Abstract
Alfalfa (Medicago sativa L.) is the most widely planted forage, which have higer economically valuable and better ecological benefits in the world. However, it’s sensitive to high root-zone temperature in tropical and subtropical areas. In order to explore the changes of metabolites in alfalfa under heat stress, the study applied the unique alfalfa landrace ‘Deqin’ to explored the mechanisms on the basis of pseudotargeted metabolomics. The results showed that the phenotype of leaves and roots were changed remarkly, leaf chlorophyll content, electrolyte leakage, H2O2 content and soluble sugar also changed at different treatments. Meanwhile, heat stress may stimulate the germination of new alfalfa roots in response to adverse environment. Metabonomics analysis showed that a total of 809 and 749 metabolites were detected in leaves and roots respectively, which treated with four different ways. Of these, 343 and 383 were identified as differential metabolites in the leaves and roots, respectively; they mainly belongs to phenylpropanoids, polyketides, organic acids and derivatives, organic acids and derivatives. Among them, the differential metabolites were mainly enriched in glyoxylate and dicarboxylate metabolism, citrate cycle, aminoacyl-tRNA biosynthesis, flavone and flavonol biosynthesis, and phenylpropanoid biosynthesis. Meanwhile, some metabolites such as phenylpropanoids, polyketides, organic acids and derivatives may play an important role in response to heat stress in M. sativa ‘Deqin’. Overall, this study reports the differential metabolites and pathways in the roots and leaves of ‘Deqin’ in response to high root-zone temperature, which provides new insights into the heat stress mechanisms of alfalfa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High temperature is one of the major environmental stresses affecting plant growth and development (Aditya and Aryadeep 2020). Many plants have developed different strategies, including physiological and metabolic responses, to ensure their survival and reproduction under high temperature stress (Jin et al. 2016). Previous research has shown that high temperature adversely affects photosynthesis, respiration, water relations, and membrane stability in plants, leading to physiological changes (Wahid et al. 2007; Wang et al. 2013). Recent studies have showed that amino acids, carbohydrates, nitrogen metabolisms and metabolites involved in the biosynthesis and catalyzing actions which can help plants to deal with heat stress (Raza, 2020). Moreover, secondary metabolites that play a key role in stress resistance respond rapidly, before the physiological changes are apparent (Akula and Aswathanarayana 2011).
Omics approaches provide important technical support for exploring the physiological and molecular mechanisms of plants under temperature stress (Raza et al, 2021). Among them,the wide application of metabolomics techniques provides a powerful research tool to investigate the mechanisms of plant response to heat (Ravi et al. 2020). Such as nuclear magnetic resonance spectroscopy (NMR), gas chromatography-mass spectrometry (GC–MS), liquid chromatography-mass spectrometry (LC–MS), fourier-transform infrared spectroscopy (FT-IR), matrix-assisted laser desorption (MALDI)-ToF–MS, capillary electrophoresis-mass spectrometry (CE-MS) (Raza 2020). Those techniques have been applied to different species, for example, several studies on Panicum maximum (Wedow et al. 2019), Sargassum fusiforme (Liu and Lin 2020), and Glycine max (Das et al. 2017) have revealed the vital role of saccharides and amino acids under high temperature stress. Furthermore, it cannot be disregarded that soil temperature, which is more critical than shoot temperature for plant growth, is strongly associated with air temperature (Hou et al. 2015; Miransari 2016). High root-zone temperature is detrimental to the uptake of nutrients and water by plant roots, which in turn influences shoot growth (Shahid and Aamer 2011). Although many studies have investigated the effects of air temperature on the growth of plant shoots (Hatfiled and Prueger 2015), less attention has been paid to the harm of high root-zone temperature.
Alfalfa (Medicago sativa L.) is a perennial forage grass of the Eurasian genus Medicago (family: Leguminosae). Medicago sativa is an excellent plant resource for ecological restoration because of its deep and rapidly growing roots, profuse leaves, short growth cycle, and N2 fixation ability (Small 2011). In addition, M. sativa is of economic value for its outstanding nutritional quality (Wassie et al. 2019). This plant species is mainly distributed in the arid and semi-arid regions of Northwest and North China. However, high temperature is the major environmental factor that limits M. sativa cultivation in the tropical and subtropical areas of South China (Mo et al. 2011; Song et al. 2019). In such areas with high temperature stress and acid soil infertility, and environmental management using plants with ecological benefits is of practical significance.
Medicago sativa ‘Deqin’ is a unique alfalfa landrace with strong heat tolerance (Zhao et al. 2010), which can grow and propagate very well in xerothermic valleys of Jinsha River in Yunnan Province, China (Bi et al. 2007). It was used as forage by local villagers with highly digestible and rich in proteins (Bi et al. 2005). Previous research has shown that high root-zone temperature influences the shoot and root growth of M. sativa ‘Deqin’, with remarkably enhanced root elongation and regeneration at 30 °C (Li et al. 2018a, b). However, the mechanisms underpinning the response of M. sativa ‘Deqin’ to high root-zone temperature is largely unclear.
In this study, we aimed to identify the heat stress mechanisms of M. sativa ‘Deqin’ on the basis of pseudotargeted metabolomics. We compared the morphological, physiological, and metabolite changes in whole alfalfa plants in response to high root-zone temperature stress (30 °C) in a short period. The results will provide new insights into the heat stress mechanisms of alfalfa and contribute to its ecological value in South China.
Materials and Methods
Plant Material, Growth Conditions, and Stress Treatment
Alfalfa (M. sativa ‘Deqin’) seeds were collected at Deqin county field breeding farm without contamination of alien pollen. These seeds were kindly provided by Professor Yufen Bi from the Faculty of Animal Science and Technology, Yunnan Agriculture University (Kunming, China). The seeds were disinfected with concentrated sulfuric acid for 30 min and rinsed with distilled water. Seed germination was conducted on moist filter paper in an artificial climate box at 25 °C ambient temperature. Fourteen days later, germinated seeds were transferred to polyethylene pots (5 cm × 5 cm × 7.5 cm) containing vermiculite and perlite (v:v = 1:2). The pots were placed in an artificial climate box with 22 °C/20 °C (day/night) ambient temperature, 65% relative humidity, a 16 h photoperiod, and photosynthetically active radiation of 400 µmol m–2. Alfalfa seedlings were watered with Hoagland’s nutrient solution (Barker et al. 2006) every 2 days and maintained in an artificial climate box.
Eight-week-old seedings showing consistent growth characteristics were selected and transferred to a hydroponic box, which was covered with aluminum foil to protect the roots from light. The seedlings were maintained in the hydroponic box for 7 days to allow for adaptation to the root environment and then treated at 30 °C root-zone temperature using a heating rod. On the 8th day, plant samples were collected at different time points for morphological observation, physiological measurement, and metabolomic analysis. In addition, quantitative reverse transcription PCR (qRT-PCR) analysis was performed to verify the related genes associated with metabolites.
Morphological Observation
Leaf and root morphological changes were observed and photographed at 0, 6, 12, 24, 48, and 72 h and 7 days of stress treatment (30 °C, rhizosphere). Then, the temperature was restored to 22 °C for 6 h (H6 h). The angle among leaf, main stem and the width of the third fully expanded leaves at 0, 0.5, 6, 12, and H6 h of stress treatment were measured using a protractor and a straight ruler, respectively. The measurement in all treatment groups was repeated three times.
Physiological Measurement
The chlorophyll content of leaf samples and the electrolyte leakage (EL), H2O2 content, and soluble sugar content of leaf and root samples were examined at 0, 0.5, 6, 12, and H6 h of stress treatment. Specifically, chlorophyll a, chlorophyll b, and carotenoid content was measured according to the method of Li (2000). Electrolyte leakage was measured following the method of Li (2000) with slight modifications. In brief, leaf and root samples with a fresh weight of 0.2 g each were cut into 1 cm pieces and placed into test tubes containing 20 mL of distilled water. The tubes were left to stand at room temperature for 30 min, and the EL was then measured using a conductivity meter (recorded as E1). Subsequently, the tubes were incubated in a boiling water bath for 10 min. After cooling, the solution was mixed by shaking, and the EL was measured again (recorded as E2). In addition, the EL of distilled water was measured (E0). The EL of a sample was calculated as follows: EL (%) = (E1−E0)/(E2−E0) × 100%. H2O2 content was measured using spectrophotometry (Li 2000). Sucrose, fructose, and glucose content was measured according to the method of Ye (2004) and Zhang (1977).
Statistical Data Analysis
The physiological data were expressed as mean and standard error of the mean. One-way analysis of variance was performed to determine significant differences between group means, and statistical significance was assessed by Duncan’s test using SPSS (v18.0; SPSS Inc., Chicago, IL, USA). Graphs of physiological parameters were generated using SigmaPlot (v14.0; Systat Software Inc., San Jose, CA, USA).
Metabolomic Analysis
Metabolite Extraction
Fresh leaf and root samples (200 mg each) were collected at 0, 0.5, 6 h of 30 °C stress treatment, and restored to 22 °C for 6 h (H6) (leaf designated as L1, L2, L3, L4 and the root as R1 R2, R3, R4, respectively). Each treatment group had six biological replicates. The samples were immediately frozen in liquid nitrogen and stored at – 80 °C until further processing. The extraction procedure of metabolites was modified from Vos et al. (2007) and Chen et al. (2013). In brief, freeze-dried samples were crushed to powder with a mixer mill for 4 min at 45 Hz and extracted with 75% methanol (10 µL mg–1) in Eppendorf tubes kept on a shaker overnight. Following centrifugation (10,000×g, 15 min, 4 °C), the resulting supernatants were filtered through a 0.22 µm filter membrane and transferred to sample vials (2 mL) for liquid chromatography–mass spectrometry (LC–MS) analysis. In addition, the supernatants of all samples were mixed in equal proportions as the quality control (QC) sample for monitoring the analytical process. The QC sample was analyzed with every six samples throughout the LC–MS analytical process.
Metabolite Separation and Detection
Chromatographic separation was performed using an Agilent 1290 Infinity II series ultra-high-performance liquid chromatography (UHPLC) system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Waters Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 µm; Waters Chromatography Division, Milford, MA, USA). The mobile phase was composed of solvent A (0.1% formic acid) and solvent B (100% acetonitrile) and had a flow rate of 400 µL/min, and the elution gradient (A:B) was 98:2 (v:v) at 0–10 min, 40:60 at 10–12 min, 2:98 at 12–13 min, and 98:2 at 13–15 min. The injection volume was 2 µL, and the temperatures of the column and auto-sampler were set at 35 °C and 4 °C, respectively. The effluent was connected to a triple time-of-flight mass spectrometer (Triple-TOF–MS).
The TripleTOF® 6600 system (AB SCIEX, Framingham, MA, USA) was used for its ability to acquire second-order mass spectra (MS2) in a data-dependent acquisition mode (Luo et al. 2016). The acquired mass range was divided into 50–300, 290–600, 590–900, and 890–1500 with four injections each, and the source conditions were set as follows: ion source gas 1, 1: 40 psi; ion source gas 2, 2: 80 psi; curtain gas, 25 psi; source temperature, 650 °C; ion spray voltage floating, 5000 or − 4000 V in positive or negative mode, respectively; declustering potential, 60 V; and fragmentor voltage, 35 ± 15 V. The data acquired by Triple-TOF–MS were analyzed using Metabolomics Analysis Platform Solution (MAPS) and BIOTREE PWT database of Shanghai BIOTREE Biological Technology Co., Ltd. (Shanghai, China).
The parent ions and daughter ions in MS2 were analyzed to generate a multiple reaction monitoring (MRM) database, and MRM-based analysis of all samples was performed using a triple-quadrupole mass spectrometer (QqQ-MS; Agilent 6490 Series, Agilent) coupled to Triple-TOF–MS, equipped with an Agilent JetStream electrospray ionization interface. The operation parameters were as follows: capillary voltage, + 3500/– 3000 V; gas (N2) temperature, 220 °C; gas (N2) flow, 16 L/min; sheath gas (N2) temperature, 350 °C; sheath gas flow, 12 L/min; and nebulizer pressure, 40 psi.
Raw Data Pre-processing
High-resolution MS data were converted to the mzXML format using the ProteoWizard software and then pre-processed using MAPS software. The pre-processed results generated a data matrix consisting of retention time (RT), mass-to-charge ratio (m/z), and peak intensity. Then, the mass spectrum peaks of the same metabolites in different samples were subject to retention time correction, integration correction, area integration using MAPS software to ensure the accuracy of the qualitative and quantitative analyses. Qualitative analysis and classification of metabolites were performed in HMDB (https://hmdb.ca/). Furthermore, a series of data management steps, including filtering of single deviation mass spectrum peaks, simulating of missing values in the original data and normalizing data according total ion current (TIC), were performed to accuracy of the quantitation results.
Orthogonal Projections to Latent Structure-Discriminant Analysis
The pre-processed data were log transformed and UV scaled using the SIMCA software (v14.1; Sartorius Stedim Data Analytics AB, Umea, Sweden), followed by automated modeling for orthogonal projections to latent structure-discriminant analysis (OPLS-DA). First, the first principal component was subjected to OPLS-DA modeling, and the model quality was tested by sevenfold cross validation. Then, the resulting R2Y (the interpretability of the model on the categorical variable Y) was used to evaluate the validity of the model by 200-fold cross validation. Finally, the permutation test of Y variables was performed to generate different random Q2 values, which were then used to further test model validity.
Screening of Differential Metabolites
Multivariate analysis was used to screen differential metabolites using the following criteria: Log2 |fold change| > 1, P value < 0.05 and the variable importance in the projection (VIP) of the first principal component in the OPLS-DA model > 1. The classification results and volcano plots of differential metabolites were obtained using SigmaPlot (v14.0) and R (v3.6.5; http://www.r-project.org/), respectively.
Pathway Annotation and Analysis of Differential Metabolites
The differential metabolites were annotated in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (https://www.kegg.jp/). Then, according to a high impact value and P value, enrichment and topological analysis of the pathways in which the differential metabolites were present enabled further identification of the key pathways that showed the highest correlation with metabolite differences. The bubble plots of differential pathways were produced using R (v3.6.5).
RNA Isolation, cDNA Synthesis, and qRT-PCR
qRT-PCR was performed to verify the genes associated with metabolites through the analysis of changes in gene expression of nine key enzymes involved in the significantly enriched pathways of differential metabolites. Eastep® Super Total RNA Extraction Kit and Eastep® RT Master Mix Kit (Promega, Shanghai, China) were used to isolate total RNA and synthesize cDNA, respectively. The sequences of target genes were obtained from the National Center for Biotechnology Information database (NCBI; https://www.ncbi.nlm.nih.gov/gene/). Optimal forward and reverse primers (Supplementary Table S1) were designed using the online tool Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The β-actin gene (F: 5′-CGAAATCGTGAGAGACGTGA-3′, R: 5′-ACCTGTCCATCAGGCAACTC-3′) was used as the internal control (Zhao 2014). qRT-PCR experiments were three technical repeats. The relative expression levels of target genes were estimated using the 2−ΔΔCT method (Livak and Schmittgen 2001). The results were processed and analyzed in Excel 2007 (Microsoft Corp., Redmond, WA, USA). Graphs were produced using SigmaPlot (v14.0).
Results
Morphological Response to High Root-Zone Temperature
The high root-zone temperature stress directly affected the morphological traits of M. sativa ‘Deqin’ plants (Fig. 1a) and reduced their root system (Fig. 1b). Under stress treatment, the shoot and root morphology showed no evident changes at 12 h compared with 0 h, except a significant (85.2%) increase in the angle between leaf and main stem (P < 0.05; Fig. 1c). At 24 h, the bottom leaves showed yellowing, and the number of roots was reduced slightly (Fig. 1a). At 48–72 h, most leaves were dehydrated and rolled back (Fig. 1a), whereas the roots were sparse and soft (Fig. 1b). At 96 h, a small fraction of leaves was etiolated and fell off; however, a few new roots emerged (Fig. 1b). After 7 d, the vast majority of leaves became etiolated, and leaf margins were shriveled, and several new roots were developed (Fig. 1b).
Morphology changes of M. sativa ‘Deqin’under high root-zone temperature stress. a The plant morphology after heat stress in different time. b Changes of root system under heat stress. c Angle between leaf and main stem, d width of the third fully expanded leaves under heat stress. In c and d, the lower case letters were represent significance level in P < 0.05
Physiological Response to High Root-Zone Temperature
High root-zone temperature affected the physiological status of M. sativa ‘Deqin’ plants. Leaf chlorophyll content showed an overall downward tendency with increasing treatment time. Chlorophyll a, chlorophyll b, and carotenoid content first decreased by 26.4%, 41.5%, and 29.9%, respectively, from 0 to 6 h (P < 0.05) and then increased by 10.6%, 13.4%, and 19.6%, respectively, from 6 to 12 h (P < 0.05; Fig. 2a). After restoring the root-zone temperature to 22 °C, the chlorophyll a and carotenoid content at H6 h was 12.7% and 9.8% lower than that at 12 h (P < 0.05), respectively; however, no significant change was observed in the chlorophyll b content. The electrolyte leakage (EL) of leaves and roots increased by 20.6% and 30.0%, respectively, from 0 to 12 h of stress treatment (P < 0.05; Fig. 2b). A larger increase was observed in the corresponding H2O2 content—72.5% and 84.5%, respectively (P < 0.05; Fig. 2c). At H6 h, the EL and H2O2 content were slightly—but not significantly—lower and higher, respectively, than at 12 h.
With increasing treatment time, the sucrose content of leaves and roots first increased by 45.2% and 58.3%, respectively, from 0 to 0.5 h (P < 0.05) and then sharply decreased by 76.8% and 85.8%, respectively, from 0.5 to 12 h (P < 0.05; Fig. 2d). The glucose content of leaves and roots also first increased from 0 to 6 h, by 38.1% and 79.7%, respectively (P < 0.05), and markedly decreased at 12 h, by 59.0% and 69.6%, respectively (P < 0.05; Fig. 2e). The fructose content of leaves and roots continuously increased by 66.0% and 85.8%, respectively, from 0 to 12 h (P < 0.05; Fig. 2f). The sucrose content of roots was 35.9% higher at H6 h than at 12 h (P < 0.05), whereas the fructose and glucose content of both leaves and roots was significantly lower at H6 h than at 12 h (P < 0.05).
Qualitative and Quantitative Analyses of Metabolites
The total ion chromatograms of leaf (L1–L4), root (R1–R4), and QC samples in positive and negative modes are summarized in Supplementary Table S2. Among the replicate samples, the area of most chromatographic peaks overlapped, and the appearance time of the peaks was consistent, indicating that the sample quality was satisfactory, and the analytical instrument was stable, and the obtained data were reliable for further analysis. In total, 809 and 749 peaks were detected in the leaves and roots by MS. After comparing the leaf and root metabolites, 809 and 747 peaks were retained. On the basis of the qualitative and quantitative analyses of the peaks, the leaf and root metabolites were classified into 13 and 12 categories, respectively (Supplementary Table S3).
Establishment of OPLS-DA Models
The OPLS-DA of leaf and root metabolite data was designed for modeling two classes of data to increase the class separation and find differential metabolites (Fig. 3). The score plot of OPLS-DA shows that L1, L2, and L3 (Fig. 3a–c) and R1, R2, and R3 (Fig. 3g–i) were distinct from each other in the leaf group and root group, respectively. All samples were located in the 95% confidence intervals (Hotelling’s T-squared ellipse).
Orthogonal projections to latent structures-discriminant analysis (OPLS-DA) of the metabolites in leaves and roots of M. sativa ‘Deqin’ plants under high root-zone temperature stress. a–c and g–i OPLS-DA score plots, in which the abscissa (t [1]P) and ordinate (t [1]0) represent the predicted scores of the first principal component and the scores of orthogonal principal components, respectively. d–f and j–l Validation plots of the OPLS-DA models corresponding to the score plots, in which the abscissa (correlation coefficient) represents the retention degree in the permutation test, the ordinate represents the R2Y or Q2 value in the permutation test, and the dotted lines represent the regression lines of R2Y or Q2
In the permutation test, the R2Y of each stochastic model was close to but less than 1 (Fig. 3d–f, j–l), indicating that the established models were stable, reliable, and accurate descriptions of the real situation in the leaf and root metabolite data. Moreover, the Q2 in each stochastic model was less than that in the original model, and the Q2 in the stochastic model gradually decreased with decreasing retention degree of permutation (Fig. 3d–f, j–l). These results indicated that the original models were simulated well in statistical significance and not overfitted.
Classification and Volcano Plots of Differential Metabolites
Through screening of metabolites in the leaf and root groups, we identified 343 and 383 differential metabolites, respectively, in the plants under high root-zone temperature stress (P value < 0.05, VIP > 1). The differential metabolites in leaf (L1–L4) comprised unknown metabolites (73.1%); phenylpropanoids and polyketides (6.7%); lipids and lipid-like molecules (5.5%); organoheterocyclic compounds (4.4%); organic acids and derivatives (3.8%); benzenoids (2.3%); organic oxygen compounds (1.5%); organooxygen compounds (1.2%); organic nitrogen compounds (0.6%); nucleosides, nucleotides, and analogues (0.6%); and lignans, neolignans, and related compounds (0.3%; Fig. 4a). The volcano plots show that there were 102 upregulated metabolites in L2 vs. L1 (Fig. 4b) and 118 upregulated metabolites in L3 vs. L2 (Fig. 4c), whereas the corresponding 62 and 63 downregulated metabolites. Meanwhile, there are only 48 upregulated and 33 downregulated metabolites in L4 vs. L3 (Fig. 4d).
The classification and volcano plots of differential metabolites in leaves and roots of M. sativa ‘Deqin’ plants under high root-zone temperature stress. a differential metabolites in leaves; b L2 vs. L1; c L3 vs. L2; d L4 vs. L3; e differential metabolites in roots; f R2 vs. R1; g R3 vs. R2; h R4 vs. R3. L1 to L4 and R1 to R4 indicate leaf and root samples collected at 0, 0.5, 6, and H6 h of stress treatment, respectively. The abscissa of the volcano plot indicates the log2 fold-change of each metabolite in the sample group, and the ordinate indicates the −log10 P value of Student’s t-test. Each point represents a differential metabolite and the size of the point represents the variable importance in the projection (VIP) of the OPLS-DA model
The differential metabolites in root (R1–R4) include unknown metabolites (63.7%); phenylpropanoids and polyketides (11.0%); organic acids and derivatives (6.0%); organoheterocyclic compounds (4.7%); lipids and lipid-like molecules (3.9%); benzenoids (3.9%); organic oxygen compounds (3.4%); organic nitrogen compounds (1.0%); nucleosides, nucleotides, and analogues (1.0%); organic oxygen compounds (0.5%), organosulfur compounds (0.3%); and lignans, neolignans, and related compounds (0.3%; Fig. 4e). The volcano plots show that there were 129 upregulated metabolites in R2 vs. R1 (Fig. 4f) and 24 upregulated metabolites in R3 vs. R2 (Fig. 4g), whereas the corresponding number of downregulated metabolites were 93 and 121 respectively. The number of upregulated and downregulated metabolites was 24 and 153 in R4 vs. R3 (Fig. 4h).
Pathway Analysis of Differential Metabolites
The differential metabolites in leaf in the groups L2 vs. L1 (Fig. 5a), L3 vs. L2 (Fig. 5b), and L4 vs. L3 (Fig. 5c) were mapped to 18, 19, and 6 metabolic pathways respectively. The impact value in topological analysis and the P value in enrichment analysis indicated that the metabolic pathways related to flavone and flavonol biosynthesis (Fig. 5a, c); aminoacyl-tRNA biosynthesis (Fig. 5a); isoquinoline alkaloid biosynthesis (Fig. 5a); tyrosine metabolism (Fig. 5a); alanine, aspartate, and glutamate metabolism (Fig. 5b); flavonoid biosynthesis; and sphingolipid metabolism (Fig. 5c) were more significantly enriched than other metabolic pathways in specific leaf groups.
Pathway enrichment of differential metabolites in leaves (a–c) and roots (d–f) of M. sativa ‘Deqin’ plants under high root-zone temperature stress. a L2 vs. L1; b L3 vs. L2; c L4 vs. L3; d R2 vs. R1; e R3 vs. R2; f R4 vs. R3. Each bubble in the plot represents a metabolic pathway. Larger size and darker color of the bubble indicate a greater degree of enrichment
The differential metabolites in root samples in the groups of R2 vs. R1 (Fig. 5d), R3 vs. R2 (Fig. 5e), and R4 vs. R3 (Fig. 5f) were mapped to 21, 26, and 24 pathways, respectively. The impact value and P value associated with the pathways indicated that the metabolic pathways related to flavone and flavonol biosynthesis (Fig. 5d); glyoxylate and dicarboxylate metabolism (Fig. 5d); riboflavin metabolism (Fig. 5d); glyoxylate and dicarboxylate metabolism (Fig. 5d, e); citrate cycle (Fig. 5e); alanine, aspartate, and glutamate metabolism (Fig. 5e); tyrosine metabolism (Fig. 5f); isoquinoline alkaloid biosynthesis (Fig. 5f); and phenylpropanoid biosynthesis (Fig. 5f) were more significantly enriched than other metabolic pathways in specific root groups.
Changes of Differential Metabolite Content in Significantly Enriched Metabolic Pathways
According to the pathway analysis, we analyzed the significantly enriched pathways with a high impact value and P value, and the relative content of differential metabolites in the metabolome (Fig. 6). In leaf samples, the relative content of quercetin (Fig. 6a) increased in the beginning and then decreased from 0 to 6 h of stress treatment. The relative content of l-glutamine (Fig. 6b), l-tyrosine (Fig. 6c), l-methionine (Fig. 6d), and l-lysine (Fig. 6e) was higher at 6 h than at 0.5 h. The relative content of naringenin (Fig. 6f), sphinganine (Fig. 6g), and naringenin chalcone (Fig. 6h) changed little from 0 to 6 h of stress treatment and markedly increased at H6 h.
In root samples, the relative content of citric acid (Fig. 6i) and l-malic acid (Fig. 6j) first increased and then decreased from 0 to 6 h of stress treatment. The relative content of riboflavin (Fig. 6k) decreased at 0.5 h compared with 0 h, whereas the relative content of succinic acid (Fig. 6-l) decreased at 6 h compared with 0.5 h and continuously decreased at H6 h. The relative content of l-phenylalanine (Fig. 6m) was higher at H6 h than at 6 h, whereas the relative content of 5-hydroxyferulate (Fig. 6n) exhibited an inverse trend.
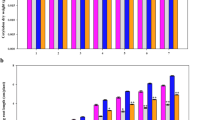
In order to detect the expression of genes of key metabolite-related enzymes under different treatments, the real-time quantitative PCR were carried out. The results were essentially consistent with the relative RNA expression levels detected by qRT-PCR of key enzymes related to citric acid (CS and ACO) and l-malic acid (MDH1 and aceB) in glyoxylate and dicarboxylate metabolism and l-glutamate (GLT and P5CDH) in alanine, aspartate, and glutamate metabolism (Fig. 7).
Changes in relative gene expression of key metabolite-related enzymes under different treatments with a real-time quantitative PCR value and P value in leaves (black columns) and roots (gray columns) of M. sativa ‘Deqin’ plants under high root-zone temperature stress. Data are the means of six replicates
Discussion
Morphological and Physiological Responses of M. sativa ‘Deqin’ to High Root-Zone Temperature
In this study, we found that high root-zone temperature stress (30 °C) caused evident morphological changes in the shoots and roots of M. sativa ‘Deqin’ plants. Despite no visable changes in root and leaf morphology within 12 h of heat stress, a considerable increase in the angle between leaf and main stem was observed. This increase in the angle between leaf and main stem is likely to be related to specific phytohormones, such as those playing a vital role in the regulation of leaf inclination in rice (Oryza sativa) (Zhang et al. 2015; Zhao et al. 2010; Zhao et al. 2013a, b). After 12 h of stress treatment, the roots of the plants were reduced substantially and became sparse, whereas the leaves showed yellowing, dehydration, and back-rolling. These changes are similar to previous findings that high root-zone temperature directly inhibited the root vigor of different grain and vegetable crops, such as O. sativa (Wu 2015), tom a to (Lycopersicon esculentum) (Han et al. 2015), lettuce (Lactuca sativa) (Gao and Li 1996), and cucumber (Cucumis sativus) (Moon et al. 2007). As a result, the uptake of water and nutrients by plant roots was inhibited, and the leaves therefore showed yellowing and dehydration.
In the present study, we observed that a few new roots emerged at 96 h, whereas a large number of new roots were growing at 7 days of stress treatment. These changes are contrary to the results obtained in colored pepper (Capsicum annuum) (Guo and Yu 2003) in which root growth almost stopped under high root-zone temperature. The new roots that emerged after 96 h of stress treatment, heat stress may stimulate the germination of new alfalfa roots in response to adverse environment. which is beneficial to recover the water and nutrient uptake and thus promotes shoot growth under high root-zone temperature stress.
Although their morphological changes were not remarkable until 12 h of stress treatment, M. sativa ‘Deqin’ plants had responded to high root-zone temperature in terms of physiological parameters within 12 h of stress treatment. Photosynthesis is an important pathway to provide energy for plant growth and stress resistance (Yagut et al. 2015). Here, we found that the chlorophyll a, chlorophyll b, and carotenoid content of leaves overall decreased from 0 to 12 h of stress treatment. This decreasing trend of chlorophyll content is similar to the reduction of photosynthesis in Zoysia japonica (Huang 2009), Poa pratensis (He et al. 1997), Rhododendron simsi (Shen and Zhao 2018), and Fargesia yunnanensis (Wang et al. 2014) under high temperature stress. The continued decrease in leaf chlorophyll content observed after the temperature was restored to 22 °C for 6 h may be related to the damage or destruction of photosystems due to high root-zone temperature stress, which led to the decreased energy supply and yellowing of leaves.
High temperature stress can induce oxidative stress, such as the generation of reactive oxygen species (ROS) including singlet oxygen (1O2), superoxide radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−), which in turn leads to the modification of cell membrane semi-permeability and functions (Wahid et al. 2007). Maintaining the relative integrity of cell membranes is an essential approach for plants to resist high temperature. Both EL and H2O2 content are often used to assess the permeability and degree of peroxidation of cell membranes (Sakae et al. 1995; Smirnoff and Arnaud 2019). In the present study, the EL and H2O2 content of M. sativa ‘Deqin’ leaves and roots increased continuously from 0 to 12 h of stress treatment. Similar increasing trends have been observed for EL in Rhododendron spp. (Li et al. 2018a, b) and Malus domestica (Ma et al. 2010) and for H2O2 content in Cicer arietinum (Kumar et al. 2013), Lotus corniculatus (Zhao et al. 2013a, b), and Lolium perenne (Wang and Xiong 2016). The increase content of EL and H2O2 indicated that M. sativa ‘Deqin’ plants had suffered damage to cell membranes caused by high root-zone temperature.
Soluble sugars such as sucrose, glucose, and fructose are a class of crucial regulators of plant growth and development, and they play a vital role in plant growth, reproduction, metabolism, and repair after stress-induced damage (Dietze et al. 2014; Kozlowski 1992). A previous study reported that the glucose and fructose content of Pinus ponderosa and P. menziesii (Marias et al. 2017) increased under heat stress, which is in line with our results obtained from the leaves and roots of M. sativa ‘Deqin’ under high root-zone temperature stress for 0.5 h. After 0.5 h of stress treatment, sucrose content markedly decreased, whereas glucose and fructose content both increased. A plausible explanation is that sucrose was converted to fructose and glucose to provide energy metabolism and maintain osmotic pressure to reduce cell damage under high temperature stress (Zhao et al. 2006). Between 6 and 12 h of stress treatment, sucrose and glucose content decreased, whereas fructose content continuously increased in M. sativa ‘Deqin’. We speculate that M. sativa ‘Deqin’ responded to high root-zone temperature by converting sucrose to fructose. In summary, the physiological changes in M. sativa ‘Deqin’ indicate that high root-zone temperature causes damage within the first 0.5 h of stress treatment.
UHPLC-Triple-TOF–MS/QqQ-MS for Pseudotargeted Metabolomics Study in M. sativa
Stability remains a common issue in LC–MS, which limits its application in metabolic studies (Luo et al. 2016). In the present study, an integrated method based on UHPLC combined with two MS techniques (Triple-TOF–MS and QqQ-MS) was used for the first time to our knowledge to perform a pseudotargeted metabolomics study on M. sativa. After UHPLC separation, high-resolution Triple-TOF–MS was used to perform secondary MS scanning with ultra-high coverage to obtain MS2 spectra of “all metabolites” from the leaves and roots of M. sativa ‘Deqin’. Parent ions and daughter ions in MS2 spectra were analyzed to generate a specific MRM database. Then, QqQ-MS was used to perform accurate qualitative and quantitative analyses of metabolites in the MRM database. Using the integrated method, we obtained high-stability results from M. sativa ‘Deqin’. The total ion chromatograms and OPLS-DA results showed that UHPLC-Triple-TOF–MS/QqQ-MS used in this study was a reliable method for pseudotargeted metabolomics.
The total ion chromatograms of metabolites in the leaves and roots of M. sativa ‘Deqin’ showed a high stability in terms of wider polarity range, higher sensitivity, and better repeatability than other UPLC-Q-TOF–MS and UHPLC-QqQ-MS studies (Xu et al. 2021; Yan et al. 2019). Furthermore, the column filter used in the UHPLC technique was < 2.0 µm, which improved the column voltage to over 15,000 psi, which is considerably higher than that in traditional UPLC (5800 psi). These conditions are beneficial to increase the width of peaks, maintain the separation ability, and reduce the LC analyses time during sample analysis (Kenneth 2011). Given the advantages of UHPLC, this technique is well suitable for combined application with high-resolution Triple-TOF–MS. Thus, pseudotargeted metabolomics based on UHPLC-Triple-TOF–MS/QqQ-MS provides a fundamental solution for the analysis of heat resistance mechanisms of M. sativa ‘Deqin’.
Phenylpropanoids and Polyketides in M. sativa ‘Deqin’ Leaves and Roots in Response to High Root-Zone Temperature
Phenylpropanoids and flavonoids are two major classes of plant metabolites that play a key role in inhibiting the production of ROS against abiotic stress (Commisso et al. 2016). Under high root-zone temperature stress, the relative content of kaempferol and quercetin (involved in flavone and flavonol biosynthesis) in M. sativa ‘Deqin’ roots decreased from 0 to 0.5 h of stress treatment, whereas the relative content of quercetin in the leaves increased simultaneously. Flavonoids are one of the most important plant pigments that can potentially combat oxidative stress to protect the plants under heat stress (Austen et al. 2019; Brenda 2002; Heim et al. 2002). A previous study showed that high temperature stress induced the flavonoid production in sugarcane (Saccharum officinarum) (Wahid and Close 2007) for thermotolerance, which supports our result of decreased kaempferol and quercetin content in M. sativa ‘Deqin’ roots. We speculate that kaempferol and quercetin were used to eliminate ROS as the corresponding H2O2 content increased from 0 to 0.5 h of stress treatment.
After restoring the root-zone temperature to 22 °C for 6 h, the relative content of 5-hydroxyferulate and l-phenylalanine (involved in phenylpropanoid biosynthesis) in M. sativa ‘Deqin’ roots decreased and increased, respectively, whereas the relative content of naringenin, naringenin chalcone, and quercetin in the leaves increased. It is known that phenylpropanoid biosynthesis is activated in plants under abiotic stress, resulting in the accumulation of phenolic compounds that have the potential to restore ROS-induced damage (Sharma et al. 2019). We speculate that high root-zone temperature activated l-phenylalanine biosynthesis in the roots and naringenin, naringenin chalcone, and quercetin biosynthesis in the leaves of M. sativa ‘Deqin’.
Organic acids and Derivatives in M. sativa ‘Deqin’ Leaves and Roots in Response to High Root-Zone Temperature
Organic acids and derivatives can reduce abiotic stress-induced damage to plants by changing antioxidant enzyme activity, osmotic regulator content, and photosynthesis (Bao 2017). Here, we found that under high root-zone temperature stress, the relative content of citric acid and L-malic acid (in glyoxylate and dicarboxylate metabolism) in the roots of M. sativa ‘Deqin’ increased from 0 to 0.5 h of treatment, followed by a decrease from 0.5 to 6 h of treatment (in glyoxylate and dicarboxylate metabolism and citrate cycle). A similar increase in the relative content of citric acid and L-malic acid has been reported in Cynodon x magennisii under heat stress (Peng 2014). The citrate cycle plays a key role in producing ATP and providing carbon skeletons for various biosynthetic processes (Akram 2014). Our results indicate that M. sativa ‘Deqin’ responded to high root-zone temperature by rapidly increasing the biosynthesis of citric acid and L-malic acid in glyoxylate and dicarboxylate metabolism within 0.5 h, whereas glyoxylate and dicarboxylate metabolism and citrate cycle were regulated between 0.5 and 6 h, and the period of ATP supplementation was short.
Aminoacyl-tRNA biosynthesis, which involves 20 different enzymes, establishes the rules of genetic code and thus plays a key role in translation and gene expression (Naeem et al. 2019). In the present study, we observed an increase in the relative content of l-glutamine, l-lysine, l-tyrosine, and l-methionine (involved in aminoacyl-tRNA biosynthesis) in the leaves of M. sativa ‘Deqin’ from 0.5 to 6 h of stress treatment. As aminoacyl-tRNA biosynthesis was shown to be upregulated in Saccharina latissimi (Sandra et al. 2012) under abiotic stress, the increase in the l-glutamine, l-lysine, l-tyrosine, and l-methionine content may be related to the equilibrium of the expression of stress-and growth-related genes to the removal and replacement of damaged proteins in the leaves (Sandra et al. 2012).
Conclusions
The present study reveals the whole-plant morphological, physiological, and metabolic changes of M. sativa ‘Deqin’ under high root-zone temperature. We identified several pathways that are crucial to resist the high root-zone temperature stress in M. sativa ‘Deqin’, such as glyoxylate and dicarboxylate metabolism, citrate cycle, aminoacyl-tRNA biosynthesis, flavone and flavonol biosynthesis, and phenylpropanoid biosynthesis. They mainly included phenylpropanoids and polyketides such as quercetin, kaempferol, and luteolin; organic acids and derivatives such as citric acid, L-malic acid, L-glutamine, and L-lysine; organoheterocyclic compounds such as riboflavin; and organic acids and derivatives such as succinic acid. The differential metabolites were primarily enriched in the seven metabolic pathways such as flavone and flavonol biosynthesis. Overall, this study reports the differential metabolites and pathways in the roots and leaves of ‘Deqin’ in response to high root-zone temperature, These findings expand the knowledge of heat stress mechanisms in alfalfa.
Data Availability
Datasets generated during and/or analyzed in the current study are available from the corresponding author on request.
References
Aditya B, Aryadeep R (2020) Seed priming as a method to generate heat-stress tolerance in plants. Heat stress tolerance in plants. Wiley, New Jersey, pp 23–32
Akram M (2014) Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68:475–478. https://doi.org/10.1007/s12013-013-9750-1
Akula R, Aswathanarayana RG (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Austen N, Walker HJ, Lake JA, Phoenix GK, Cameron DD (2019) The regulation of plant secondary metabolism in response to abiotic stress: interactions between heat shock and elevated CO2. Plant Metab Chemodivers 10:1–12. https://doi.org/10.3389/fpls.2019.01463
Bao YZ (2017) Comparative metabolomics analysis of Dongnongdongmai 1 response to low temperature stress. Dongbei Agricultural University
Barker DG, Pfaff T, Moreau D, Groves E, Ruffel S, Lepetit M, Whitehand S, Maillet F, Nair RM, Journet EP (2006) Growing M. truncatula: choice of substrates and growth conditions. In: Mathesius U, Journet EP, Sumner LW (eds) The Medicago truncatula handbook. Noble Research Institute, Oklahoma
Bi YF, Li JR, Che WG (2005) Studies on nutrition value of less fall dormancy alfalfa. J Yunnan Agric Univ 20(2):252–257
Bi YF, Che WG, Gu L (2007) Polymorphic characteristics and origin analysis of wild alfalfa population (Medicago sativa L.) in Deqin area. Acta Agrestia Sin 15:306–311
Brenda WS (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223. https://doi.org/10.1016/s1369-5266(02)00256-x
Chen W, Gong L, Guo ZL, Wang WS, Zhang HY, Liu XQ, Yu SB, Xiong LZ, Luo J (2013) A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol Plant 6:1769–1780. https://doi.org/10.1093/mp/sst080
Commisso M, Toffali K, Strazzer P, Stocchero M, Ceoldo S, Baldan B, Levi M, Guzzo F (2016) Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Plant Cell Biol 7:1–16. https://doi.org/10.3389/fpls.2016.01439
Das A, Rushton PJ, Rohila JS (2017) Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 6:1–21. https://doi.org/10.3390/plants6020021
Dietze MC, Sala N, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687
Gao LH, Li SJ (1996) Mechanism of optimal root-zone temperature relieving heat injury in shoot of lettuce. J Nanjing Agric Univ 19:34–39
Guo CY, Yu F (2003) Effect of root temperature on seedling growth of colour pepper. Acta Agric Univ Jiangxiensis 25:30–32
Han YP, Li YL, Li AP, Jia XS, Song ML (2015) The influence of defferent root temperature treatment on tomato plant growth. J Shanxi Agric Sci 43:677–678. https://doi.org/10.3969/j.issn.1002-2481.2015.06.12
Hatfiled JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
He YL, Shen J, Wang HL (1997) Studies on heat tolerance mechanism of coll season turf grass. J Shanghai Agric Coll 5:128–132
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584. https://doi.org/10.1016/s0955-2863(02)00208-5
Hou JH, Li Q, Xu XJ, Xu YK, Zhou J (2015) Prediction model for soil temperature by statistical regression of air temoerature. J Zhejiang for Sci Technol 35:55–59
Huang JW (2009) Physiological responses and molecular mechanism of Zoysiagrass to remperature stress. Fujian Agriculture and Forestry University, Fuzhou
Jin R, Wang YP, Liu RJ, Gou JB, Chan ZL (2016) Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Plant Biotechnol 6:1–11. https://doi.org/10.3389/fpls.2015.01123
Kenneth JF (2011) UPLC versus UHPLC: comparison of loading and peak capacity for small molecule drugs. Waters application note 720003869EN: 1–6
Kozlowski TT (1992) Carbohydrate sources and sinks in woody-plant. Bot Rev 58:107–222. https://doi.org/10.1007/bf02858600
Kumar S, Thakur P, Kaushal N, Malik JA, Gaur P, Nayyar H (2013) Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch Agron Soil Sci 59:823–843. https://doi.org/10.1080/03650340.2012.683424
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing
Li H, Su JL, Li C, Xiao Z, He LS (2018a) Physiological response and heat tolerance of different Rhododendron cultivars under high temperature stress. J Jiangsu for Sci Technol 45:1–19
Li MN, Zhang YW, Bi YF, Zhao Y (2018b) Effect of high rhizosphere temperature stress on morphological characteristics and water status of Medicago sativa L ‘Deqin.’ J West China for Sci 47:22–28. https://doi.org/10.16473/j.cnki.xblykx1972.2018.06.004
Liu LJ, Lin LD (2020) Effect of heat stress on Sargassum fusiforme leaf metabolome. J Plant Biol 63:229–241. https://doi.org/10.1007/s12374-020-09247-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luo P, Yin PY, Zhang WJ, Zhou L, Lu X, Lin XH, Xu GW (2016) Optimization of large-scale pseudotargeted metabolomics method based on liquid chromatography-mass spectrometry. J Chromatogr A 1437:127–136. https://doi.org/10.1016/j.chroma.2016.01.078
Ma YH, Wang YH, Wang L, Wan MC, Zou YJ (2010) Effects of high temperature stress on lipid peroxidation and ascorbic acid content in apple leaves. Guizhou Agric Sci 38(176–178):183
Marias DE, Meinzer FC, Woodruff DR, McCulloh KA (2017) Thermotolerance and heat stress responses of Douglas-fir and ponderosa pine seedling populations from contrasting climates. Tree Physiol 37:301–315. https://doi.org/10.1093/treephys/tpw117
Miransari M (2016) Soybeans, stress, and plant growth-promoting rhizobacteria. Environmental stresses in soybean production. Elsevier, Amsterdam, pp 177–203
Mo YW, Liang GB, Shi WQ, Xie JH (2011) Metabolic responses of alfalfa (Medicago sativa L.) leaves to low and high temperature induced stresses. Afr J Biotechnol 10:1117–1124
Moon JH, Boo HO, Jang IO (2007) Effect of root-zone temperature on water relations and hormone contents in cucumber. Hortic Environ Biotechnol 48:257–264
Naeem K, Asghari B, Ali BMD (2019) Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 14:1–21. https://doi.org/10.1371/journal.pone.0213040
Peng HT (2014) Study on accumulation of metabolites and expression of heat-tolerant proteins in leaves and roots of hybrid bermudagrass under heat stress. Shanghai Jiao Tong University, Shanghai
Ravi S, Young T, Cate M-N, Nyugen TV, Duxbury M, Alfaro AC, Leuzinger S (2020) Untargeted metabolomics in halophytes: the role of different metabolites in New Zealand mangroves under multi-factorial abiotic stress conditions. Environ Exp Bot 173:1–20. https://doi.org/10.1016/j.envexpbot.2020.103993
Raza A (2020) Metabolomics: a systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. https://doi.org/10.1007/s00299-020-02635-8
Raza A, Tabassum J, Kudapa H, Varshney RK (2021) Can omics deliver temperature resilient ready-to-grow crops? Crit Rev Biotechnol 41(8):1209–1232. https://doi.org/10.1080/07388551.2021.1898332
Sakae A, Naomi H, Fumitake K, Waichi A, Peter K (1995) Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.). J Fac Agric Kyushu Univ 40:233–240. https://doi.org/10.5109/24109
Sandra H, Klaus V, Stephan F, Uwe J, Christian W (2012) Transcriptomic analysis of acclimation to temperature and ligh stress in Saccharina latissima. PLoS ONE 7:e44342. https://doi.org/10.1371/journal.pone.0044342
Shahid H, Aamer MM (2011) Root zone temperature influences nutrient accumulation and use in maize. Pak J Bot 43:1551–1556
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:1–22. https://doi.org/10.3390/molecules24132452
Shen HF, Zhao B (2018) Study on evaluation of heat tolerance and its physiological mechanisms in Rhododendron cultivars. Plant Physiol J 54:335–345
Small E (2011) Alfalfa and relatives: evolution and classification of Medicago. NRC Research Press, Ottawa
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214. https://doi.org/10.1111/nph.15488
Song YG, Lv J, Ma ZQ, Dong W (2019) The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul 89:239–249. https://doi.org/10.1007/s10725-019-00530-1
Vos RCHD, Moco S, Lommen A, Keurentjes JJB, Bino RJ, Hall RD (2007) Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat Protoc 2:778–791. https://doi.org/10.1038/nprot.2007.95
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109. https://doi.org/10.1007/s10535-007-0021-0
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang RM, Xiong XY (2016) Effect of temperature stress on growth and metabolism in perennial ryegrass. Acta Pratacul Sin 25:81–90
Wang H, Zhao Y, Shao CG, Wang HZ, Bi YF (2013) Effects of high temperature stress on morphological indexes and physiological characteristics of Deqin alfalfa. J Yunnan Agric Univ 28(006):765–768
Wang SG, Lin SY, Ding YL (2014) Studies on the adaptive physiological changes of Fargesia yunnanensis leaves in long-term high temperature environment. J Nanjing for Univ Nat Sci Ed 38:87–90
Wassie M, Zhang WH, Zhang Q, Ji K, Chen L (2019) Effect of heat stress on growth and physiological traits of Alfalfa (Medicago sativa L.) and a comprehensive evaluation for heat tolerance. Agronomy 9:597–617. https://doi.org/10.3390/agronomy9100597
Wedow JM, Yendrek CR, Mello TR, Creste S, Martinez CA, Ainsworth EA (2019) Metabolite and transcript profiling of Guinea grass (Panicum maximum Jacq) response to elevated [CO(2)] and temperature. Metab off J Metab Soc 15:51. https://doi.org/10.1007/s11306-019-1511-8
Wu D (2015) Effect of different light, sowing rate and root temperature on growth and development of rice seedlings. Nanjing Agricultural University, Nanjing
Xu XF, Qu WJ, Jia Z, Han T, Liu MN, Bai YY, Wang M, Lin RC, Hua Q, Li XR (2021) Effect of cultivation ages on anti-inflammatory activity of a new type of red ginseng. Biomed Pharmacother 136:111280–111280. https://doi.org/10.1016/j.biopha.2021.111280
Yagut A, Natalia B, Mikael B, Hiroaki F, Saijaliisa K, Paula M, Pekka MA, Kaisa N, Kirk O, Jarkko S, Michael W (2015) Integration of photosynthesis, development and stress as an opportunity for plant biology. New Phytol 208:647–655. https://doi.org/10.1111/nph.13549
Yan N, Du YM, Liu XM, Chu MJ, Shi J, Zhang HB, Liu YH, Zhang ZF (2019) A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chem 275:618–627. https://doi.org/10.1016/j.foodchem.2018.09.153
Ye SH (2004) Experiment course of plant physiology and biochemistry. Yunnan Science and Technology Press, Kunming
Zhang YJ (1977) Determined Glucose, fructose, sucrose and starch in fruits and vegetables by anthrone spectrophotometry. Chin J Anal Chem 5:12–15
Zhang SN, Wang SK, Xu YX, Yu CL, Shen CJ, Qian Q, Geisler M, Jiang DA, Qi YH (2015) The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ 38:638–654. https://doi.org/10.1111/pce.12397
Zhao Y (2014) Research on heat tolerance relative protein of Medicago sativa L. ‘Deqin.’ Yunnan Agricultural University, Kunming
Zhao JT, Li XF, Li H, Xu RM (2006) Research on the role of souble sugar in the regulation of physiological metabolism in higher plant. J Anhui Agric Sci 34:6423–6425
Zhao SQ, Hu J, Guo LB, Qian Q, Xue HW (2010) Rice leaf inclination2, a VIN3-like protein, regulates leaf angle through modulating cell division of the collar. Cell Res 20:935–947. https://doi.org/10.1038/cr.2010.109
Zhao LL, Wang PC, Chen C, Ma L (2013a) Physiological response and heat tolerance evaluation of Lotus corniculatus under high temperature stress. Pratacul Sci 30:2018–2023
Zhao SQ, Xiang JJ, Xue HW (2013b) Studies on the rice leaf inclination (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control. Mol Plant 6:174–187. https://doi.org/10.1093/mp/sss064
Acknowledgements
We would express our heartfelt thanks to Professor Yufen Bi, Faculty of Animal Science and Technology in Yunnan Agriculture University, China, for the M. sativa ‘Deqin’ seeds supplying of this study. We are grateful to BioTree Biotechnology Co., Ltd. Shanghai, China for the suggestions of metabonomic analysis.
Funding
This research project is funded by the National Natural Science Foundation of China (Grant No. 31660683).
Author information
Authors and Affiliations
Contributions
Yan Zhan designed the experiments, and collected the material, as well as performed the preparatory experiments. YuZ performed the experiments and analyzed the data. YaZ, YuZ and XL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors have read the MS and approved.
Additional information
Handling Editor: Mohammad Irfan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Li, X. & Zhao, Y. Pseudotargeted Metabolomics Reveals the Effects of High Root-Zone Temperature Stress on Leaves and Roots of Medicago sativa. J Plant Growth Regul 42, 5667–5682 (2023). https://doi.org/10.1007/s00344-023-10949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10949-6