Abstract
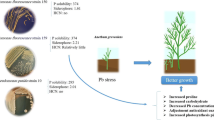
Atriplex lentiformis is a halophytic plant species used for desalination and phytoremediation. The plant tolerates abiotic constraints, such as salinity, drought, and toxic metals. It is also used as a fodder for domestic animals. It grows luxuriantly at 100–400-mM NaCl concentrations without any toxic symptoms. In the present investigation effects of biological amendments—PGPB (Plant growth-promoting bacteria—Bradyrhizobium japonicum—NCIM5350 and Pseudomonas fluorescens—NCIM2100), organic manure (OM), and chemical amendment—Ethylene Diamine Tetra Acetic acid (EDTA) on Atriplex lentiformis were explored in cadmium- and nickel-contaminated soil. Heavy metal resistance and plant growth-promoting traits of PGPB were also analyzed. Augmentation with a combination of both PGPB and OM, A. lentiformis displayed maximum uptake of Ni (45.67 mg kg−1 in roots; 24.68 mg kg−1 in shoots) and Cd (14.15 mg kg−1 in roots; 7.19 mg kg−1 in shoots). Highest Ni uptake in shoots was observed under the EDTA amendment (25.33 mg kg−1). Metal uptake by A. lentiformis under NCIM2100 was greater than NCIM5350 for both Cd and Ni (10.57 and 43.87 mg kg−1). Among all the amendments highest metal uptake was recorded under bio-organic treatments (PGPB1 + PGPB2 + OM) for both Cd and Ni (14.15 and 45.67 mg kg−1), respectively. The results showed that this association has significantly improved the plant height, biomass, chlorophyll, MDA (Malondialdehyde) content, and the activity of antioxidative enzymes (CAT, APX, and SOD) which exhibited a positive correlation with metal uptake at 1% level of significance and the potency of synergistic impact of microbial consortium, while EDTA reduced the growth of the plant. Metal uptake under EDTA was also much lower than biological amendments. Higher metal values in roots establishes A. lentiformis as a phytostabilizer thus indicating its suitability as a safer forage. Biological amendments-based phytoremediation holds great promise and could be used in future to give further impetus to the antioxidative defense, phytoremedial potential, and growth of this and other important forage plants.







Similar content being viewed by others
Data Availability
Not applicable.
References
Adekiya AO, Ejue WS, Olayanju A (2020) Different organic manure sources and NPK fertilizer on soil chemical properties, growth, yield and quality of okra. Sci Rep 10:16083
Aebi H (1984) Catalase in Vitro. Methods Enzymol 105:121–126
Agency for Toxic Substances and Disease Registry ATSDR (2019) Substance priority list. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service
Ahemad M (2019) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem 12(7):1365–1377. https://doi.org/10.1016/j.arabjc.2014.11.020
Ahmad B, Zaid A, Sadiq Y, Bashir S, Wani SH (2019) Role of selective exogenous elicitors in plant responses to abiotic stress tolerance. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, Cham, pp 273–290. https://doi.org/10.1007/978-3-030-06118-0_12
Ahmad B, Zaid A, Zulfiqar F, Bovand F, Dar TA (2022) Nanotechnology: A novel and sustainable approach towards heavy metal stress alleviation in plants. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-022-00230-8
Al-Aqeel H, Vinod K (2016) Heavy metal uptake efficiency of Alfalfa, Barley, Indian Mustard and Atriplex from contaminated desert soil. Int J of Tropi Agri 34:2403–2406
Al-Dhabi NA, Esmail GA, Mohammed GAK, Valan AM (2019) Optimizing the management of cadmium bioremediation capacity of metal-resistant Pseudomonas sp. Strain Al-Dhabi-126 isolated from the industrial City of Saudi Arabian environment. Int J Environ Res Public Health 16:4788. https://doi.org/10.3390/ijerph16234788
Alsohim AS (2020) Influence of Pseudomonas fluorescens mutants produced by transposon mutagenesis on in vitro and in vivo biocontrol and plant growth promotion. Egypt J Biol Pest Control 30:19. https://doi.org/10.1186/s41938-020-00220-5
Ambreetha S, Chinnadurai C, Marimuthu P, Balachandar D (2018) Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 5:57–66
Amjad A, Di G, Amanullah M, Fang M, Ronghua L, Feng S, Ping W, Zengqiang Z (2017) Streptomyces pactum assisted phytoremediation in Zn/Pb smelter contaminated soil of Feng County and its impact on enzymatic activities. Sci Rep 7:46087
Baker AJM, Walker PL (1990) Ecophysiology of metal uptake by tolerant plants, heavy metal tolerance in plants. In: Shaw AJ (ed) Evolutionary aspects. CRC, Boca Raton, pp 155–177
Barnawal D, Maji D, Bharti N, Chanotiya CS, Kalra A (2013) ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul 32:809–822. https://doi.org/10.1007/s00344-013-9347-3
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bulent T, Nuri A, Houcine A, Reguieg Y (2019) Phytoremediation potential of Atriplex canescens (Pursh) Nutt and Nicotiana tabacum grown in heavy metal contaminated soil. International Conference on Food, Nutrition and Agriculture (ICFNA19). Istanbul (Turkey). Conference Book.
Chellaiah E (2018) Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl Water Sci 8:154
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins, roles in heavy metal detoxification and homeostasis. Ann Rev Plant Biol 53:159–182
Cui LM, Wang YG, Gao L, Hu LH, Yan LG, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II), and Cu(II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10
Cushman JC (2001) Osmoregulation in plants: implications for agriculture. Amer Zool 41:758–769. https://doi.org/10.1093/icb/41.4.758
Del Río LA, Sandalio LM, Altomare DA, Zilinskas BA (2003) Mitochondrial and peroxisomal manganese superoxide dismutase: Differential expression during leaf senescence. J Exp Bot 54:923–933. https://doi.org/10.1093/jxb/erg091
Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E (2010) “In situ” phytostabilization of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth-promoting rhizobacteria. J Hazard Mater 177:323–330
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dutta P, Karmakar A, Majumdar S, Roy S (2018) Klebsiella pneumonia (HR1) assisted alleviation of Cd (II) toxicity in Vigna mungo: a case study of biosorption of heavy metal by an endophytic bacterium coupled with plant growth promotion. Euro-Medi J Environ Integ 27:1–10. https://doi.org/10.1007/s41207-018-0069-6
Egamberdieva D, Berg G, Lindström K, Räsänen LA (2013) Alleviation of salt stress of symbiotic Galega officinalis L (Goat’s Rue) by co-inoculation of rhizobium with root colonizing Pseudomonas. Plant Soil 369:453–546
Egamberdieva D, Wirth S, Jabborova D, Leena AR, Liao H (2017) Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interact 12:100–107. https://doi.org/10.1080/17429145.2017.1294212
Errabii T, Gandanou CB, Essalmani H, Abrini J, Idamor M, Senhaji NS (2007) Effect of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiol Plant 29:95–102. https://doi.org/10.1007/s11738-006-0006-1
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963. https://doi.org/10.1111/j.1469-8137.2008.02531.x
Garnier L, Françoise SP, Patrice T, Jean-Pierre A, Jean-pierre B, Raoul R, Jean-luc M (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell and Environ 29:1956–1969. https://doi.org/10.1111/j.1365-3040.2006.01571.x
Ghassemi HR, Mostajeran A (2018) TASOS1 and TATM20 genes expression and nutrient uptake in wheat seedlings may be altered via excess cadmium exposure and inoculation with Azospirillum brasilense sp7 under saline condition. Appl Ecol Environ Res 16:1797–1817
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gopal R, Rizvi AH (2008) Excess lead alters growth, metabolism, and translocation of certain nutrients in radish. Chemosphere 70:1539–1544. https://doi.org/10.1016/j.chemosphere.2007.08.043
Gorden SA, Paleg LG (1957) Observations on the quantitative determination of indole acetic acid. Physiol Plant 10:39–47. https://doi.org/10.1111/j.1399-3054.1957.tb07608.x
Gratão PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LEP, Medici LO, Lea PJ, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67:387–394. https://doi.org/10.1016/j.envexpbot.2009.06.017
Gupta P, Rani R, Chandra A, Kumar V (2018) Potential applications of Pseudomonas sp. (strain CPSB21) to ameliorate Cr6þ stress and phytoremediation of tannery effluent contaminated agricultural soils. Sci Rep 8:4860
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front Microbiol 10:1506. https://doi.org/10.3389/fmicb.2019.01506
HazDat (2006) HazDat database: ATSDR’s Hazardous Substance Release and Health Effects Database. Atlanta, GA: Agency for Toxic Substances and Disease Registry. www.atsdr.cdc.gov/hazdat.html.
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I-Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agr Biol Chem 42:1825–1831. https://doi.org/10.1080/00021369.1978.10863261
Ishtiyaq S, Kumar H, Varun M, Kumar B, Paul MS (2018) Heavy metal toxicity and antioxidative response in plants: an overview: responses, tolerance, and remediation. In: Hasanuzzaman M, Nahar K, Fujita M (eds) Plants under metal and metalloid stress. E-Publishing Inc. Springer Intern Publishing, Switzerland, pp 77–106
Ishtiyaq S, Kumar H, Clement OO, Varun M, Paul MS (2021) Role of secondary metabolites in salt and heavy metal stress mitigation by halophytic plants: An overview. In: Hasanuzzaman M, Prasad MNV (eds) Handbook of bioremediation. E-Publishing Inc. Academic Press, Cambridge, pp 307–321
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 104:285–293. https://doi.org/10.1016/j.ecoenv.2014.03.008
Jahanian A, Chaichi MR, Rezaei K, Rezayazdi K, Khavazi K (2012) The effect of plant growth-promoting rhizobacteria (PGPR) on germination and primary growth of Artichoke (Cynara scolymus). Int J Agric Crop Sci 4:923–929
Jebara SH, Chiboub M, Jebara M (2018) Antioxidant responses and gene level expressions of Sulla coronaria inoculated by heavy metals resistant plant growth promoting bacteria under cadmium stress. In: Kallel A et al (eds) Recent advances in environmental science from the euro-mediterranean and surrounding regions, advances in science, technology & innovation. Springer International Publishing, Berlin. https://doi.org/10.1007/978-3-319-70548-4_106,2018
Kang SM, Shahzad R, Bilal S, Khan AL, Park YG, Lee KE, Lee IJ (2019) Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol 19(1):80
Karimpour M, Ashraf SD, Taghavi K, Mojtahedi A, Roohbakhsh E, Naghipour D (2018) Adsorption of cadmium and lead onto live and dead cell mass of Pseudomonas aeruginosa: a dataset. Data Brief 18:1185–1192. https://doi.org/10.1016/j.dib.2018.04.014
Karthik C, Oves M, Thangabalu R, Sharma R, Santhosh SB, Arulselvi PI (2016) Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under chromium (VI) toxicity. J Adv Res 7:839–850
Karthikeyan B, Jaleel CA, Gopi R, Deiveekasundaram M (2007) Alterations in seedling vigour and antioxidant enzyme activities in Catharanthus roseus under seed priming with native diazotrophs. J Zhejiang Univ Sci 8:453–457. https://doi.org/10.1631/jzus.2007.B0453
Katznelson H, Bose B (1959) Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Can J Microbiol 5:79–85. https://doi.org/10.1139/m59-010
Kelvin RP, Víctor MC, Julio CAO, Warren RR (2020) Bioavailability and solubility of heavy metals and trace elements during composting of cow manure and tree litter. Appl Envir Soil Sci. https://doi.org/10.1155/2020/5680169
Khanna K, Jamwal VL, Kohli SK, Gandhi SG, Ohri P, Bhardwaj R, Allah EFA, Hashem A, Ahmad P (2019) Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 217:463–474. https://doi.org/10.1016/j.chemosphere.2018.11.005
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. Adv Microb Physiol 71:97–132. https://doi.org/10.1016/bs.ampbs.2017.04.001
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:10438. https://doi.org/10.1016/j.apgeochem.2019.104388
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Liphadzi MS, Kirkham MB (2006) Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. S Afr J Bot 72:391–397
Liu X, Wang C, Su Q (2013) Screening for salt tolerance in eight halophyte species from yellow river delta at the two initial growth stages. ISRN Agronomy 1:1–8. https://doi.org/10.1155/2013/592820
Lokhande VH, Nikam TD, Suprasanna P (2010) Differential osmotic adjustment to iso-osmotic salt and PEG stress in vitro in the halophyte Sesuvium portulacastrum L. J Crop Sci Biotechnol 13(4):251–256. https://doi.org/10.1007/s12892-010-0008-9
Lokhande VH, Mulye K, Patkar R, Nikam TD, Suprasanna P (2013) Biochemical and physiological adaptations of the halophyte Sesuvium portulacastrum L (Aizoaceae) to salinity. Arch Agron Soil Sci 59:1373–1391. https://doi.org/10.1080/03650340.2012.712207
Luttge U, Smith JAC (1984) Structural, biophysical, and biochemical aspects of the role of leaves in plant adaptation to salinity and water stress. In: Staples RC, Toenniessen GH (eds) Salinity tolerance in plants: strategies for crop improvement. Wiley, New York, pp 125–150
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918. https://doi.org/10.3389/fpls.2016.00918
Maria BGSF, Luana CC, Andreza RBF, Júlia KS, Karen CFS (2010) Isolation of Atriplex nummularia-associated halotolerant bacteria and bioprospecting by nitrogen-fixing bacteria in saline-sodic soil. 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia. pp. 1–4
Maxton A, Singh P, Masih SA (2018) ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J Plant Nutr 41(5):574–583
Mesa-Marín J, Pérez-Romero JA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID, Mateos-Naranjo E (2020) Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Frontiers in Microbiol. https://doi.org/10.3389/fmicb.2020.553018
Meyer R (2005) Atriplex lentiformis. In: Fire Effects Information System [Online] U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). http://www.fs.fed.us/database/feis.
Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52:464–469
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140. https://doi.org/10.1093/oxfordjournals.pcp.a077268
Nascimento CWA, Amarasiriwardena D, Xing B (2006) Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ Pollut 140:114–123
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489. https://doi.org/10.4236/ajps.2012.310178
Nazir F, Hussain A, Fariduddin Q (2019) Interactive role of epibrassinolide and hydrogen peroxide in regulating stomatal physiology, root morphology, photosynthetic and growth traits in Solanum lycopersicum L. under nickel stress. Environ Exp Bot 162:479–495
Olsen SR, Sommers LE (1982) Determination of available phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. E-Publishing Inc. American Society of Agronomy, Madison, pp 403–430
Orozco-Mosqueda MC, Glick BR, Santoyo G (2020) ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol Res 235:126439. https://doi.org/10.1016/j.micres.2020.126439
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth-promoting rhizobacteria. Plant Physiol 118:10–15. https://doi.org/10.1034/j.1399-3054.2003.00086.x
Piccini D, Malavolta E (1992) Effect of nickel on two common bean cultivars. J Plant Nut 15:2343–2350. https://doi.org/10.1080/01904169209364478
Rao KVM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L.) Millspauga in response to Zn and Ni stress. Plant Sci 157:113–128. https://doi.org/10.1016/s0168-9452(00)00273-9
Rizvi A, Ahmed B, Zaidi A, Khan MS (2019) Bioreduction of toxicity influenced by bioactive molecules secreted under metal stress by Azotobacter chroococcum. Ecotoxicol 28:302–322. https://doi.org/10.1007/s10646-019-02023-3
Rizwan M, Ali S, Abbas T, Rehman MZ, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Safety 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Rodrigues AC, Bonifacio A, Antunes JEL, Silveira JAG, Figueiredo MVB (2013) Minimization of oxidative stress in cowpea nodules by the interrelationship between Bradyrhizobium spp and plant growth-promoting bacteria. Appl Soil Ecol 64:245–251. https://doi.org/10.1016/j.apsoil.2012.12.018
Rowe DR, Abdel-Magid IM (1995) HandBook of waste water reclamation. CRC Press Inc, Boca Raton, p 550
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Saleh M, Saleh AG (2006) Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. Afr J Biotechnol 5:133–142
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Seneviratne M, Gunaratne S, Bandara T, Weerasundara L, Rajakaruna N, Seneviratne G, Vithanage M (2016) Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S Afri J of Bot 105:19–24. https://doi.org/10.1016/j.sajb.2016.02.206
Seniyat LA, Lesley CB (2019) Effect of plant growth-promoting bacterium; Pseudomonas putida UW4 inoculation on phytoremediation efficacy of monoculture and mixed culture of selected plant species for PAH and lead spiked soils. Int J of Phytorem 21:200–208. https://doi.org/10.1080/15226514.2018.1501334
Seyed MM, Babak M, Hossein MH, Hoseinali A, Ali AZ (2018) Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol Environ Saf 147:206–216
Short DC, Colmer TD (1999) Salt tolerance in the halophyte Halosarcia pergranulata subsp. pergranulata. Annu Bot 83:207–213. https://doi.org/10.1006/anbo.1998.0812
Slama I, Ghnaya T, Savouŕe A, Abdelly C (2008) Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. C R Biol 331:442–451. https://doi.org/10.1016/j.crvi.2008.03.006
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A (2015) Diversity, distribution, and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447. https://doi.org/10.1093/aob/mcu239
Soliz D, Edward G, Seaman RB, Yoklic M, Nelson SG, Brown P (2011) Water consumption, irrigation efficiency and nutritional value of Atriplex lentiformis grown on reverse osmosis brine in a desert irrigation district. Agric Ecosyst Environ 140:473–483. https://doi.org/10.1016/j.agee.2011.01.012
Suprasanna P, Rai AN, Kumari HP, Kumar SA, Kishor KPB (2014) Modulation of proline: implications in plant stress tolerance and development. In: Anjum NA, Gill SS, Gill R (eds) Plant adaptation to environmental change. CABI Publishers, Wallingford, pp 68–93
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Szopiński M, Sitko K, Gieroń Ż, Rusinowski S, Corso M, Hermans C, Verbruggen N, Małkowski E (2019) Toxic effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front Plant Sci 10:748. https://doi.org/10.3389/fpls.2019.00748
Takahashi R, Shimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice plant. Cell Environ 35:1948–1957
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tang X, Zeng G, Fan G, Zhou M, Tang L, Zhu J, Wan J, Huang D, Chen M, Xu P, Zhang C, Lu Y, Xiong W (2018) Chromosomal expression of CadR on Pseudomonas aeruginosa for the removal of Cd (II) from aqueous solutions. Sci Tot Environ 636:1335–1361. https://doi.org/10.1016/j.scitotenv.2018.04.229
Tank N, Saraf M (2009) Enhancement of plant growth and decontamination of nickel spiked soil using PGPR. J Basic Microbiol 49:195–204. https://doi.org/10.1002/jobm.200800090
Toth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Pollution 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Treesubsuntorn C, Dhurakit P, Khaksar G, Thiravetyan P (2018) Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.). Environ Sci Pollut Res 25:25690–25701
Tripathi RP, Singh B, Bisht SS, Pandey J (2009) L-Ascorbic acid in organic synthesis: an overview. Curr Org Chem 13:99–122. https://doi.org/10.2174/13852720978719379.2
Van Engelen DL, Sharpe-Pedler RC, Moorhead KK (2007) Effect of chelating agents and solubility of cadmium complexes on uptake from soil by Brassica juncea. Chemosphere 68:401–408
Vartika M, Antriksh G, Parvinder G, Simranjeet S, Nasib S, Praveen G, Joginder S (2016) Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int J Phytorem 18:697–703
Vivas A, Biro B, Ruiz-Lozano JM, Barea JM, Azcon R (2006) Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn toxicity. Chemosphere 52:1523–1533
Wan Y, Luo S, Chen J, Xiao X, Chen L, Zeng G, Liu C, He Y (2012) Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 89:743–750. https://doi.org/10.1016/j.chemosphere.2012.07.005
Wang LW, Showalter AM (2004) Cloning and salt-induced, ABA independent expression of choline mono-oxygenase in Atriplex prostrate. Physiol Plant 120:405–412. https://doi.org/10.1111/j.0031-93172004.00247.x
Wang Y, Wang H, Yang CH, Wang Q, Mei R (2007) Two distinct manganese-containing superoxide dismutase genes in Bacillus cereus: Their physiological characterizations and roles in surviving in wheat rhizosphere. FEMS Microbiol Lett 272:206–213. https://doi.org/10.1111/j.1574-6968.2007.00759.x
Wani P, Khan M, Zaidi A (2008) Effect of metal-tolerant plant growth-promoting Rhizobium on the performance of pea grown in metal amended soil. Ach Environ Contam Toxicol App Pharmacol 55:33–42. https://doi.org/10.1007/s00244-007-9097-y
Wani W, Masoodi KZ, Zaid A, Wani SH, Shah F, Meena VS, Wani SA, Mosa KA (2018) Engineering plants for heavy metal stress tolerance. Rendiconti Lincei. Scienze Fisiche e Naturali. 29:709–723
Weckx JEJ, Clijsters HMM (1996) Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol Plant 96:506–512
Weyens N, Van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598. https://doi.org/10.1016/j.tibtech.2009.07.006
Whiting SN, de Souza MP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Envirn Sci and Technol 35:3144–3150. https://doi.org/10.1021/es001938v
Wiangkham N, Prapagdee B (2018) Potential of Napier grass with cadmium-resistant bacterial inoculation on cadmium phytoremediation and its possibility to use as biomass fuel. Chemosphere 201:511–518. https://doi.org/10.1016/j.chemosphere.2018.03.039
Woldetsadik D, Drechsel P, Keraita B, Itanna F, Gebrekidan H (2017) Heavy metal accumulation and health risk assessment in wastewater-irrigated urban vegetable farming sites of Addis Ababa. Ethiopia Int J Food Contam 4:9. https://doi.org/10.1186/s40550-017-0053-y
Yokoi S, Bressan RA, Hasegawa PM (2002) Genetic engineering of crop plants for abiotic stress. In: Iwanaga M (ed) Salt stress tolerance of plants, The Japan International Centre for Agricultural Sciences (JIRCAS) Working Report No. 23, Tsukuba: Japan International Centre for Agricultural Sciences Publishing, Japan, pp 25–33
Yu X, Li Y, Zu C, Cui Y, Xiang Q, Gu Y, Zhau K, Zhang X, Penttinen P, Chen Q (2017) Pongamia pinnata inoculated with Bradyrhizobium liaoningense PZHK1 shows potential for phytoremediation of mine tailings. App Microbiol Biotechnol 101:1739–1751. https://doi.org/10.1007/s00253-016-7996-4
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Zaier H, Ghnaya T, Lakhdar A, Baioui R, Ghabriche R, Mnasri M, Sghair S, Lutts S, Abdelly C (2010) Comparative study of Pb phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: tolerance and accumulation. J Hazard Mater 183:609–615
Zaid A, Mohammad F, Fariduddin Q (2019a) Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol Mol Bio Plants 26:25–39
Zaid A, Mohammad F, Wani SH, Kadambot MH, Siddique, (2019b) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol Environ Saf 180:575–587
Zaid A, Wani SH (2019) Reactive oxygen species generation, scavenging and signaling in plant defense responses. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense signaling in growth and stress. Springer, Cham, pp 111–132. https://doi.org/10.1007/978-3-030-27165-7_7
Zaid A, Bhat ZA, Wani SH (2020) Influence of Metalloids and Their Toxicity Impact on Photosynthetic Parameters of Plants. In: Deshmukh R, Tripathi DK, Guerriero G (eds) Metalloids in Plants: Advances and Future Prospects. John Wiley, New York
Zaid A, Mohammad F, Siddique HM (2022) Salicylic acid priming regulates stomatal conductance, trichome density and improves cadmium stress tolerance in Mentha arvensis L. Front Plant Sci. https://doi.org/10.3389/fpls.2022.895427
Zeffa DM, Fantin LH, Koltun A, Andre LMO, Maria PBAN, Marcelo CG, Leandro SAG (2020) Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: a meta-analysis of studies from 1987 to 2018. Peer J 8:7905. https://doi.org/10.7717/peerj.7905
Zhang JS, Xie C, Li ZY, Chen SY (1999) Expression of the plasma membrane H+ ATPase gene in response to salt stress in a rice salt tolerant mutant and its original variety. Theoret Appl Genet 99:1006–1011
Acknowledgements
This study had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic Project UIDB/04292/2020 granted to MARE. We also thank University Grants Commission, New Delhi for providing funding (Grant sanction F. no. 43-100/2014 SR) for the work. Harsh Kumar is grateful for the fellowship under the project. Authors are thankful to the Principal, St. John’s College, Agra, India for providing the necessary facilities for conducting this research.
Funding
This study had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic Project UIDB/04292/2020 granted to MARE and also the University Grants Commission, New Delhi for providing funding (Grant sanction F. no. 43-100/2014 SR) for the work.
Author information
Authors and Affiliations
Contributions
HK conceptualized the study and designed the methodology of the experiment. HK, SI, and MV carried out the experiment, monitor the growth stages, and collected data. HK, SI, and MV were involved in the data analysis and drafting of manuscript. MV analyzed the data statistically and interpreted the results. PJCF and MSP supervised the overall study and reviewed the manuscript draft before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
The study has no ethical issue problem related to any person or organization.
Consent to Participate
Not applicable.
Consent for Publication
The author agreed freely to publish the present work in this journal.
Additional information
Handling Editor: M. Iqbal R. Khan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, H., Ishtiyaq, S., Favas, P.J.C. et al. Effect of Metal-resistant PGPB on the Metal Uptake, Antioxidative Defense, Physiology, and Growth of Atriplex lentiformis (Torr.) S.Wats. in Soil Contaminated with Cadmium and Nickel. J Plant Growth Regul 42, 3868–3887 (2023). https://doi.org/10.1007/s00344-022-10853-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10853-5