Wang DY, Sun Y, Zhu M, Li FH (2021) Exogenous salicylic acid ameliorates waterlogging stress damages and improves photosynthetic efficiency and antioxidative defense system in waxy corn. Photosynthetica 59(1):84–94. https://doi.org/10.32615/ps.2021.005
Wollmer A, Pitann B, Mühling KH (2018) Waterlogging events during stem elongation or flowering affect yield of oilseed rape (Brassica napus L.) but not seed quality. J Agron Crop Sci 204(2):165–174. https://doi.org/10.1111/jac.12244
Xu M, Ma H, Zeng L, Cheng Y, Lu G, Xu J, Zhang X, Zou X (2015) The effect of waterlogging on yield and seed quality at the early flowering stage in Brassica napus L. Field Crop Res 180:238–245. https://doi.org/10.1016/j.fcr.2015.06.007
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA independent glutathione accumulation. Funct Plant Biol 30:955–963. https://doi.org/10.1071/FP03091
Zhang Y, Song X, Yang G, Li Z, Lu H, Kong X et al (2015) Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Res 179:164–172. https://doi.org/10.1016/j.fcr.2015.05.001
Zou XL, Liu ZENG, Lu G, Cheng Y, Xu JS, Zhang XK (2015) Comparison of transcriptomes undergoing waterlogging at the seedling stage between tolerant and sensitive varieties of Brassica napus L. J Integr Agric 14(9):1723–1734. https://doi.org/10.1016/s2095-3119(15)61138-8
FAO (2021) Food Outlook–Biannual Report on Global Food Markets – June 2021. http://www.fao.org/3/cb4479en/cb4479en.pdf. Accessed 19 August 2022
Acknowledgements
We thank Stephanie thor Straten for her excellent assistance with metabolites analyses. MA Hussain is thankful to the Bangabandhu Science and Technology Fellowship Trust, Ministry of Science and Technology, Bangladesh for the grant of fellowship. A. Naeem is thankful to the Alexander von Humboldt Foundation for grant of George Forster Post-Doctorate Fellowship.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MAH conducted the experiments, measurements, calculations, analyses, and wrote the paper. AN assisted with the desiging and setting-up of the experiment, supervised the measurements, and reviewed the manuscript. BP evaluated the measurements, reviewed the manuscript. KHM reviewed the manuscript, designed and supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they do not have any conflicts of interest.
Consent for publication
This manuscript does not provide data from any individual person, so it is “not applicable.”
Research Involving Human and Animal Participants
We all certify that manuscripts reporting studies do not include any human participants, human data, or human tissue. So, it is not applicable.
Additional information
Communicated by Setsuko Komatsu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, M.A., Naeem, A., Pitann, B. et al. High Sulfur (S) Supplementation Imparts Waterlogging Tolerance to Oilseed rape (Brassica napus L.) Through Upregulating S Metabolism and Antioxidant Pathways. J Plant Growth Regul 42, 7591–7605 (2023). https://doi.org/10.1007/s00344-023-11034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11034-8