Abstract
We investigated the modification of cold-induced mechanisms of photosynthetic apparatus adjustment and phytohormone response by brassinosteroid 24-epibrassinolide (EBR) and its consequences for frost tolerance of perennial ryegrass (Lolium perenne L.). We recorded the responses of two cultivars with contrasting frost tolerances to foliar hormone application, both in non-acclimated plants and plants cold acclimated for 3 and 6 weeks at 4 °C. In non-cold-acclimated plants of both cultivars, EBR induced increases in carbon fixation and lowered sucrose levels. Temporary suppression in quantum efficiency of PSII of photosystem II and activities of ribulose-1,5-bisphosphate carboxylase/oxygenase and sucrose phosphate synthase, a consequence of energy dissipation in non-photochemical quenching, was observed in the leaves of the highly frost-tolerant cultivar after 3 weeks of cold acclimation. After 6 weeks of cold acclimation, EBR accelerated recovery of photosynthesis, reflecting adjustment to cold conditions, and increased frost tolerance. As carbohydrate export from leaves is favored during cold acclimation, EBR application did not increase frost tolerance of the moderately tolerant cultivar, reflecting the downregulation of photosynthesis due to high leaf sucrose concentrations. It is also likely that EBR participated in the enhancement of frost tolerance by regulation of stress-related signaling compounds such as JA and ethylene but not SA, in winter ryegrass undergoing cold acclimation. Taken together, our results demonstrate that EBR-induced changes are temperature dependent. The beneficial effect of EBR is not universal under cold conditions, as genetically determined mechanisms are apparently dominant relative to EBR action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to structural and biochemical changes, cold acclimation in plants is associated with photosynthetic acclimation at low growth temperatures. Low temperature and light intensity affect the relative redox state of photosystem II (PSII), thereby leading to an imbalance between the energy absorbed in the light phase of photosynthesis and its consumption for photochemistry (Huner and others 1998). Plants possess two main mechanisms to counteract such cold-induced photoinhibition (Adams and others 2002): a photochemical mechanism, which relies on increased energy utilization, and a non-photochemical mechanism (non-photochemical quenching; NPQ), which involves increased dissipation of excess light (Huner and others. 1993); activation of both mechanisms is genotype dependent, however plants with higher tolerance towards cold-induced photoinhibition are usually more tolerant to frost, (Huner and others 1993; Rapacz and others 2004).
Cold acclimation is also a time-dependent response, with freezing tolerance increasing along with acclimation time (Fowler and others 1996). The recovery of photosynthesis after long-term growth at low, non-freezing temperatures is supported by increases in the activities of photosynthetic carbon reduction cycle enzymes, such as ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), and enzymes responsible for sucrose biosynthesis (Guy and others 1992; Holaday and others 1992).
Phenolics accumulation is observed under environmental stresses (Reuber and others 1996; von Ropenack and others 1998). Bound phenolics are integrated components of the cell wall determining its mechanical properties and protecting photosynthetic apparatus by absorption of UV radiation (Bilger and others 1997). Soluble phenolics contribute to scavenging reactive oxygen species (ROS), a common cause of damage to the photosynthetic membranes (Blokhina and others 2002; Nogues and Baker 2000).
The decreased growth rate that accompanies cold acclimation has been found to be associated with dynamic changes in growth-promoting and growth-suppressing phytohormones such as cytokinins, auxins, abscisic acid, jasmonic acid, and salicylic acid (Kosová and others 2012). The level of growth stimulants and stress-related plant hormones such as salicylic acid (SA), jasmonic acid (JA), and ethylene depends on the stage of cold acclimation (Kosová and others 2012). JA was shown to regulate C-repeat binding factor (CBF) during cold stress. Ethylene pathways are also involved in cold and freezing tolerance. 1-aminocyclopropane-1-carboxylic acid (ACC) application enhanced freezing tolerance of Arabidopsis (Catalá and others 2014) and 1-methylcyclopropene (1-MCP) reduced cold tolerance of tomato (Zhao and others 2009).
Our previous studies have also revealed the role of brassinosteroids in cold acclimation of winter rye (Pociecha and others 2016a). Brassinosteroids belong to a group of plant growth regulators that are responsible for cell division, cell expansion, and vegetative growth. These steroidal hormones also play a significant role in adaptive response to environmental stresses, such as high-temperature stress, oxidative stress, drought, and salinity (Yu and others 2004; Bajguz and Hayat 2009; Janeczko and others 2011), but their function during cold acclimation is poorly recognized (Pociecha and others 2016a) and requires further study. Investigation of the role of brassinosteroids during cold acclimation is important, as the mechanism of action of these phytohormones may be different at optimal versus cold temperatures. Janeczko and others (2007) have previously demonstrated the temperature-dependent effect of these hormones on cell membrane permeability and lipid composition as well as chlorophyll degradation. At optimal growth temperatures, brassinosteroids increase the quantum yield of photosystem II (PSII) and Rubisco activity and consequently boost concentrations of soluble sugars (Yu and others 2004). Brassinosteroids regulate the function of Rubisco in many ways. According to Xia and others (2009), brassinosteroids regulate Rubisco activity and also upregulate the expressions of rbcL and rbcS genes that encode subunits of this enzyme. In their study, transcript levels of rbcS and rbcL genes were elevated in 24-epibrassinolide (EBR)-treated plants, but were reduced in plants treated with a brassinosteroid inhibitor compared with control plants. The mechanisms underlying the effect of EBR on Rubisco activity may also be based on the impact of these hormones on enzymes directly or indirectly supporting Rubisco functioning, such as Rubisco activase (RCA) or carbonic anhydrase. According to Xia and others (2009), transcript levels of the rca gene were elevated in EBR-treated cucumber, with EBR also increasing RCA protein levels. Application of a brassinosteroid biosynthesis inhibitor reversed these effects. The protective effect of brassinosteroids on the efficiency of PSII can also be explained by their structural and physical properties. Dobrikova and others (2014) revealed that exogenous EBR alters the thermodynamic parameters of photosynthetic membranes of pea (Pisum sativum) plants under non-stress conditions. The revealed structural reorganizations most probably ensure structural stability, which is required to preserve the integrity of the membranes and the entire organelle (Dobrikova and others 2014).
Because the mechanisms underlying the action of brassinosteroids are involved in plant carbohydrate metabolism and photosynthesis, the processes crucial for cold acclimation, we studied their effect on frost tolerance of perennial ryegrass (Lolium perenne L.). The aim of our study was to investigate the mechanisms of photosynthetic apparatus adjustment and photoinhibition avoidance during cold acclimation in perennial ryegrass treated with 24-epibrassinolide. We specifically focused on chlorophyll a fluorescence, Rubisco, and sucrose phosphate synthase (SPS) activities, the profile of water-soluble carbohydrates, soluble and bound phenolics, and stress-related hormones such as salicylic acid, jasmonic acid, and precursor of ethylene 1-aminocyclopropane-1-carboxylic acid (ACC). Finally, we attempted to verify whether EBR-induced changes were related to frost tolerance.
Materials and Methods
Plant Materials and Experimental Design
Two cultivars of perennial ryegrass, Flinston and Amarant, were selected for our experiments. According to the Polish Institute of Plant Protection, National Research Institute (2015), Flinston is the more frost tolerant of these two cultivars. Plants were grown in pots (30 plants per 30 × 15 × 15 cm pot, 12 pots per cultivar) containing a mixture of soil:peat:sand (2:2:1 v/v/v) and fertilized weekly with Hoagland’s solution. The plants in pots were kept in a growth chamber at 18/16 °C (day/night) and illuminated for up to 12-h photoperiods with sodium lamps (AGRO Philips, Brussels, Belgium) at a light intensity of 250 µmol m−2 s−1 photosynthetic photon flux density (PPFD). At the three-leaf stage, the plants were divided into two groups. One group was sprayed with an EBR solution (0.25 mg dm−3) containing Tween 20, which was obtained by dilution with distilled water of an EBR stock solution (Sigma–Aldrich, Poznań, Poland) prepared in 1 ml 96% ethanol using an ultrasound bath. The second group (plants non-treated with EBR) was sprayed with aqueous solution of EBR solvent (distilled water with ethanol and Tween 20) and was used as a control. After spraying, the plants were kept at 18 °C for a week. Both groups (control and EBR treated) were then cold acclimated for 3 or 6 weeks at 4 °C under an 8-h photoperiod with a light intensity of 200 µmol m−2 s−1 PPFD. The data obtained from two independent, randomly arranged experiments conducted from September to mid December in 2013 and 2014 were combined. Before cold acclimation (after 1 week at 18 °C) and after 3 or 6 weeks of cold acclimation at 4 °C, the following parameters were evaluated: PSII efficiency, Rubisco, and SPS activities, and a profile of soluble sugars. After measurements and sampling, both EBR-treated and control plants that had been cold acclimated for 3 or 6 weeks were exposed to frost (see “Testing of frost tolerance” below) and their frost tolerance was estimated.
Slow Kinetic Fluorescence of Chlorophyll a
Parameters of the slow kinetic fluorescence of chlorophyll a were measured using an FMS2 fluorometer (Hansatech Ltd., Kings Lynn, UK). The following measurements were obtained from dark-adapted leaves held in leaf clips for 20 min: maximum quantum yield of PSII (Fv/Fm), variable fluorescence (Fv = Fm − Fo), fluorescence when all PSII reaction centers are closed (Fm), and chlorophyll a fluorescence when all PSII reaction centers are open (Fo). Measurements of maximum quantum yield of PSII in light-adapted leaves (Fvʹ/Fmʹ) were obtained from light-adapted material, with fluorescence when all PSII reaction centers are closed (Fmʹ) and steady-state fluorescence (Fs) was recorded after achieving stable values of Fs after re-exposure to light. Quantum efficiency of PSII (φPSII), (Fmʹ −Fs)/Fmʹ, was calculated according to Genty and others (1989). Photochemical quenching (qp), (Fmʹ − Fs)/(Fmʹ − Foʹ), where Foʹ is fluorescence in leaves previously exposed to light and darkened just before measurement, was calculated according to Schreiber and others (1994). NPQ was calculated as (Fm − Fmʹ)/Fmʹ according to Bilger and Björkman (1991).
Biochemical Analyses
The youngest fully developed leaves were collected for analyses of enzyme activities and carbohydrate composition. Rubisco (EC 4.1.1.39) activity was determined spectrophotometrically as described by Sharkey and others (1991), and SPS (EC 2.4.1.14) activity was measured according to the method of Kalt-Torres and Huber (1987). Enzyme activity was measured in five replications representing five leaves from different plants for each treatment. Carbohydrate composition was measured by high-performance liquid chromatography (HPLC) as described by Pociecha and Dziurka (2015). We determined the content of sugars such as glucose, fructose, sucrose, 1-kestose, and nystose. Each carbohydrate composition assay was repeated three times on bulk samples, each containing five leaves from different plants for each treatment.
Analyses of ACC, jasmonic acid, and salicylic acid were performed using HPLC. The apparatus used was Agilent Technologies 1260 equipped with Tandem Mass Spectrometer 6410 (MS–MS) with electrospray interface (ESI). Standards were obtained from OlChemim (Olomouc, Czech Republic), and the other chemicals from Sigma-Aldrich (Poznań, Poland). Frozen samples (0.5–1 g each) were homogenized in a mortar with liquid nitrogen. Three milliliters of extraction mixture: water/methanol/chloroform (3/5/12 v/v/v) were added and the samples were vigorously shaken for 30 min, sonicated for 5 min, and centrifuged at 2500 g for 5 min. Supernatant was collected and the extraction procedure was repeated with 2 ml of the extraction mixture, then both supernatants were pooled. The aliquot of the mixture comprising internal isotopic standards: [2H4] 1-aminocyclopropane-1-carboxylic acid and [2H6] jasmonic acid was added during the first extraction. Then, the chloroform and water–methanolic layers were separated, collected, and dried under the stream of nitrogen at 40 °C. Evaporated samples were reconstituted in methanol (water–methanolic fraction) and in acetonitrile (chloroform fraction). The samples were centrifuged at 21,000 g for 10 min, placed in HPLC vials and analyzed. 1-aminocyclopropane-1-carboxylic acid was analyzed in water–methanolic fractions injected into Phenomenex Kinetex 2.6u HILIC 100 A HPLC column, with 5 mM/dm3 ammonium formate dissolved in water and 5 mM/dm3 ammonium formate dissolved in acetonitrile as eluents. MS–MS was set to primary ion 130 and secondary ion 56. Jasmonic acid was analyzed in chloroform fractions injected into Supelco Ascentis Express RP-Amide 7.5 cm × 4.6 mm, 2.7 μm HPLC column, with 0.1% formic acid in water, and mixture of methanol and acetonitrile (1/1 v/v) as eluent. MS–MS was set to primary ion 211 and secondary ions 151 and 133. Salicylic acid was analyzed in water–methanolic fraction according to the HPLC procedure used for jasmonic acid. MS–MS was set to primary ion 139 and secondary ion 121.
Total phenolic content in both fractions was measured according to the Singleton method (Singleton and others 1999), modified by Bach and others (2015). About 15 mg of lyophilized and finely ground sample was extracted in 1 ml of H2O/HCOOH/methanol solution (4/1/15 v/v) for 15 min at 30 Hz (MM 400, Retach, Kroll, Germany). Then, the samples were centrifuged (15 min, 22,000 × g, 10 °C, Universal 32R, Hettich, Germany) and the supernatant was collected. The extraction procedure was repeated and the supernatant was pooled. It comprised the fraction of soluble phenolics. Next, an aliquot of the extract was collected for total soluble phenolic analysis. The remaining solution was evaporated under nitrogen stream (TurboVap LV, Capiler Ltd., MA, USA) and kept for HPLC analyses. The pellet was hydrolyzed with 3 M NaOH, the suspension was neutralized with HCl, and the released phenolics were extracted as above. They comprised the fraction of cell wall-bound phenolics. An aliquot of pooled extract was collected for estimation of total phenolics released after hydrolysis.
Testing of Frost Tolerance
Frost tolerance was estimated using a modified version of Larsen’s method (1978). Pots with plants that had been cold acclimated for 3 or 6 weeks were transferred to a freezing chamber at a temperature of 4 °C. The temperature was then reduced to −12 °C at a rate of 3 °C h−1 under dark conditions and held at this level for a period of 6 h. Then temperature was subsequently increased to 4 °C at a rate of 3 °C h−1 and cold acclimation conditions were recreated. Twenty-four hours later, all plants were cut and then exposed to conditions of 12 °C, a 12-h photoperiod and 250 μmol m−2 s−1 PPFD for regrowth. After 10 days, regrowth was estimated using Larsen’s (1978) visual score, where 0 corresponds to a completely dead plant and 9 indicates a plant with no symptoms of injury.
Statistical Analysis
To validate the use of parametric tests, the normality assumption of the ANOVA was verified by the Shapiro–Wilk test. A Levene test was used to check the homoscedasticity assumption. Data were analyzed with multi-factor analysis of variance (MANOVA). Graphs were plotted using means and standard errors for each data point. A post hoc comparison was conducted using Duncan’s multiple range test (p = 0.05). All calculations were carried out using the STATISTICA 12.0 (StatSoft Inc., Tulsa, OK, USA) software package.
Results
Slow Kinetic Fluorescence of Chlorophyll a Parameters
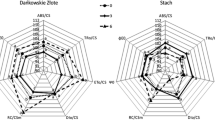
In both cultivars, EBR treatment modified photosynthetic efficiency, with the observed changes dependent on cultivar and length of cold acclimation (Fig. 1). In the moderately tolerant Amarant cultivar, EBR did not affect any investigated parameters except for NPQ. In contrast to the resistant cultivar, the value of NPQ increased gradually compared with non-acclimated plants and reached its highest point after 6 weeks of cold acclimation.
Parameters of slow kinetic fluorescence of chlorophyll a in the leaves of control and 24-epibrassinolide (EBR)-treated non-cold-acclimated perennial ryegrass plants and in plants after 3 and 6 weeks of cold acclimation. The following parameters were measured: maximum quantum yield in dark-adapted (Fv/Fm) and light-adapted (Fv ʹ/Fmʹ) leaves, quantum efficiency of PSII (φPSII), photochemical quenching (qp), and non-photochemical quenching (NPQ). The presented data (arbitrary units) are mean values for the EBR-treated plants, expressed as a percentage of the control values based on 10 replicates. Mean values marked with the same letter within a parameter and a cultivar did not differ significantly according to Duncan’s test at p ≤ 0.05
In the resistant Flinston cultivar after 3 weeks of cold acclimation, EBR induced the NPQ mechanism, which coincided with a sharp decrease in φPSII and qP compared with plants before cold acclimation and those that were cold acclimated for 6 weeks. After 6 weeks of cold acclimation, φPSII and qP values returned to pre-acclimation levels; this change was accompanied by only a slight decrease in NPQ, which was still markedly higher than that of non-acclimated plants.
Rubisco and SPS Activities
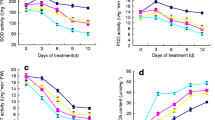
In both cultivars under optimal temperature before cold acclimation, EBR mediated increases in Rubisco and SPS activities (Figs. 2, 3). Under cold conditions, EBR significantly decreased the activity of both enzymes in the leaves of Amarant. As for Flinston, slight decreases were found in Rubisco and SPS activities only after 3 weeks of cold acclimation. In plants acclimated for 6 weeks, EBR stimulated the activity of both enzymes (Figs. 2, 3).
Rubisco activity in the leaves of control and 24-epibrassinolide (EBR)-treated non-cold-acclimated perennial ryegrass plants and in plants after 3 and 6 weeks of cold acclimation. The presented data are mean values based on five replicates. Mean values marked with the same lowercase letter within a cultivar and the same uppercase letters within term did not differ significantly according to Duncan’s test at p ≤ 0.05
Sucrose phosphate synthase (SPS) activity in the leaves of control and 24-epibrassinolide (EBR)-treated non-cold-acclimated perennial ryegrass plants and in plants after 3 and 6 weeks of cold acclimation. The presented data are mean values based on five replicates. Mean values marked with the same lowercase letter within a cultivar and the same uppercase letters within term did not differ significantly according to Duncan’s test at p ≤ 0.05
Composition of Soluble Carbohydrates
Exogenous EBR modified soluble carbohydrate content, which was dependent on cultivar and duration of cold acclimation (Fig. 4). In non-acclimated control plants of both cultivars, EBR triggered an increase in fructose levels, a decrease in sucrose levels, and no change in glucose levels. In regard to fructooligosaccharides (FOSs), nystose was not detected while 1-kestose increased only in Amarant.
In moderately resistant Amarant, 3 and 6 weeks of cold acclimation under EBR treatment resulted in a decrease in monosaccharides (glucose and fructose) and an increase in sucrose (Fig. 4). Both examined FOSs decreased after 3 weeks and increased after 6 weeks of cold acclimation. In 3-week acclimated Flinston plants, the lack of changes in monosaccharides coincided with decreased sucrose level. A decrease in monosaccharide’s occurred later after 6 weeks of cold acclimation and was accompanied by an increase in sucrose and FOSs.
Soluble and Bound Phenolics
EBR affected the concentration of soluble phenolics in different ways, depending on the investigated cultivar. After 3 weeks of cold acclimation, EBR significantly increased soluble phenolic concentrations in the leaves of Amarant, and for Flinston a sharp decrease was found (Fig. 5). Contrary to that, after 6 weeks of cold acclimation soluble phenolic contents were reduced in Amarant and enhanced in Flinston. Cell wall-bound phenolic contents did not change under EBR treatment with the exception of Flinston, where after 3 weeks of cold acclimation a significant increase was noticed. Additionally, the increase of bound phenolics in Flinston accompanied by the decrease of soluble phenolics suggested that they were used in cell wall strengthening.
Soluble and cell wall-bound phenolics in the control and 24-epibrassinolide (EBR)-treated leaves of Amarant and Flinston cultivars of perennial ryegrass after 3 and 6 weeks of cold acclimation. Mean values marked with the same letter within a parameter did not differ significantly according to Duncan’s test at p ≤ 0.05
ACC, JA, and SA Content
In control plants, prolonged cold resulted in the lowered levels of ACC in both cultivars, lowered (Amarant) and stable (Flinston) JA levels and no changes in SA levels. In Flinston, EBR applied before cold acclimation mediated an increase in ACC and JA but not SA content (Fig. 6). As for Amarant, raised content of ACC was observed only after 6 weeks of cold acclimation. In Flinston, EBR significantly decreased SA concentrations at the later stage of cold acclimation (after 6 weeks), as compared to its concentration after 3 weeks of cold acclimation.
ACC, JA, and SA contents in the control and 24-epibrassinolide (EBR)-treated leaves of Amarant and Flinston cultivars of perennial ryegrass after 3 and 6 weeks of cold acclimation. Mean values marked with the same letter within a parameter did not differ significantly according to Duncan’s test at p ≤ 0.05
Frost Tolerance
The frost tolerance of control plants of the Amarant cultivar was similar after 3 and 6 weeks of cold acclimation (Table 1). In the case of Flinston, a longer cold acclimation induced higher frost resistance. Foliar EBR application did not increase the frost tolerance of Amarant compared with control plants acclimated for 6 weeks. However, 6 weeks of cold acclimation and EBR application resulted in an increase in frost tolerance compared with plants cold acclimated for 3 weeks. In Flinston, EBR caused lack of changes after 3 weeks of cold acclimation and a significant increase in frost tolerance after 6 weeks of cold acclimation.
Discussion
Several studies have shown that brassinosteroids are directly involved in the regulation of photosynthesis at optimal growth temperatures (Yu and others 2004; Janeczko and others 2016). In the present study, the response of plants to EBR treatment was similar in both cultivars prior to cold acclimation. The only differences involved quantum efficiency of PSII (φPSII) and photochemical quenching (qp), which were higher in the highly frost-tolerant Flinston cultivar compared with the control. Independent of differences in photosynthetic efficiency between the two cultivars, EBR increased Rubisco and SPS activities-a change that was accompanied by lowering of sucrose levels coupled with an increase in fructose. This result suggests that at optimal growth conditions, EBR in the lesser extent influenced the light reactions of photosynthesis than the dark reactions of carbon fixation and carbohydrate metabolism. Our findings are consistent with the previous studies obtained on barley (Hordeum vulgare) mutants. Disruption of brassinosteroid biosynthesis resulted in decreases in Rubisco activity and sucrose concentration accompanied by increases in glucose and fructose contents (Janeczko and others 2016). Defects in brassinosteroid biosynthesis did not disturb the efficiency of PSII and even slightly increased the effectiveness of this photosystem (Janeczko and others 2016).
In contrast to observations made under optimal growth conditions, very little is known about whether brassinosteroids play a role in the alteration of photosynthetic acclimation during prolonged growth under cold temperatures. Downregulation of photosynthetic efficiency is a mechanism that allows plants to survive winter conditions. Cold acclimation usually is accompanied by a decrease in φPSII and qp together with an increase in NPQ (Rapacz and others 2004). A similar relationship between sugar distribution, photosynthetic efficiency, and winter resistance was also observed in our earlier study on Festulolium (Pociecha and others 2010). In that study, the resistant genotype showed a decrease in leaf sugar content after 3 weeks of cold acclimation that was associated with a lower reduction in NPQ and a higher decline in qp and φPSII; this was in contrast to the susceptible line, where an increased level of carbohydrates was connected with lower reductions of qp and φPSII.
In the present study, the NPQ mechanism was activated by EBR in the Flinston cultivar after 3 weeks of cold. The decrease in φPSII, which is related to limited consumption of NADPH, was accompanied by lowered Rubisco and SPS activities. Low Rubisco activity, probably due to low internal concentration of CO2, decreased the pool of the electron acceptor NADP+ available on the acceptor side of photosystem I. Moreover, a decline in the activity of SPS, an enzyme responsible for the export of sucrose to acceptor tissues, was followed by a decrease in sucrose levels. The suppression of φPSII and Rubisco and SPS activities was transient and can be attributed to photoinhibition avoidance via NPQ. Thanks to the NPQ mechanism, the level of excitation energy in the PSII antenna can be regulated to prevent over-reduction of the electron transport chain. After 6 weeks of cold acclimation, a marked increase in φPSII and qP reflected increased demands for ATP and NADPH, as an increase in qP is related to increased consumption of reductants and ATP (Nogues and Baker 2000). At the same time, EBR increased Rubisco activity, and, as a result of increased carbon fixation, SPS activity and sucrose levels. These changes can thus be attributed to the adaptation of the photosynthetic apparatus to function at low temperatures, in contrast to changes observed after 3 weeks at 4 °C, when plants redirected their metabolism to deal with stress. In general, EBR accelerated the recovery of the photosynthetic apparatus and enzyme activities, which reflected an adjustment to cold conditions and contributed to increased frost tolerance.
In the Amarant cultivar, an increase in sucrose levels was accompanied by a decrease in Rubisco and SPS activities during the cold acclimation period. An accumulation of cytoplasmic sucrose causes a build-up of triose and hexose phosphates as a consequence of feedback inhibition of SPS by sucrose (Paul and Foyer 2001). This observation suggests that EBR triggered an excess of sugars in the leaves of the Amarant cultivar, which in turn induced feedback inhibition of photosynthesis. Instead of being exported, carbohydrates accumulated in leaves and suppressed photosynthesis, as high sugar concentrations have been shown to downregulate photosynthetic genes including Rubisco activase (Jurczyk and others 2016). Interestingly, downregulation of photosynthesis in Amarant did not diminish frost tolerance, which remained at the control level. Most likely, another protective mechanism stemming from brassinosteroid structural and physical properties was operating, such as stabilization of membrane structure similar to that due to sterols at cold temperatures (Grunwald 1974, Janeczko and others 2007).
Because accumulated reserves are the source for regrowth of new leaves from surviving subapical meristems, the strength of the sink is important for winter survival. Sugars allocated from leaves to crowns not only provide energy resources, but also lead to anatomical changes that support defense mechanisms. In winter rye, callose synthesis in crown tissue (Pociecha and others 2013; Żur and others 2011) and strengthening of the cell wall matrix with structural carbohydrates (Pociecha and others 2016b) accompany plant resistance to winter stress factors. In Flinston plants exposed to 3 weeks of cold acclimation, EBR enhanced the level of cell wall-bound phenolics which also participate in cell wall reinforcement. Additionally, the increase of bound phenolics was accompanied by a decrease in soluble phenolics indicating their utilization as building material in the cell wall structure.
In Flinston, EBR pre-treatment enhanced ACC and JA accumulations during cold acclimation. The elevated level of these signaling compounds corresponded with the results of Gaudet and others (2010), who reported an upregulation in ethylene and jasmonic acid pathways in freezing susceptible line CI14106 and to a lesser extent in cold hardy line DH + 268, particularly in later stages of hardening at 2 °C from days 7 to 49. According to their work, full expression of the gene-controlling jasmonic acid synthesis, allene oxide synthase (AOS) was achieved after 42 days of hardening treatment. In our study, the highest level of JA was noticed in EBR-treated Flinston plants exactly after 6 weeks of cold acclimation. The interaction between BRs and JA in abiotic stress adaptation is still unclear, however, it is hypothesized that BRs might influence plant stress responses by stimulating JA biosynthesis (Ahammed and others 2015). In Flinston, EBR-mediated increase of ACC level was consistent with the results published by Hansen and others (2009) who found a positive influence of BRs on ET biosynthesis through the regulation of ACS and ACC oxidase activity. The lack of changes or decreased SA level in the study could be explained by mutual antagonism of JA and SA. It is known that mutations that disrupt JA signaling (coi1) lead to enhanced basal and inducible expression of SA marker gene PR1, whereas the mutations that disrupt SA signaling (npr1) lead to concomitant increase in the basal or induced levels of JA marker gene PDF1.2 (Kazan and Manners 2008). According to Kosova and others (2012), 21 days of low temperature stimulated the accumulation of endogenous JA in winter wheat, whereas prolonged cold treatment was associated with a decrease of SA.
Taken together, our results demonstrate that brassinosteroid-induced regulation of photosynthetic efficiency and activities of photosynthetic enzymes such as Rubisco and those involved in sugar metabolism are temperature and stage of cold acclimation dependent. Our findings emphasize the role of EBR in enhancing photoprotection mechanisms during prolonged exposure to cold. It is also likely that EBR participated in the enhancement of frost tolerance not only by altering photosynthesis but also by regulation of stress-related signaling compounds such as JA and ethylene but not SA in winter rye undergoing cold acclimation. However, as evidenced by the increase in frost tolerance observed only in the highly frost-tolerant cultivar, the beneficial effect of EBR is not universal. It is suggested that genetically determined mechanisms may be dominant relative to the action of exogenous EBR. However, to understand the role of EBR in sink size regulation and hormone homeostasis more detailed investigations are needed.
References
Adams WW, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Ahammed GJ, Xia XJ, Li X, Shi K, Yu JQ, Zhou YH (2015) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16:462–473
Bach A, Kapczyńska A, Dziurka K, Dziurka M (2015) Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia ‘Ronina’and ‘Rupert’in vitro cultures under different lighting environments. Sci Hortic 188:23–29
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bilger W, Björkman O (1991) Temperature-dependence of violaxanthin deepoxidation and nonphotochemical fluorescence quenching in intact to leaves Gossypium-hirsutum L. & Malva Parviflora L. Planta 184:226–234
Bilger W, Veit M, Schreiber L, Schreiber U (1997) Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant 101:754–763
Blokhina O, Virolainen E, Fagerstedt KV (2002) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Catalá R, López-Cobollo, Mar Castellano R, M Angosto T, Alonso JM, Ecker JR, Salinas J (2014) The Arabidopsis 14-3-3 Protein RARE COLD INDUCIBLE 1 A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 26:3326–3342
Dobrikova AG, Vladkova RS, Rashkov GD, Todinova SJ, Krumova SB, Apostolova EL (2014) Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non-stress conditions. Plant Physiol Biochem 80:75–82
Fowler DB, Limin AE, Wang SY, Ward RW (1996) Relationship between low-temperature tolerance and vernalization response in wheat and rye. Can J Plant Sci 76:37–42
Gaudet DA, Wang Y, Frick M, Puchalski B, Penniket C, Ouellet T, Robert L, Singh J, Laroche A (2011) Low temperature induced defence gene expression in winter wheat in relation to resistance to snow moulds and other wheat diseases. Plant Sci 180:99–110
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Grunwald C (1974) Sterol molecular modifications influencing membrane permeability. Plant Physiol 54:624–628
Guy CL, Huber JLA, Huber SC (1992) Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol 100:502–508
Hansen M, Chae HS, Kieber JJ (2009) Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J 57:606–614
Holaday AS, Martindale W, Alred R, Brooks A, Leegood RC (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol 98(1105–1):114
Huner NPA, Öquist G, Hurry VM, Krol M, Falk S, Griffith M (1993) Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth Res 37:19–39
Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3:224–230
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358
Janeczko A, Oklestkova J, Pociecha E, Kościelniak J, Mirek M (2011) Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiologiae Plantarum 33:1249–1259
Janeczko A, Gruszka D, Pociecha E, Dziurka M, Filek M, Jurczyk B, Kalaji HM, Kocurek M, Waligórski P (2016) Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. Plant Physiol Biochem 99:126–141
Jurczyk B, Rapacz M, Pociecha E, Kościelniak J (2016) Changes in carbohydrates triggered by low temperature waterlogging modify photosynthetic acclimation to cold in Festuca pratensis. Environ Exp Bot 122:60–67
Kalt-Torres W, Huber SC (1987) Diurnal changes in Maize leaf photosynthesis. Plant Physiol 83:294–298
Kazan K, Manners JM (2008) Jasmonate signaling: toward an integrated view. Plant Physiol 146:1459–1468
Kosová K, Prášil IT, Vítámvás P, Dobrev P, Motyka V, Floková K et al (2012) Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol 169:567–576
Larsen A (1978) Freezing tolerance in grasses. Methods for testing in controlled envinronments. Department of Farm Crops Report no. 195. As, Norway, Sci. Rep. Agr. University Kraków
Nogues S, Baker N (2000) Effects of drought on photosynthesis in Mediterranen plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Pociecha E, Dziurka M (2015) Trichoderma interferes with cold acclimation by lowering soluble sugars accumulation resulting in reduced pink snow mould (Microdochium nivale) resistance of winter rye. Environ Exp Bot 109:193–200
Pociecha E, Płażek A, Rapacz M, Niemczyk E, Zwierzykowski Z (2010) Photosynthetic activity and soluble carbohydrate induced by the cold acclimation affect frost tolerance and resistance to Microdochium nivale of androgenic Festulolium genotypes. J Agron Crop Sci 196:48–54
Pociecha E, Janowiak F, Dubas E, Żur I, Tokarz K, Kolasińska I, Płażek A (2013) Progress of snow mould infection in crowns of winter rye (Secale cereale L.) is related to photosynthetic activity during cold acclimation. Plant Physiol Biochem 70:360–367
Pociecha E, Dziurka M, Oklestkova J, Janeczko A (2016a) Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. Plant Growth Regul 80:127–135
Pociecha E, Rapacz M, Dziurka M, Kolasińska I (2016b) Mechanisms involved in the regulation of photosynthetic efficiency and carbohydrate partitioning in response to low- and high-temperature flooding triggered in winter rye (Secale cereale) lines with distinct pink snow mold resistances. Plant Physiol Biochem 104:45–53
Polish Institute of Plant Protection, National Research Institute (2015) Methodology of integrated protection of selected grass species. Poznań. pp. 9 (in polish). http://www.minrol.gov.pl/Informacjebranzowe/Produkcjaroslinna/Ochronaroslin/Integrowana-ochrona-roslin/Metodyki-integrowanej-ochrony-roslin
Rapacz M, Gąsior D, Zwierzykowski Z, Leśniewska-Bocianowska A, Humphreys MW, Gay AP (2004) Changes in cold tolerance and the mechanisms of acclimation of photosystem II to cold hardening generated be anther culture of Festuca pratensis ´ Lolium multiflorum cultivars. New Phytol 161:105–114
Reuber S, Bornman JF, Weissenböck G (1996) Phenylpropanoid compounds in primary leaf tissue of rye (Secale cereale). Light response of their metabolism and the possible role in UV-B protection. Physiol Plant 97:160–168
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schultze E-D, Caldwell MM (eds) Excophysiology of photosynthesis. Springer, Berlin, pp 49–70
Sharkey TD, Savitch LV, Butz ND (1991) Photometric method for routine determination of kcat carbamylation of rubisco. Photosynth Res 28:41–48
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 299:152–178
von Ropenack E, Parr A, Schulze-Lefert P (1998) Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem 273:9013–9022
Xia XJ, Huang LF, Zhou YH, Mao WH, Shi K, Wu JX, Asami T, Chen Z, Yu JQ (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogué S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Zhao D, Shen L, Fan B, Yu M, Zheng Y, Lv S, Sheng J (2009) Ethylene and cold participate in the regulation of LeCBF1 gene expression in postharvest tomato fruits. FEBS Lett 583:3329–3334
Żur I, Dubas E, Pociecha E, Dubert F, Kolasińska I, Płażek A (2011) Cytological analysis of infection process and the first defence responses induced in winter rye (Secale cereale L.) seedlings inoculated with Microdochium nivale. Physiol Mol Plant Pathol 76:189–196
Acknowledgements
This study was financed by the National Science Centre of Poland (DEC-2011/03/D/NZ9/05548).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pociecha, E., Dziurka, M., Waligórski, P. et al. 24-Epibrassinolide Pre-Treatment Modifies Cold-Induced Photosynthetic Acclimation Mechanisms and Phytohormone Response of Perennial Ryegrass in Cultivar-Dependent Manner. J Plant Growth Regul 36, 618–628 (2017). https://doi.org/10.1007/s00344-016-9662-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9662-6