Abstract
Facing climate change, the development of innovative agricultural technologies securing food production becomes increasingly important. Plasma-treated water (PTW) might be a promising tool to enhance drought stress tolerance in plants. Knowledge about the effects of PTW on the physiology of plants, especially on their antioxidative system on a long-term scale, is still scarce. In this work, PTW was applied to barley leaves (Hordeum vulgare cv. Kosmos) and various constituents of the plants’ antioxidative system were analyzed 30 days after treatment. An additional drought stress was performed after foliar PTW application followed by a recovery period to elucidate whether PTW treatment improved stress tolerance. Upon PTW treatment, the Total Antioxidant Capacity (TAC) in leaves and roots was lower in comparison to deionized water treated plants. In contrast, PTW treatment caused a higher content of chlorophyll, quantum yield and total ascorbate content in leaves compared to deionized water treated plants. After additional drought application and subsequent recovery period, an enhancement of values for TAC, contents of malondialdehyde, glutathione as well as activity of ascorbate peroxidase indicated a possible upregulation of antioxidative properties in roots. Hydrogen peroxide and nitric oxide might mediate abiotic stress tolerance and are considered as key components of PTW.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Facing climate change and the continuous population growth, many challenges arise for securing the global demand of crops (Anderson et al. 2020). Climate change in particular risks food security due to the progressive occurrence of extreme weather events. Drought and desertification are two of the most pervasive ecological consequences and exacerbate quality and quantity of crop products (reviewed by Raza et al. 2019). Consequently, extensive strategies encompassing agricultural adaption to climate change are required to deal with climatic future challenges and to ensure food security (Pretty et al. 2010).
Cold atmospheric plasma (CAP) gained considerable attention as promising ‘green technology’ for future agricultural applications (Puač et al. 2018). Plasma is an ionized gas and referred as the fourth state of matter containing electrons, ions, neutral atoms and molecules, radicals, reactive species, different kinds of electromagnetic radiation (e.g., UV, visible light), and electric fields (Lu et al. 2016; Zhou et al. 2020). A huge variety of methods exists to generate CAP for treatment of biological targets under physiological temperatures, which range from the feed gas to electrical parameters to ignite plasma and configuration of the plasma devices (Šimek and Homola 2021; Zhou et al. 2020). Plasma-treated water (PTW) that is produced by exposing water to plasma, has the advantage that it can be generated in bigger quantities to treat plants roots or shoots and by that, omitting effects of, e.g., UV radiation or electromagnetic fields. The chemistry of PTW is based on complex reactions between plasma-gas–liquid interfaces (Graves et al. 2019; Thirumdas et al. 2018; Zhou et al. 2020). In principle, energy transferred to molecular oxygen- and nitrogen-containing gas leads to the generation of reactive oxygen and nitrogen species (RONS). Gaseous RONS, e.g., ozone (O3) or nitric oxide (NO), can diffuse to certain extend to the liquid but are relatively unstable due to further reactions. Hydrogen peroxide (H2O2), nitrite (NO2−), and nitrate (NO3−) ions accompanied with decrease in pH are frequently detected in PTW (Hu et al. 2021; Zhou et al. 2020). PTW has multifaceted effects on plants, comprising the activation of plant vitality, inactivation of phytopathogens, enhancing seed germination, and plant growth as well as influencing the antioxidative system (Ito et al. 2018; Zhou et al. 2020; Adhikari et al. 2020). Moreover, recent studies evaluated the effects of PTW to stimulate biotic and abiotic-related stress responses in barley (Gierczik et al. 2020), grapevine (Laurita et al. 2021), maize (Lukacova et al. 2021), periwinkle (Zambon et al. 2020), and tomato (Adhikari et al. 2019).
Plants perceive biotic as well as abiotic changes. If environmental changes extend in strain, outside influences may result in oxidative stress (Kranner et al. 2010; Demidchik 2015). Since plants are aerobic organisms and utilize molecular oxygen (O2) in several biochemical processes, stress metabolism leads to the enhanced generation of toxic byproducts called reactive oxygen species (ROS) which include singlet oxygen (1O2), superoxide anion (O2−), hydroxyl radical (OH•), and H2O2 (Choudhury et al. 2017; Mittler 2002). ROS naturally occur upon the partial reduction of O2 in many parts of the metabolism. The reactivity due to the high oxidizing potential can cause damage to nucleic acids, proteins, carbohydrates, and lipids. ROS triggers cell death by overwhelming the redox homeostasis if oxidative stress is severe (Bartosz 1997). If the damage cannot be reversed, programmed cell death might be initiated (Mittler 2002). In contrast, if kept in transient concentrations, ROS may also function as second messengers (Alscher et al. 1997; Foyer and Noctor 2005). They are responsible for fine-tuning several signal transduction processes involved in defense mechanisms against biotic and abiotic stresses (Dumanović et al. 2021). Besides ROS, it was shown that an imbalanced redox homeostasis also results in the production of reactive nitrogen species (RNS), essentially NO and derivates (Wang et al. 2013). NO is the most studied RNS and participates in many physiological processes of higher plants. Versatile interactions between ROS and RNS are noticeable (Astier et al. 2018). Under stress conditions, an accumulation or de-regulated synthesis of RNS can prevail, leading to nitrosative stress which is possibly involved in oxidative stress (Del Río 2015). Since plants are sessile and possess limited capabilities of stress avoidance, they developed a flexible scavenging system as an adaption to changing environmental conditions, which is universally referred to as the antioxidative system. The ascorbate–glutathione cycle plays an important role in the antioxidative system since it facilitates the efficient detoxification of H2O2 (Foyer and Halliwell 1976). It consists of the low molecular mass antioxidants ascorbic acid (Asc), glutathione and the enzymes ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR).
Typically, plants cope with drought stress by activating the antioxidative system as well as producing compatible solutes (also known as osmolytes) to counteract osmotic disequilibria (Fang and Xiong 2015). The upregulation of enzymatic and non-enzymatic antioxidants requires a complex network of signaling pathways which to date is still not completely understood. Next to phytohormones, interlinking molecules, protein kinases, and transcription factors, H2O2 and NO play a pivotal role in this signaling network (Qiao et al. 2014; Ilyas et al. 2020; Lau et al. 2021). Many strategies have been designed to improve drought tolerance in plants. An improved stress tolerance based on physiological or metabolic adjustment due to an earlier exposure to a mild stress is referred to as ‘priming’ (or ‘hardening’). It represents one of the most promising crop protection approaches for production of resilient crops (Li and Liu 2016). The exogenous application of certain compounds which can enhance tolerance to above-ground plant parts is a well-established method both in research and agriculture to improve the performance of crops (Merewitz 2016). Since primed plants show improved stress responses, this phenomenon is part of the concept of a ‘stress memory’ (Hilker and Schmülling 2019). Associated molecular mechanisms are still unclear (Li et al. 2019), although the same compounds involved in the signaling pathways for the ‘natural’ development of stress tolerance seem to participate in priming induced stress tolerance. The exogenous application of drought-responsive phytohormones, H2O2 and NO-donating chemicals resulted in enhanced drought tolerance (Molassiotis et al. 2016). A common feature for these signaling pathways are complex cross-talks between signaling components (Molassiotis and Fotopoulos 2011).
In this work, we investigated the long-term effects of PTW on the antioxidative system of Hordeum vulgare leaves and roots under no stress and drought stress conditions. Furthermore, it is discussed whether the foliar application of PTW, and particularly the PTW containing compounds H2O2 and NO, sustainably mediates drought stress tolerance.
Materials and Methods
Production of Plasma-Treated Water
The alternating current (AC)-driven plasma system used in this study consisted of a pin-to-liquid discharge configuration with four metal electrodes placed approx. 3 mm from the water surface (Schmidt et al. 2019). Deionized water (DW) was mixed with 7.5% (v/v) of tap water prior to plasma treatment, as sufficient concentration of ions (≥ 80 µS cm−1) was needed to ignite the plasma between electrodes and water surface. Water mixture of 900 ml was treated for 20 min. The spatial boundary layers between transient spark discharges, water surface, and ambient air led to the formation of reactive and excited nitrogen and oxygen species, which were transported into the water volume by constant stirring during treatment. PTW application to plants and physicochemical analysis were performed 5–10 min after treatment.
The pH of the water was measured using the pH 3210 m (WTW, Weilheim, Germany) and the conductivity with help of the TetraCon 325 electrode on an inoLab Multi Level3 with inoLab Terminal Level3 (WTW, Weilheim, Germany).
Determination of Nitrite and Nitrate Concentration in PTW
Nitrate and nitrite ions were analyzed by ion exchange chromatography using Dionex ICS 6000 system (Thermo Scientific, Dreieich, Germany) equipped with an anion-exchange column (Dionex IonPac AS 18, Thermo Scientific, Dreieich, Germany) and a guard column (Dionex IonPac AG 18, Thermo Scientific, Dreieich, Germany) according to manufactures instructions. Undiluted PTW was injected with 5 µl, ions were separated in 23 mM KOH at 0.25 ml min−1 under isocratic conditions, and conductivity signals were recorded. Concentration of ions was calculated based on a calibration curve established by Dionex 7-ion standard solution (Thermo Scientific, Dreieich, Germany). Ions were determined from four independent PTW solutions.
Determination of Hydrogen Peroxide Concentration in PTW
The level of H2O2 in PTW was determined with the potassium iodide (KI) method according to Junglee et al. (2014). The assay was performed within 1 ml test volume containing 500 µl 1 M KI in MES-KOH (50 mM, pH 6.0) 50 µl PTW and 450 ml H2Odeion. Absorbance was read at 350 nm after 30 min incubation at room temperature. A standard curve was obtained with H2O2 standard solution prepared in H2Odeion.
Determination of Nitric Oxide Release from PTW
Constant and specific measurements of gaseous NO were accomplished via ANALYZER LCD 88 sp (Eco Physics) chemiluminescence-based NO detector equipped with an ozone generator (Stöhr et al. 2001; Stöhr and Stremlau 2006). 500 µl PTW were placed on a petri dish (diameter 5 cm) within the custom-made reactor chamber. The experiment was performed at 30 °C under anoxic conditions, and sample was constantly stirred. N2 carrier gas transported the emitted NO with a flow rate of 400 ml min−1 (mass flow meter GFM ANALYT-MTC) to the analyzer. Data were recorded until NO was no longer detectable.
Plant Material and Cultivation
Seeds of Hordeum vulgare cv. Kosmos were pre-germinated in Petri dishes on filter paper soaked with 0.5 mM calcium sulfate for 2 days in dark. Each seedling was placed in a pot (∅ 12 cm) with a homogenous mixture of 2:1 (v:v) coarse-grained and fine-grained quartz sand. Plants were grown under a light/dark rhythm of 14/10 h at 22/18 °C air temperature, respectively. Dependent on the weather conditions, sunlight was supplemented by the light of high-pressure sodium lamps. The pots were rotated 2 times a week to ensure uniform growth conditions. All plants were watered daily with a defined nutrient solution containing 5 mM nitrate (Stöhr and Ullrich 1997) except for the drought period.
PTW Application and Drought Treatments
Four plant groups were treated as follows:
-
I.
“DW no stress” was sprayed with deionized water instead of PTW (DW: 1.7 ml) as a control 18, 19, and 20 days after sowing (DAS).
-
II.
“PTW no stress” was sprayed with PTW (1.7 ml) 18, 19, and 20 DAS.
-
III.
“DW drought” was sprayed with DW as mentioned in (i) and did experience drought stress by omitting/ceasing the nutrient solution on 33, 34, and 35 DAS followed by a recovery: rewatering on 36 DAS with a double amount of nutrient solution and normal watering thereafter.
-
IV.
“PTW drought” did experience both PTW application (ii) and drought stress (iii) followed by a recovery as indicated above.
Photosynthetic measurements were performed 49 DAS, one day before harvesting. Plants were harvested in the morning before rewatering 36 DAS for proline determination only (another batch) and 50 DAS after the recovery phase for biochemical assays. For MultispeQ measurements, 10 biological replicates of each treatment were used, and biochemical assays were performed on 4 biological replicates.
Photosynthetic Measurements
Spectroscopic measurements were done with the hand-held MultispeQ (v2.0) spectrophotometer (Kuhlgert et al. 2016) using the ‘Photosynthesis RIDES’ protocol linked to the PhotosynQ platform (https://photosynq.org). They were performed in the middle of an intact fully expanded leaf (third leaf from top) to estimate the fraction of light energy captured by Photosystem II (quantum yield or operating efficiency of PSII, Phi2, ΦII).
Soil Humidity
The measurement of soil moisture was conducted with the FOM/mts device with non-standard probes LP/ms (E-Test Ltd., Stasin, Poland; on the base of Time Domain Reflectometry technique). FOM/mts provides readout of volumetric water content according to the empirical calibration of Malicki et al. (1996). Under well-watered conditions, plants met a soil moisture of about 10% (v/v), whereas the drought-stressed plants experienced a soil moisture of 2% (v/v) at the lowest point.
Biochemical Assays
Fully expanded leaves and roots were harvested 50 DAS (36 DAS for proline determination), ground with liquid nitrogen and stored at − 80 °C. Frozen tissue powder was treated with individual extracting reagents according to each assay.
Determination of Proline Content and Lipid Peroxidation
Proline content was determined according to the method of Bates et al. (1973). Lipid peroxidation was determined and calculated in terms of thiobarbituric acid-reactive substances (TBARS) using the Malondialdehyde (MDA) assay according to Cavalcanti et al. (2004) with some modifications. 0.2 g frozen tissue were treated with 1 ml ice cold 1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 18,000×g, 4 °C and 15 min. For the assay, 250 µl of the supernatant were incubated with 750 µl 0.5% (w/v) thiobarbituric acid in 20% (w/v) TCA. After 1 h incubation at 98 °C, the reaction was stopped on ice and centrifuged at 15,000×g, 4 °C, and 5 min. The absorbance was read at 532 and 600 nm. Calculation was done with A532 nm–A600 nm and the extinction coefficient ε = 155 mmol−1 cm−1.
Determination of Chlorophyll Content and Total Antioxidant Capacity
Methanolic extraction was done for chlorophyll content and total antioxidant capacity (TAC). 0.1 g frozen tissue was treated with 1 ml of 99% (v/v) methanol and incubated in an ultrasonic bath (62 °C, 15 min, 100% DEGAS). Extraction was repeated 3 times.
Measurements and calculations of chlorophyll in methanolic extracts were performed according to Lichtenthaler and Buschmann (2001).
TAC was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) as described by Mahieddine et al. (2018) with some modifications. In this study, methanolic extract (100 µl for leaves, 200 µl for roots) was incubated with 900 µl of 0.1 mM DPPH ethanolic solution for 30 min at room temperature in dark and absorbance was read at 520 nm. A blank for each sample was performed by adding 900 µl ethanol to each extract instead of DPPH solution and subtracted from sample value. Ascorbic acid was used for standard and content was calculated in terms of ascorbic acid equivalents (AAE).
Determination of Ascorbate and Glutathione Content
0.1 g frozen tissue were extracted with 1.8 ml 6% TCA. The colorimetric assay was performed according to Gillespie and Ainsworth (2007), and absorbance was read at 525 nm. Asctot and Ascred: Ascox ratios were calculated using ascorbic acid as a standard.
For determination of the glutathione content, a modified protocol of the enzymatic recycling procedure according to Noctor et al. (2016) was performed. Reduced glutathione (GSH) and oxidized glutathione (GSSG) were used as a reference. 2-vinylpyridine was used as a masking reagent for GSH. The assay was performed in a 96-well microtiter plate and absorbance read at 405 nm. GStot and GSH:GSSG ratios were calculated.
APX Activity
The ascorbate peroxidase (APX) activity was determined according to Noctor et al. (2016) with some modifications. 1 ml of the extracting agent (0.1 M sodium phosphate buffer, 0.1 mM EDTA (pH 7.0), 5% (w/v) polyvinylpolypyrrolidone (PVPP), 1 mM Asc) was added to 0.25 g frozen powder and centrifuged at 25,000×g, 4 °C, 15 min. The assay was performed in 96-well microtiter plates and absorbance read at 290 nm. A baseline was recorded with 175 µl test buffer (0.1 M potassium phosphate buffer (KPP, pH 7.0) and 0.1 mM EDTA), 25 µl of 5 mM ascorbic acid, and 25 µl of diluted extract. APX activity started with the addition of 25 µl of 1 mM H2O2. Specific APX activity was calculated using the extinction coefficient 2800 l * mol−1 * cm−1. The protein content was determined according to Bradford (1976).
Statistical Analysis
All the obtained data have mean values ± standard deviation (SD). The data were statistically analyzed by student’s t test with Excel. Significant differences are denoted according to p < 0.05 (⁎), p < 0.01 (⁎⁎), and p < 0.001 (⁎⁎⁎).
Results
Plasma Treatment of Water Caused Increased Occurrence of NOx Species and Hydrogen Peroxide
Plasma treatment of DW for 20 min resulted in accumulation of hydrogen peroxide, nitrite, and nitrate ions in µmolar concentrations (Table 1). The observed decrease in pH in PTW (within several publications denoted as ‘plasma activated water,’ PAW) in comparison to DW is in line with published data (e.g., Adhikari et al. 2019; Hu et al. 2021; Kang et al. 2019). In addition, significant amounts of NO released from stirred PTW into the gas phase were detected. The detection method of NO applied in this study is based on the reaction of NO with ozone resulting in excited nitrogen dioxide species that emit detectable photons when dropping back to the ground state (Stöhr and Stremlau 2006). The occurrence of NO in plasma-treated liquids has been noticed for other plasma treatment systems as well by measuring NO within PTW via amperometric microsensors (Kang et al. 2019) or by EPR spectroscopy (Tian et al. 2017).
Proline Content Increased in Leaves and Roots Directly After Drought Stress
Proline content was measured to estimate the stress status of the plants. Directly after drought stress (36 DAS), in leaves, proline content was 150 times higher in DW drought compared to DW no stress (1048 compared to 7 µmol proline * g FW−1) and 136 times higher in PTW drought compared to PTW no stress (1023 compared to 7 µmol proline * g FW−1) (Fig. 1a). Regarding roots, proline content was 5.4 times higher in DW drought and 7.6 times higher in PTW drought compared to the respective no stress group (Fig. 1b). After two weeks of recovery following drought application, only minor differences between proline contents under no stress conditions and drought stress conditions could be observed in leaves and roots independent of the PTW treatment (Fig. 1c, d).
Proline content determined directly after drought stress treatment in leaves a and roots b after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Measurement was performed again 2 weeks after drought stress treatment in leaves c and roots d. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
PTW Treatment Resulted in Enhanced Chlorophyll Content and Quantum Yield
Assessing the content of photosynthetic pigments is a suitable indicator for photosynthetic activity (Ghosh et al. 2004) as well as photooxidative stress (Pinto-Marijuan and Munné-Bosch 2014) and considered as an overall requirement for the effective cultivation of plants (Sonobe et al. 2020). Under no stress conditions, significantly higher values of Chl (a + b) content were observable upon PTW treatment relative to DW-treated plants (18%, Fig. 2a). Under drought stress conditions, higher contents of Chl (a + b) in comparison to DW treatment were detected, despite not being significant (10%, Fig. 2a). MultispeQ measurements revealed the same pattern regarding the quantum yield of Photosystem (PS) II: Significantly higher values were obtained upon PTW treatment relative to DW treatment under no stress (11%), whereas under drought conditions, the changes were not significantly higher (7%; Fig. 2b). No morphological differences could be obtained.
Chl (a + b) content a and quantum yield of Photosystem II b after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
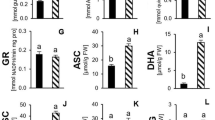
Total Antioxidant Capacity Increased Significantly in Leaves and Roots upon PTW Treatment Under Drought Stress Conditions
Plant enzymatic and non-enzymatic antioxidative mechanisms jointly contribute to the TAC. It serves as biochemical marker for the plant response to environmental changes since it assesses its redox status (Ghiselli et al. 2000; Gillespie et al. 2007). It reflects the scavenging capacity of reducing agents such as antioxidants towards DPPH (Pyrzynska and Pȩkal 2013). In both organs, treatment with PTW affected TAC in a similar pattern under no stress and drought stress conditions. Under no stress conditions, treatment resulted in significantly lower values for TAC (61% in leaves, Fig. 3a; 51% in roots, Fig. 3b). Under drought stress conditions, values for TAC were significantly higher upon treatment with PTW (56% in leaves, Fig. 3a; 85% in roots, Fig. 3b).
Total antioxidant capacity (TAC) in leaves a and roots b after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Results are expressed in ascorbic acid equivalents (AAE). Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
TBARS Content Increased Significantly in Roots upon PTW Treatment Under Drought Stress Conditions
Membrane damage can be a result of oxidation by RONS. Lipid peroxidation estimated as the content of TBARS serves as an indicator for oxidative stress. However, after recovery, TBARS content may also indicate acclimation processes facilitating stress tolerance. In spite of not being significant, a tendentiously higher TBARS content was observed upon PTW treatment under both no stress (17%) and drought conditions (14%) in leaves (Fig. 4a). In roots, no differences in TBARS content were obtained under no stress conditions after PTW application, whereas under drought conditions, significantly higher values were observed in comparison to DW treatment (37%; Fig. 4b).
Lipid peroxidation in terms of TBARS in leaves a and root b after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
PTW Treatment Influenced Components of the Ascorbate–Glutathione–Cycle Under no Stress and Drought Stress Conditions
Asc is one of the most important antioxidant metabolites in plants and an essential component of the ascorbate–glutathione–cycle. By controlling the cellular redox state, it contributes to the development of stress tolerance (Latowski et al. 2010). Changes in the Ascred: Ascox ratio can act as indicators for abiotic stresses as it directly responds to altered turnover rates of antioxidant enzymes (Tausz 2004). The Asctot content was fivefold higher in leaves compared to the roots (Fig. 5a, b). Treatment with PTW resulted in significantly higher \({\text{Asc}}_{\text{tot}}\) contents in leaves under no stress conditions compared to DW-treated plants (32%), while no differences were noticeable in roots. Under drought stress conditions, minor Asctot contents were observed non-significantly in leaves (23%) and significantly in roots (49%) relative to DW treatment. Based on the difference between Asctot content and Ascred content, the Ascox content and further Ascred: Ascox ratio can be calculated (Fig. 5c, d). The Ascox content was generally higher in roots than in leaves, although a significant shift towards the Ascred content was notable in roots under drought stress conditions after PTW treatment and subsequent recovery period. Moreover, only minor non-significant changes regarding the Ascred: Ascox ratio were detected.
Total ascorbate content (Asctot) in leaves a and root b and distribution c, d of reduced ascorbate (Ascred; light gray bars) and oxidized ascorbate (Ascox; white bars) after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t test
Glutathione is the other important metabolite of the ascorbate–glutathione cycle and crucial for the preservation of the cellular redox homeostasis (Latowski et al. 2010; Noctor et al. 1998). The concentration of total glutathione correlates with the adaption to environmental stresses and alterations in the GSH: GSSG ratio may indicate a response to changes in environmental conditions (May et al. 1998). Besides the Ascred: Ascox ratio, changes in the GSH: GSSG ratio represent a direct consequence of altering turnover rates of enzymes of the ascorbate–glutathione cycle (Tausz 2004). The overall GStot contents were approximately 10 times higher in leaves compared to the roots (Fig. 6a, b). The difference between GStot content and GSSG content allows the calculation of GSH content as well as the GSH:GSSG ratio (Fig. 6c, d). Regarding the contents of GStot and the GSH:GSSG ratio, no long-term effects of treatment with PTW were obtained in leaves, neither under no stress nor under drought stress conditions (Fig. 6a, c). Values of GStot content were significantly higher in roots upon PTW treatment relative to DW treatment under drought stress conditions (Fig. 6b). With regard to the GSH:GSSG ratio in roots, no differences between DW-treated and PTW-treated plants were obtained (Fig. 6c, d).
Total glutathione content (GStot) in leaves a and root b and distribution c, d of reduced glutathione (GSH; light gray bars) and oxidized glutathione (GSSG; white bars) after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
In leaves, non-significant alterations regarding the APX activity occurred, as the PTW treatment led to higher values under no stress conditions (33%) and minor values under drought stress conditions (18%) compared to DW treatment (Fig. 7a). In roots, no alterations are noticeable under no stress conditions, whereas under drought stress conditions, values of APX activity were significantly higher upon PTW treatment (45%; Fig. 7b).
Ascorbate peroxidase (APX) activity in leaves a and root b after treatment with deionized water (DW) or plasma-treated water (PTW) under no stress and drought stress conditions. Mean values (± SD) were calculated from four replicates of each treatment. Bars with an asterisk indicate significance differences (⁎: p < 0.05; ⁎⁎: p < 0.01; ⁎⁎⁎: p < 0.001). Statistical analysis was performed using student’s t-test
Discussion
Numerous physical and chemical reactions between CAP and water lead to the generation of a variety of RONS with different reactivity. Mainly, nitrogen oxides (NOx) are converted to nitrite and nitrate ions in water while hydroxyls are converted to hydrogen peroxide (Graves et al. 2019). The typical constituents NO2−, NO3−, and H2O2 were also found in the PTW used in this study. Interestingly, PTW contained 331 µM NO that was liberated to the gas phase (Table 1). Only few studies documented the occurrence of NO in PTW (e.g., Kang et al. 2019; Tian et al. 2017). It is known that the radical NO can possess different half-lifetimes from microseconds to hours depending on concentration and chemical environment (Procházková et al. 2015). Kang et al. (2019) could still detect 15–30 µM NO within PTW 16 h after generation. It is proposed that PTW containing H2O2, NOx, and specifically NO might play a prominent role in plant responses to PTW (Kang et al. 2019; Adhikari et al. 2019). It has been reported that PTW irrigation resulted even in enhanced endogenous levels of H2O2 and NOx in tomato seedlings (Adhikari et al. 2019). Both, H2O2 and NO, are important signaling molecules in plants and responsible for many short-term and long-term reactions to environmental stresses and developmental factors during plants life cycle (Farnese et al. 2016; Neill et al. 2002; Sanz et al. 2015).
In response to environmental stresses, proline accumulates in many plant species including barley (Hanson et al. 1979). Hence, it was described as reasonable indicator of plant reactions to water deficit (Dar et al. 2016). The higher proline content in drought-stressed plants compared to non-stressed plants implied water deficit and corroborates the efficacy of drought stress application (Fig. 1a, b). Two weeks of recovery following drought application the proline content in drought-stressed plants did not differ from non-stressed plants, which indicates that the drought-stressed plants were recovered (Fig. 1c, d) independently of the PTW treatment.
In this study, PTW treatment sustainably resulted in higher chlorophyll content regardless of the application of drought stress, whereby significantly higher values could be observed for non-drought-stressed plants (Fig. 2a). The effect on photosynthetic pigments caused by PTW was reported by other studies as well (Adhikari et al. 2019; Gierczik et al. 2020; Ndiffo Yemeli et al. 2021) and might be a direct effect of H2O2 and NO. This conclusion is supported by studies on the treatment of marigold with NO and H2O2 (Liao et al. 2012) or on the treatment of Ficus deltoidea (Nurnaeimah et al. 2020) and maize (Gondim et al. 2013) with H2O2. Additionally, foliar H2O2 treatment prior to osmotic stress enhanced chlorophyll content of pistachio (Bagheri et al. 2021), soybean (Guler and Pehlivan 2016), and quinoa (Iqbal et al. 2018). Although the molecular mechanisms have not been investigated yet, it could be shown that H2O2 induces osmolyte accumulation under osmotic stress which in turn can lower the destruction of chlorophyll by ROS generated in the chloroplast (Farooq et al. 2017).
The quantum yield of PSII followed the pattern of the chlorophyll content: the treatment with PTW sustainably resulted in a higher quantum yield of PSII after no stress and drought stress with significantly higher values for PTW without drought. Škarpa et al. (2020) stated that the quantum yield of the electron transport of the photosystem II was not significantly influenced by foliar PTW application in maize. On the other hand, intensive PTW application on the maize plants leads to damage of the photosystem apparatus (Škarpa et al. 2020). Considering the concentrations of H2O2 and NOx in PTW in this study, they were much lower compared to our treatment. Yemeli et al. (2021) found that watering with PTW increased the concentration of photosynthetic pigments and simultaneously had no or negative impact on net photosynthesis of barley and maize, respectively. They had comparably high concentrations of H2O2 (5 times higher) in PTW and watered only with PTW for 4 weeks. Liao et al. (2012) treated marigold explants with NO and H2O2. Their data suggest that the application of exogenous NO or H2O2 could effectively mitigate the damage of drought stress on leaves by protecting the ultrastructure of mesophyll cells. This was accompanied by a rapid photosynthetic electron transfer rate and higher PS II electron transfer activity under drought conditions. That is in agreement with the presented data, where drought stress resulted in lower values for quantum yield of PS II for DW-treated as well as PTW-treated plants relative to no stress conditions (Fig. 2b). Plants that encountered drought stress after PTW treatment were able to keep the photosynthetic performance up to a higher level as DW-treated plants that did not experience drought.
Asc and GSH have discrete and specific functions in photosynthesis and associated redox signaling (Foyer and Noctor 2009). Following Foyer and Shigeoka (2011), Asc has several important roles in photosynthesis: It is a cofactor for violaxanthin deepoxidase, an enzyme required for nonphotochemical quenching formation, and it participates in the abscisic acid-mediated regulation of stomatal closure. Finally, it can strongly influence the expression of both nuclear and chloroplast genes encoding photosynthetic components (Kiddle et al. 2003). Enhancing the activities of antioxidant enzymes and/or the accumulation of low molecular weight antioxidants by genetic manipulation may increase tolerance to a variety of stresses through more efficient removal of ROS. By improving ROS removal in plant tissue, the photosynthetic processes are desensitized to environmental change (Foyer and Shigeoka 2011).
In this study, the exposure of barley plants to PTW took effect on the plants’ antioxidative system. Most of the significant changes occurred in the root after foliar treatment of PTW, drought stress, and subsequent recovery period, revealing that PTW induces systemic signaling.
Values for TAC were significantly higher in leaves and roots upon PTW treatment under drought stress conditions compared to treatment with DW. Elevated values for TAC denote the accumulation of DPPH-reducing agents, which might include antioxidative compounds. In general, higher levels of constitutive or induced antioxidants facilitate tolerance against different environmental stresses including drought stress (Reddy et al. 2004; Miranda et al. 2014). Chutipaijit (2016) stated that TAC indicated by DPPH as reagent may correlate with stress tolerance. In fact, it has been reported that higher levels of DPPH radical scavenging activity imply abiotic stress tolerance in rice-seedling radicles (Kang and Saltveit 2001) and cucumber-seedling radicles (Kang and Saltveit 2002). In respect of drought, Štajner et al. (2013) and Weidner et al. (2009) used the DPPH assay as a potential parameter for drought stress tolerance. With regard to our study, PTW-treated plants possibly adapted to drought stress more efficiently than DW-treated plants by acquiring the ability to scavenge more RONS as reflected by elevated TAC in leaves and roots. The combined treatment of plants with PTW followed by drought may has resulted in improved detoxification of prooxidants and might has facilitated the induction of drought stress tolerance. It might be possible that the elevated GStot content in roots under drought stress conditions contributed to elevated values for TAC since the highly reductive thiol group of GSH reacts with DPPH (Viirlaid et al. 2009). In contrast to the elevated values for TAC under drought stress conditions, it must be pointed out that minor values for TAC were observed in leaves and roots under no stress conditions. Considering the recovery phase of 30 days after PTW treatment, it remains questionable whether RONS present in PTW resulted in oxidative stress still visible after that period. With respect to the elevated chlorophyll content caused by treatment with PTW and the low proline content, plants did probably not experience stress at time of harvest. The elevated Asctot content in leaves of PTW-treated plants compared to DW-treated plants under no stress conditions supports the idea that the antioxidative system was upregulated. Further experiments assessing the TAC with more specific methods are mandatory to evaluate the radical scavenging properties of PTW-treated plants.
The range of lipid peroxidation as determined by the MDA content was significantly higher in roots under drought stress conditions, whereas in leaves, it did not differ significantly between no stress and drought stress conditions upon PTW treatment relative to DW treatment (Fig. 4). Although an elevated MDA content is deemed to be an indicator for oxidative stress, it might also correspond to acclimation processes rather than to damage. Depending on intracellular levels, MDA is described as either toxic or gene activating (Missihoun and Kotchoni 2017) and facilitates expression of abiotic stress genes (Weber et al. 2004). It was suggested that MDA present in low concentrations can implement cell protection under oxidative stress by activating regulatory genes involved in plant defense and development and cellular redox homeostasis. Fine tuning of MDA as a gene activator requires the activity of aldehyde dehydrogenases (ALDHs) (Missihoun and Kotchoni 2017). ALDHs were shown to control MDA levels by catalyzing the oxidation of aldehydes to the corresponding carboxylic acid utilizing NADP+ as the oxidizing agent. For its part, ALDH activity can be induced by H2O2 (Zhao et al. 2018). Summarizing, MDA levels are highly balanced in plants and may act as a signal molecule and not as a damager. Cui et al. (2010) suggested that lipid peroxidation and increasing H2O2 levels might be involved in the activation of secondary metabolite accumulation. In our study, such an accumulation might count for the elevated TAC in roots under drought stress conditions.
Another indicator of the upregulation of the antioxidative system caused by PTW in roots under drought stress conditions might be the higher percental Ascred pool within the Asctot content (Fig. 5d). It is hypothesized that the maintenance of a high Ascred: Ascox ratio might be a key element for the protection against abiotic stress-induced ROS (Fotopoulos et al. 2010). Contrarily, PTW-treated roots under drought stress conditions exhibited higher Ascred:Ascox ratios while simultaneously featuring decreasing contents of Asctot compared to DW treatment. The GSH:GSSG ratio remained unaltered, while GStot content displayed significantly higher values compared to DW treatment (Fig. 6b). Higher total concentrations of glutathione may indicate acclimation processes (Tausz 2004; Cheng et al. 2015). Even though the GSH pool remains unaltered, the higher GStot content might imply the enhancement of antioxidative traits.
APX activity was significantly higher upon PTW treatment in drought-stressed roots (Fig. 7b). The increase in synthesis or activity of antioxidant enzymes may facilitate drought stress tolerance (Kusvuran et al. 2016; Sallam et al. 2019). Therefore, the PTW induced elevated APX activity in roots under drought stress conditions might indicate enhanced drought stress tolerance. The treatment of rice (Farooq et al. 2009) and turfgrass (Boogar et al. 2014) with NO donors resulted in the drought stress alleviation by upregulating APX activity. This supports the idea that NO might be one of the key components responsible for the reported effects of PTW.
With respect to the overall enhancement of antioxidative traits effected by PTW, the results of this work indicate that the treatment with PTW itself does not show significant evidence of tolerance development before plants meet the stressor (Fig. 8a, c). The application of drought stress was necessary to obtain visible signs of the upregulation of the antioxidative system and systemic signaling caused by components of PTW (Fig. 8b, d).
Ascorbate–glutathione cycle colored with the log2 fold change after treatment with PTW relative to DW treatment under no stress a, c and drought stress b, d conditions in the leaf a, b and in the root c, d. Color key of log2 fold change: green color represents higher values, red color lower values compared to DW treatment. The higher the intensity of the color, the higher the log2 fold change. Asc Ascorbate, GSSG Oxidized glutathione, GSH Reduced glutathione, DHA Dehydroascorbate, MDHA Monodehydroascorbate, APX Ascorbate peroxidase, DHAR Dehydroascorbate reductase, MDHAR Monodehydroascorbate reductase, GR Glutathione reductase (Color figure online)
In conclusion, this study indicates that components of PTW affect the antioxidative system of barley on a long-term scale. These alterations imply that the treatment with PTW might lead to enhanced drought stress tolerance and renders PTW as a putative priming agent.
Data Availability
The data supporting the findings of this study are available from the corresponding author, Christine Stöhr, upon request.
Abbreviations
- AAE:
-
Ascorbic acid equivalents
- APX:
-
Ascorbate peroxidase
- Asc:
-
Ascorbate
- Ascox :
-
Oxidized ascorbate
- Ascred :
-
Reduced ascorbate
- Asctot :
-
Total amount of ascorbate
- CAP:
-
Cold atmospheric plasma
- DAS:
-
Days after sowing
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- DW:
-
Deionized water
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- GStot :
-
Total amount of glutathione
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbate
- MDHAR:
-
Monodehydroascorbate reductase
- PTW:
-
Plasma-treated water
- TAC:
-
Total Antioxidant Capacity
- TBARS:
-
Thiobarbituric acid-reactive substances
References
Adhikari B, Adhikari M, Ghimire B et al (2019) Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci Rep 9:1–15. https://doi.org/10.1038/s41598-019-52646-z
Adhikari B, Adhikari M, Park G (2020) The effects of plasma on plant growth, development, and sustainability. Appl Sci 10:6045. https://doi.org/10.3390/app10176045
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233. https://doi.org/10.1111/j.1399-3054.1997.tb04778.x
Anderson R, Bayer PE, Edwards D (2020) Climate change and the need for agricultural adaptation. Curr Opin Plant Biol 56:197–202. https://doi.org/10.1016/j.pbi.2019.12.006
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3411. https://doi.org/10.1093/JXB/ERX420
Bagheri M, Gholami M, Baninasab B (2021) Role of hydrogen peroxide pre-treatment on the acclimation of pistachio seedlings to salt stress. Acta Physiol Plant 43:51. https://doi.org/10.1007/s11738-021-03223-3
Bartosz G (1997) Oxidative stress in plants. Acta Physiol Plant 19:47–64. https://doi.org/10.1007/s11738-997-0022-9
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 391(39):205–207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS et al (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Cheng MC, Ko K, Chang WL et al (2015) Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J 83:926–939. https://doi.org/10.1111/tpj.12940
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867. https://doi.org/10.1111/tpj.13299
Chutipaijit S (2016) Changes in physiological and antioxidant activity of indica rice seedlings in response to mannitol-induced osmotic stress. Chil J Agric Res 76:455–462. https://doi.org/10.4067/S0718-58392016000400009
Cui XH, Murthy HN, Wu CH, Paek KY (2010) Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult 103:7–14. https://doi.org/10.1007/s11240-010-9747-z
Dar MI, Naikoo MI, Rehman F et al (2016) Proline accumulation in plants: roles in stress tolerance and plant development. In: Iqbal N, Nazar R, Khan NA (eds) Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi, pp 155–166
Del Río LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66:2827–2837. https://doi.org/10.1093/jxb/erv099
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Dumanović J, Nepovimova E, Natić M et al (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:2106. https://doi.org/10.3389/fpls.2020.552969
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689. https://doi.org/10.1007/s00018-014-1767-0
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471. https://doi.org/10.3389/fpls.2016.00471
Farooq M, Basra SMA, Wahid A, Rehman H (2009) Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 195:254–261. https://doi.org/10.1111/J.1439-037X.2009.00367.X
Farooq M, Nawaz A, Chaudhry MAM et al (2017) Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J Agron Crop Sci 203:464–472. https://doi.org/10.1111/jac.12222
Fotopoulos V, Ziogas V, Tanou G, Molassiotis A (2010) Involvement of AsA/DHA and GSH/GSSG ratios in gene and protein expression and in the activation of defence mechanisms under abiotic stress conditions. In: Anjum NA, Umar S, Chan MT (eds) Ascorbate–glutathione pathway and stress tolerance in plants. Springer, Netherlands, pp 265–302
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Foyer CH, Noctor G (2009) Redox Regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905. https://doi.org/10.1089/ARS.2008.2177
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100. https://doi.org/10.1104/pp.110.166181
Ghiselli A, Serafini M, Natella F, Scaccini C (2000) Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 29:1106–1114. https://doi.org/10.1016/S0891-5849(00)00394-4
Ghosh PK, Ajay BKK et al (2004) Comparative effectiveness of cattle manure, poultry manure, phosphocompost and fertilizer-NPK on three cropping systems in vertisols of semi-arid tropics. II. Dry matter yield, nodulation, chlorophyll content and enzyme activity. Bioresour Technol 95:85–93. https://doi.org/10.1016/j.biortech.2004.02.012
Gierczik K, Vukušić T, Kovács L et al (2020) Plasma-activated water to improve the stress tolerance of barley. Plasma Process Polym 17:1900123. https://doi.org/10.1002/ppap.201900123
Gillespie KM, Ainsworth EA (2007) Measurement of reduced, oxidized and total ascorbate content in plants. Nat Protoc 2:871–874. https://doi.org/10.1038/nprot.2007.101
Gillespie KM, Chae JM, Ainsworth EA (2007) Rapid measurement of total antioxidant capacity in plants. Nat Protoc 2:867–870. https://doi.org/10.1038/nprot.2007.100
Gondim FA, de Miranda R, S, Gomes-Filho E, Prisco JT, (2013) Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor Exp Plant Physiol 25:251–260. https://doi.org/10.1590/s2197-00252013000400003
Graves DB, Bakken LB, Jensen MB, Ingels R (2019) Plasma activated organic fertilizer. Plasma Chem Plasma Process 39:1–19. https://doi.org/10.1007/s11090-018-9944-9
Guler NS, Pehlivan N (2016) Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol Hung 67:169–183. https://doi.org/10.1556/018.67.2016.2.5
Hanson AD, Nelsen CE, Pedersen AR, Everson EH (1979) Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Sci 19:489–493. https://doi.org/10.2135/CROPSCI1979.0011183X001900040015X
Hilker M, Schmülling T (2019) Stress priming, memory, and signalling in plants. Plant Cell Environ 42:753–761. https://doi.org/10.1111/pce.13526
Hu X, Zhang Y, Wu RA et al (2021) Diagnostic analysis of reactive species in plasma-activated water (PAW): current advances and outlooks. J Phys D Appl Phys 55:023002. https://doi.org/10.1088/1361-6463/AC286A
Ilyas M, Nisar M, Khan N et al (2020) Drought tolerance strategies in plants: a mechanistic approach. J Plant Growth Regul 40:926–944. https://doi.org/10.1007/s00344-020-10174-5
Iqbal H, Yaning C, Waqas M et al (2018) Hydrogen peroxide application improves quinoa performance by affecting physiological and biochemical mechanisms under water-deficit conditions. J Agron Crop Sci 204:541–553. https://doi.org/10.1111/jac.12284
Ito M, Oh J-S, Ohta T et al (2018) Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process Polym 15:1700073. https://doi.org/10.1002/ppap.201700073
Junglee S, Urban L, Sallanon H, Lopez-Lauri F (2014) Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am J Anal Chem 05:730–736. https://doi.org/10.4236/AJAC.2014.511081
Kang HM, Saltveit ME (2001) Antioxidant enzymes and DPPH-radical scavenging activity in chilled and heat-shocked rice (Oryza sativa L.) seedlings radicles. J Agric Food Chem 50:513–518. https://doi.org/10.1021/JF011124D
Kang HM, Saltveit ME (2002) Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high- and low-vigour cucumber seedling radicles. Plant Cell Environ 25:1233–1238. https://doi.org/10.1046/J.1365-3040.2002.00915.X
Kang MH, Jeon SS, Shin SM et al (2019) Dynamics of nitric oxide level in liquids treated with microwave plasma-generated gas and their effects on spinach development. Sci Reports 91(9):1–15. https://doi.org/10.1038/s41598-018-37711-3
Kiddle G, Pastori GM, Bernard S et al (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antoxid Redox Sign 5:23–32. https://doi.org/10.1089/152308603321223513
Kranner I, Minibayeva FV, Beckett RP, Seal CE (2010) What is stress? Concepts, definitions and applications in seed science. New Phytol 188:655–673. https://doi.org/10.1111/j.1469-8137.2010.03461.x
Kuhlgert S, Austic G, Zegarac R et al (2016) MultispeQ Beta: a tool for large-scale plant phenotyping connected to the open PhotosynQ network. R Soc Open Sci. https://doi.org/10.1098/RSOS.160592
Kusvuran S, Kiran S, Ellialtioglu SS (2016) Antioxidant enzyme activities and abiotic stress tolerance relationship in vegetable crops. In: Anker S (ed) Abiotic and biotic stress in plants—recent advances and future perspectives. InTech, London, pp 481–506
Latowski D, Surówka E, Strzałka K (2010) Regulatory role of components of ascorbate–glutathione pathway in plant stress tolerance. In: Anjum NA, Chan MT, Umar S (eds) Ascorbate–glutathione pathway and stress tolerance in plants. Springer, Netherlands, pp 1–53
Lau SE, Hamdan MF, Pua TL et al (2021) Plant nitric oxide signaling under drought stress. Plants 10:1–30. https://doi.org/10.3390/plants10020360
Laurita R, Contaldo N, Zambon Y et al (2021) The use of plasma-activated water in viticulture: induction of resistance and agronomic performance in greenhouse and open field. Plasma Process Polym 18:2000206. https://doi.org/10.1002/PPAP.202000206
Li X, Liu F (2016) Drought stress memory and drought stress tolerance in plants: biochemical and molecular basis. In: Hossain MA, Wani SH, Bhattacharjee S, Burritt DJ (eds) Drought stress tolerance in plants, vol 1. Physiology and biochemistry. Springer International Publishing, Cham, pp 17–44
Li P, Yang H, Wang L et al (2019) Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front Genet 10:55. https://doi.org/10.3389/fgene.2019.00055
Liao WB, Huang GB, Yu JH, Zhang ML (2012) Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol Biochem 58:6–15. https://doi.org/10.1016/j.plaphy.2012.06.012
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Curr Protoc Food Anal Chem 1:F431–F438. https://doi.org/10.1002/0471142913.faf0403s01
Lu X, Naidis GV, Laroussi M et al (2016) Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Phys Rep 630:1–84. https://doi.org/10.1016/J.PHYSREP.2016.03.003
Lukacova Z, Svubova R, Selvekova P, Hensel K (2021) The effect of plasma activated water on maize (Zea mays L.) under arsenic stress. Plants 10:1899. https://doi.org/10.3390/PLANTS10091899
Mahieddine B, Amina B, Faouzi SM et al (2018) Effects of microwave heating on the antioxidant activities of tomato (Solanum lycopersicum). Ann Agric Sci 63:135–139. https://doi.org/10.1016/j.aoas.2018.09.001
Malicki MA, Plagge R, Roth CH (1996) Improving the calibration of dielectric TDR soil moisture determination taking into account the solid soil. Eur J Soil Sci 47:357–366. https://doi.org/10.1111/J.1365-2389.1996.TB01409.X
May MJ, Vernoux T, Leaver C et al (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667. https://doi.org/10.1093/jxb/49.321.649
Merewitz E (2016) Chemical priming-induced drought stress tolerance in plants. In: Hossain MA, Wani SH, Bhattacharjee S et al (eds) drought stress tolerance in plants, vol 1. Physiology and Biochemistry. Springer International Publishing, Cham, pp 77–103
Miranda D, Fischer G, Mewis I et al (2014) Salinity effects on proline accumulation and total antioxidant activity in leaves of the cape gooseberry (Physalis peruviana L.). J Appl Bot Food Qual 87:67–73. https://doi.org/10.5073/JABFQ.2014.087.010
Missihoun TD, Kotchoni SO (2017) Aldehyde dehydrogenases modulate signaling by lipid peroxidation-derived bioactive aldehydes. Plant Signal Behav 12:e1387707
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Molassiotis A, Fotopoulos V (2011) Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signal Behav 6:210–214. https://doi.org/10.4161/psb.6.2.14878
Molassiotis A, Job D, Ziogas V, Tanou G (2016) Citrus plants: a model system for unlocking the secrets of NO and ROS-inspired priming against salinity and drought. Front Plant Sci 7:229. https://doi.org/10.3389/fpls.2016.00229
Ndiffo Yemeli GB, Švubová R, Kostolani D et al (2021) The effect of water activated by nonthermal air plasma on the growth of farm plants: case of maize and barley. Plasma Process Polym 18:2000205. https://doi.org/10.1002/ppap.202000205
Neill SJ, Desikan R, Clarke A et al (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247. https://doi.org/10.1093/JEXBOT/53.372.1237
Noctor G, Arisi A-CM, Jouanin L et al (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647. https://doi.org/10.1093/jxb/49.321.623
Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160. https://doi.org/10.1111/pce.12726
Nurnaeimah N, Mat N, Mohd KS et al (2020) The effects of hydrogen peroxide on plant growth, mineral accumulation, as well as biological and chemical properties of Ficus deltoidea. Agronomy 10:599. https://doi.org/10.3390/agronomy10040599
Pinto-Marijuan M, Munne-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65:3845–3857. https://doi.org/10.1093/jxb/eru086
Pretty J, Sutherland WJ, Ashby J et al (2010) The top 100 questions of importance to the future of global agriculture. Int J Agric Sustain 8:219–236. https://doi.org/10.3763/ijas.2010.0534
Procházková D, Wilhelmová N, Pavlík M (2015) Reactive nitrogen species and nitric oxide. Nitric oxide action abiotic stress responses plants. Springer, Cham, pp 3–19
Puač N, Gherardi M, Shiratani M (2018) Plasma agriculture: a rapidly emerging field. Plasma Process Polym 15:1700174. https://doi.org/10.1002/ppap.201700174
Pyrzynska K, Pȩkal A (2013) Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal Methods 5:4288–4295. https://doi.org/10.1039/c3ay40367j
Qiao W, Li C, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93. https://doi.org/10.1016/j.envexpbot.2013.12.014
Rahimian Boogar A, Salehi H, Jowkar A (2014) Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. South Afr J Bot 92:78–82. https://doi.org/10.1016/J.SAJB.2014.02.005
Raza A, Razzaq A, Mehmood SS et al (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8:34. https://doi.org/10.3390/PLANTS8020034
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202. https://doi.org/10.1016/J.JPLPH.2004.01.013
Sallam A, Alqudah AM, Dawood MFA et al (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20:3137. https://doi.org/10.3390/ijms20133137
Sanz L, Albertos P, Mateos I et al (2015) Nitric oxide (NO) and phytohormones crosstalk during early plant development. J Exp Bot 66:2857–2868. https://doi.org/10.1093/JXB/ERV213
Schmidt M, Hahn V, Altrock B et al (2019) Plasma-activation of larger liquid volumes by an inductively-limited discharge for antimicrobial purposes. Appl Sci 9:2150. https://doi.org/10.3390/app9102150
Šimek M, Homola T (2021) Plasma-assisted agriculture: history, presence, and prospects—a review. Eur Phys J D 75:1–31. https://doi.org/10.1140/EPJD/S10053-021-00206-4
Škarpa P, Klofáč D, Krčma F et al (2020) Effect of plasma activated water foliar application on selected growth parameters of maize (Zea mays L.). Water 12:3545. https://doi.org/10.3390/W12123545
Sonobe R, Yamashita H, Mihara H et al (2020) Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens 12:3265. https://doi.org/10.3390/rs12193265
Štajner D, Orlovic S, Popovic BM et al (2013) Screening of drought oxidative stress tolerance in Serbian melliferous plant species. Afr J Biotechnol 10:1609–1614. https://doi.org/10.4314/ajb.v10i9
Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57:463–470. https://doi.org/10.1093/JXB/ERJ058
Stöhr C, Ullrich WR (1997) A succinate-oxidising nitrate reductase is located at the plasma membrane of plant roots. Planta 203:129–132. https://doi.org/10.1007/s00050173
Stöhr C, Strube F, Marx G et al (2001) A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta 212:835–841. https://doi.org/10.1007/S004250000447
Tausz M (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962. https://doi.org/10.1093/jxb/erh194
Thirumdas R, Kothakota A, Annapure U et al (2018) Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci Technol 77:21–31. https://doi.org/10.1016/j.tifs.2018.05.007
Tian Y, Guo J, Wu D et al (2017) The potential regulatory effect of nitric oxide in plasma activated water on cell growth of Saccharomyces cerevisiae. J Appl Phys 122:123302. https://doi.org/10.1063/1.4989501
Viirlaid S, Mahlapuu R, Kilk K et al (2009) Mechanism and stoichiometry of 2,2-diphenyl-1-picrylhydrazyl radical scavenging by glutathione and its novel α-glutamyl derivative. Bioorg Chem 37:126–132. https://doi.org/10.1016/J.BIOORG.2009.05.001
Wang Y, Loake GJ, Chu C (2013) Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front Plant Sci 4:314. https://doi.org/10.3389/fpls.2013.00314
Weber H, Chételat A, Reymond P, Farmer EE (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37:877–888. https://doi.org/10.1111/j.1365-313X.2003.02013.x
Weidner S, Karolak M, Karamac M et al (2009) Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc Bot Pol 78:97–103
Zambon Y, Contaldo N, Laurita R et al (2020) Plasma activated water triggers plant defence responses. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-76247-3
Zhao J, Missihoun TD, Bartels D (2018) The ATAF1 transcription factor is a key regulator of aldehyde dehydrogenase 7B4 (ALDH7B4) gene expression in Arabidopsis thaliana. Planta 248:1017–1027. https://doi.org/10.1007/s00425-018-2955-1
Zhou R, Zhou R, Wang P et al (2020) Plasma-activated water: generation, origin of reactive species and biological applications. J Phys D Appl Phys 53:303001. https://doi.org/10.1088/1361-6463/ab81cf
Acknowledgements
We would like to thank Markus Schulte and Stefan Schloemer for taking care of the plants as well as for help during sampling procedure. We also gratefully thank Dr. Mark Dörr for his kind support for measuring APX activity. We thank Stefan Horn for establishing the plasma treatment system and Robert Wagner for assistance with IC measurements.
Funding
Open Access funding enabled and organized by Projekt DEAL. Research was partly funded by the Federal Ministry of Education and Research of Germany within the framework of the project ‘Physics for Food’ (FKZ 03WIR2806B and FKZ 03WIR2806C).
Author information
Authors and Affiliations
Contributions
FB, CaSch: devising the experiment and statistical analysis; FB, CaSch, AK, HB: data collection; FB: writing; CaSch, AK, HB, ChSt: revising and reviewing the manuscript; ChS: funding acquisition, project design and supervision.
Corresponding author
Ethics declarations
Competing interest
The authors declare no conflict of interest.
Additional information
Handling Author: Nudrat Aisha Akram.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bussmann, F., Krüger, A., Scholz, C. et al. Long-Term Effects of Cold Atmospheric Plasma-Treated Water on the Antioxidative System of Hordeum vulgare. J Plant Growth Regul 42, 3274–3290 (2023). https://doi.org/10.1007/s00344-022-10789-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10789-w