Abstract
Temperature-dependent emission spectra and fluorescence dynamics profiles have been investigated in Pr3+:Y4Al2O9 crystals in order to better understand the processes responsible for quenching of the praseodymium 3P0 emissions. The cross-relaxation transfer rates were experimentally determined as a function of temperature. Using the rate equations formalism, the dynamics of the observed emissions were modeled. Basing on comparison between the measured and calculated decays, the energy transfer rates between Pr3+ ions were evaluated. The role of the backward process in explanation of the complicated character of 3P0 decays and its temperature dependence, especially its unexpectedly slow decaying component, were established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Trivalent praseodymium (Pr3+) ion is continuously considered as a promising activator for solid state lasers, optical amplifiers, scintillation detectors, sensors, quantum memories, solar converters and various phosphors [1–4]. This is due to various strong emissions, resulting from both inter-configurational d-f and intra-configurational f–f transitions extending from UV to near infrared wavelengths, which could be generated in Pr3+-activated media. The energy level diagram of Pr3+ ion indicates that praseodymium materials have advantageous ability to be pumped by the commercially available GaN blue diodes and lasers [5] via strong 3H4 → 3P1,2 absorption.
It was pointed out that various transitions from the excited 3P0 and 1D2 states are of interest in Pr3+ systems, among them the 3P0 → 3F2 and the 1D2 → 3H4 transitions, which are associated with intense red emissions in the 600 nm region (see energy level diagram in Fig. 1). In several applications, strong, spin-allowed transitions originating from the 3P0 level are utilized. It is thus interesting to investigate the effects of concentration and temperature on these emissions in Pr3+-doped Y4Al2O9 (abbreviated YAM) crystals. Very few reports [6, 7] are available on the spectroscopic properties of this system so far. Previous studies of Pr3+:YAM concentrated on its basic optical and spectroscopic properties such as the position of Pr3+ energy levels in 4f2 configuration and the multisite character of this system [6, 7]. Thus, there are some interesting basic processes such as nonradiative relaxations and multi-ion processes that need to be explored. Since such processes in phosphor materials often occur via thermal activation, understanding them is vital to elucidate the luminescence mechanisms. In addition, as it was recently demonstrated, the temperature dependence of the Pr3+ fluorescence features can be used to measure temperature [8–10] and is interesting from the application point of view in optical thermometry.
Thus, the purpose of this paper is three-fold: to extend our knowledge on luminescence properties of the new praseodymium system, to get insight into processes of excitation energy distribution after pumping the 3P0 level of Pr3+ ions and to study and model the 3P0 state dynamics in the function of temperature.
2 Experimental
Samples used in our study were grown in the Institute of Electronic Materials Technology (ITME) in Warsaw. As YAM undergoes the phase transition at about 1,300 °C and crystals grown by standard Czochralski method crack during cooling, the micro-pulling down (μ-PD) method was used to obtain YAM samples. The μ-PD method was invented in Japan, originally for growth of single-crystal fibers [11]. This method was then used in ITME for preparing YAG [12] single crystals and, for the first time to our knowledge, for growing YAM.
Polycrystals in the form of rods 2–3 mm in diameter and several cm long were obtained. Four YAM:Pr3+ samples with activator concentrations of 0.1, 1, 5 and 10 at.% were used in our studies.
The yttrium–alumina system has several stable phases including monoclinic Y4Al2O9 (YAM), cubic garnet Y3Al5O12 (YAG), orthorhombic perovskite YAlO3 (YAP) and a metastable hexagonal perovskite YAlO3 phase (YAH) observed during the synthesis by soft chemistry methods. YAM forms monoclinic crystals with space group P21/c. The Y atoms, having C1 site symmetry, are coordinated to either six or seven oxygen atoms [13]. The shortest distance between the Y3+ ions is 3.65 Å. There are four formula units in the unit cell of Y4Al2O9 and four different rare earth sites in the asymmetric unit.
Emission spectra were measured using CVI DK-480 grating monochromator followed by a cooled EMI C1034-02 GaAs photomultiplier and SR-400 photon counting system. The samples were excited by pulsed (10 ns pulse-width, repetition rate 10 Hz) Continuum Surelite Nd:YAG laser with third harmonic generator, followed by an optical parametric oscillator, or by CW Coherent Innova 300, a 10 W argon ion laser. Fluorescence dynamics profiles were recorded with Stanford Research SR-430 multi-channel analyzer controlled with a PC computer. The best temporal resolution of the experimental apparatus was 5 ns.
Temperature dependence of the sample fluorescence in the range 300–1,300 K was measured in a self-made resistive heat cell. The temperature of the samples was monitored by a Pt/Rh thermocouple and controlled with accuracy of about 1 K by Eurotherm PID temperature controller type 3024. Sample cooling was provided by a Displex Model CSW-202 closed cycle He optical cryostat which allowed the temperature to be varied between 10 and 300 K.
3 Results
3.1 Emission
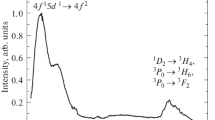
After excitation of the 3PJ levels by blue-violet laser radiation, rich emission spectrum extending from blue to red wavelength could be observed. These emissions are attributed to transitions originating mostly from the 3P0 level. However, also weak fluorescence starting from the 1D2 state could be observed in the red-infrared part of the spectrum. Only in the 550–650 nm range, where emissions from the 3P0 and 1D2 levels overlap, emission corresponding to the 1D2 → 3H4 transition dominates over 3P0 → 3F2 + 3H6 one. In Fig. 2, part of the emission spectrum in the 600 nm region after selective excitation is shown. To well distinguish between 3P0 and 1D2 lines, time resolved selectively excited emission spectra were also recorded at 10 and 300 K as well as the 1D2 → 3H4 emission spectra following direct excitation into 1D2 level.
The observed line positions are consistent with the data reported in the literature for Pr3+:YAM [6]. In Fig. 3, the temperature-dependent emission measurements of 1 at.% Pr3+:YAM in the 560–720 nm wavelength range after blue 3P2 excitation are shown. It is observed that both 3P0 and 1D2 lines are present in the spectrum and that, as temperature is increased, the overall luminescence intensity decreases and the intensity of 1D2 emissions increases relatively to that of the 3P0 (at 660 nm) one. Figure 3 indicates that temperature quenching of the 3P0 emission is stronger than that of the 1D2 one.
3.2 Excited state dynamics
Decays originating from the 3P0 and 1D2 levels were measured in the function of activator concentration and temperature. 3P0 decays were measured at 541 nm where an intense emission occurs. This wavelength was chosen as it is spectrally isolated from the 1D2 emission. Nonexponentiality of the 3P0 and 1D2 decays was observed even at low dopant concentrations, and at low temperatures indicating that energy transfer processes strongly contribute to the decays of these two luminescent levels. It was also observed that, as the concentration of Pr3+ ions was increased, the 3P0 fluorescence decays shortened and became strongly nonexponential, see Fig. 4. Low temperature 3P0 lifetime determined in 0.1 % Pr3+-doped sample from the long time part of the decay was 13 μs when the 1D2 decay in the lowest concentration sample indicated lifetime of 378 μs which is in agreement with results for other praseodymium-activated oxide crystals like LaAlO3 [14]. When the 3P0 decays were registered in long time scale and over several e-foldings, the presence of a weaker, slow component of the decay extending up to about 10 ms has been observed.
The luminescence decays of the 3P0 level in 1 % Pr3+:YAM for several different temperatures are plotted in Fig. 5. The decays shorten with increasing temperature, indicating efficient nonradiative quenching. From Fig. 5, it can be also seen that slow component of the 3P0 state decays becomes stronger with the increase in temperature. The 1D2 excited level decays are less temperature influenced up to about 900 K; however, at higher temperatures, these decays become much faster and strongly nonexponential.
4 Discussion
In YAM, as well as in some other praseodymium compounds, it is observed that upon photo-excitation of the 3P0 level not only emission from the 3P0 level is observed, but also from the 1D2 one [15, 16]. Despite the extensive studies on praseodymium systems that have been done and presented in literature conclusions regarding the mechanism of relaxation from 3P0 to 1D2 level are still not consistent. There are three decay paths of interest possible: radiative, multiphonon relaxation and energy transfer. Radiative transitions from 3P0 to 1D2 are spin forbidden and have very low probability [17]. Also, as these levels are separated by about 3,500 cm−1, the probability of multiphonon process in the system considered here is not significant [18]—of the order of 103 s−1 [17] at room temperature. The T dependence of multiphonon relaxation rate (MPR) can be written as [19]:
where p is the number of phonons involved in the nonradiative decay p = ΔE/ħω and WNR(0) is the multiphonon decay rate at 0 K, given as
where ΔE is the energy gap to the next lower energy level, α and β are phenomenological parameters. With the use of α and β determined for YAG in [17] and taking the maximum phonon energy of the YAM host of 812 cm−1 as determined from Raman scattering data, ΔE = 3,500 cm−1, the temperature dependence of WNR for 3P0 → 1D2 transition was calculated. In the result, at high temperature of 1,300 K, we have WNR(1,300 K) = 3.8 × 103 s−1 which is still much lower than the 3P0-radiative decay rate of 7.7 × 104 s−1.
Therefore, it is concluded here that for populating the 1D2 level and resulting emission energy transfer process is most likely. Also, this energy transfer, called cross-relaxation (CR), is responsible for sharp decrease in the luminescence with increasing activator concentration in most praseodymium compounds. CR process has been first studied by Hegarty et al. [20] and by Vial and Buisson [21] in Pr3+:LaF3 crystals. It was shown that in praseodymium-activated solids, concentration quenching of the 3P0 fluorescence dynamics is related to the CR mechanism of the type (3P0, 3H4) → (3H6, 1D2). Another (3P0, 3H4) → (1G4, 1G4) process can be disregarded because of a large energy mismatch of about 800 cm−1 and because both participating transitions are spin forbidden. Wu et al. [22] reported on the backward process of the type (1D2, 3H6) → (3H4, 3P1) which reaches 3P1 state and after fast nonradiative relaxation results in refeeding the 3P0 state. The energy levels of YAM [6] indicate that this process could excite either the 3P1 or directly the 3P0 levels depending on the energy of the initial 3H6 Stark level.
This agrees well with our observations of the slow component, with the lifetime close to the half of the 1D2 one, in the 3P0 decay (see Fig. 6) and its increase in intensity with temperature. Discussed mechanisms are presented schematically in Fig. 7, where the Pr3+ pair system has been reduced to the main levels of CR process.
In order to determine the CR rates and its temperature dependence and to model fluorescence dynamics, the following system of differential rate equations was proposed and numerically solved.
where n 0, n 1, n 2 and n 3 are the populations of the 3H4, 3H6, 1D2 and 3P0 levels of Pr3+, respectively (units of cm−3), x and y are forward and backward CR transfer rates, respectively (units of s−1cm3), A ik are the radiative transition probabilities between the i and k states, W i is the phonon de-excitation rate for the i-th level (units of s−1). As only the mentioned four levels are involved, we have n 1 + n 2 + n 3 + n 4 = N, where N is Pr3+ concentration in the sample (units of cm−3).
Also, A ik = β ik A i , where β ik are the branching ratios and A i radiative transition probabilities of the 3P0 (i = 3) and 1D2 (i = 2) states. Thus, for evaluating A 3k and A 2k (where k = 0.1) values, the corresponding branching ratios were obtained from the normalized fluorescence spectra by comparing the relative areas under the emission peaks. It was also assumed that A 32 = 0 and W 21 = 0 which means that 3H6 is only populated by radiative transitions from 3P0 and 1D2 levels. No value of the 3H6 state lifetime is reported for praseodymium-activated oxide hosts. Wu et al. [22] assumed in their calculations for Pr:YAG the 3H6 lifetime of 5 ms, that is, the decay rate of 2 × 102 s−1. However, because of the small energy gap of the order of 1,200 cm−1 [6] to the 3H5 level, the highest limit of the decay rate of 3H6 level (W 1 + A 1) is estimated to be about 106 s−1. Because 1D2 decay is temperature dependent, effective lifetime values of the 1D2 level experimentally determined for each temperature were used in our calculations. Forward and backward transfer rates (x and y, respectively) were treated as adjustable parameters. Values of most parameters necessary to solve the above indicated system of equations have been experimentally determined and are listed in Table 1.
From the presented model of CR transitions, it may be concluded that the dynamics of 3P0 excited state is strongly affected by both forward and backward transfer rates as well as by the lifetime of 1D2 level. Because of the relatively fast relaxation, the influence of 3H6 level on 3P0 decay curve can be excluded. Simple numerical solutions showing influence of x and y transfer rates on dynamics of 3P0 level are presented in Fig. 8. As could be seen, the x factor is responsible for quenching of the luminescence from 3P0 state, resulting in shortening of its lifetime when the y process activates the long part on temporal characteristic with decay constant equal to half of 1D2 lifetime.
Decay profiles of 3P0 level calculated from the differential equations system (3): a For different forward CR transfer rates—x (for y = 0 μs−1cm3). Inset presents dependence of the estimated 3P0 lifetime on x. Each data point in the inset corresponds to one decay profile. b For different backward CR transfer rates—y (for x = 2 × 10−21 μs−1cm3). The short and the long time part of the decay curves represents the lifetime of 3P0 state and the half of 1D2 level lifetime, respectively
The next step in our investigation was to determine the 3P0 CR rates for 1 % doped sample, X and Y with units of s−1, and their temperature dependence. X and Y are related to x and y as follows: X,Y = x, y × N, where N equals 2 × 1020 cm−3 for 1 at. % doped YAM sample [23].
Our analysis indicated that the short-time exponential part of the decays represents the 3P0 lifetime; thus, cross-relaxation transfer rates could be simply calculated as X = 1/τ−1/τ0, where τ is the fluorescence decay time, and τ0 is the isolated ion lifetime of 13 μs measured in the lowest concentration sample. This value is close to the value of 16 μs determined in [6] for the praseodymium B site in YAM. It should also be noted that in our experiments at room and higher temperatures, no emission lineshapes nor fluorescence dynamics dependence on excitation wavelengths, which may result from the multisite character of YAM, were observed.
The determined quenching rates X are plotted as a function of temperature for 1 % Pr3+:YAM in Fig. 9. It is observed that the cross-relaxation rate remains practically constant up to about 180 K and starts to increase rapidly at higher temperatures. As the 3P0 radiative decay rate is independent on temperature and phonon-assisted relaxation is weak, with the cutoff phonon frequency of YAM equal 812 cm−1 about 4 phonons are required to bridge the 3P0 → 1D2 energy gap, then this dependence results from thermal activation of Stark levels participating in the forward CR process, thus influencing the spectral overlap between the donor and acceptor transitions. However, temperature dependencies of the shape, peak intensities and widths of participating transition could also be considered. Such thermally activated process normally obeys an Arrhenius-type temperature dependence X(0)exp(−ΔE/kT), where ΔE is an activation energy [24]. Thus, we attempted to fit the experimental results presented in Fig. 9 with this relation. The best fit was obtained with ΔE = 680 cm−1 at high temperatures and ΔE = 3 cm−1 at cryogenic temperatures. The energy level positions presented in [6] demonstrate that for both forward and backward process, resonant or quasi-resonant CR transitions initiate from the higher-lying Stark sublevels in the 3H4 and 3H6 multiplet, respectively. The activation energy ΔE = 680 cm−1 obtained from the fitting is close to the value of 684 cm−1 of the Stark level in the 3H4 multiplet [6]. Thus, the responsible CR process could be (3P0(20,725 cm−1), 3H4(684 cm−1) → (3H6(4,942 cm−1, 1D2 (16,464 cm−1) with a small mismatch of 3 cm−1. It shows that the temperature dependence of forward process is well explained in terms of resonance conditions. The room temperature X rate value in 1 % Pr3+ sample is 3 × 10−2 μs−1 and is close to the values determined for YAG [22] and LaAlO3 [14] crystals.
The backward CR transfer is represented by the long time part of 3P0 decays. By fitting the solutions of rate equations to the experimentally determined decay curves, the values of Y for different temperatures were obtained. For example, the room temperature Y rate value in 1 % Pr3+ sample is 3.2 × 10−1 μs−1 when it is 1.8 μs−1 and 35 μs−1 at 500 K and 700 K, respectively. In Fig. 10, comparison of the calculated data with experimental points is presented. We obtained generally good fit for temperatures up to about 700 K, however, at higher temperatures our model did not reproduce the experimental results well. This may be due to the fact that at higher temperatures, the multiphonon relaxation rate from 3P0 increases and that the energy migration between Pr3+ ions is more likely to occur. It could be also interpreted in terms of energy dissipation between several Pr3+ sites in YAM at higher temperatures which increases the number of resonant and quasi-resonant CR transitions.
Decay profiles of 3P0 exited state in 1 % Pr3+:YAM for different temperatures. The points are the experimental data, the solid lines are the fits resulting from the solutions of rate equations (3) taking into consideration the forward (X) and backward (Y) energy transfers
It is observed that for temperatures above about 200 K the backward transfer rate values are about one order of magnitude higher than those for the forward process. The temperature dependence of backward process is explained in terms of activation of higher crystal-field levels in the 3H6 multiplet.
Finally, it must be also noted that the observed shortening of the 1D2 decays with increasing temperature could be related to the increase in the back transfer, rather than 1D2 cross-relaxation itself, similar situation has been reported in [25] for Pr-doped CsCdBr3.
5 Summary
Fluorescence spectra and fluorescence lifetimes corresponding to transitions from the 3P0 level of praseodymium in YAM have been measured and analyzed. We have identified and characterized a nonradiative cross-relaxation channels from the 3P0 manifold concluding that a considerable portion of the overall fluorescence emission stems from the 1D2 manifold. Also, the shortening and nonexponentiality of the decays, observed with increasing activator concentrations and temperature, were interpreted in terms of cross-relaxation among the Pr3+ ions. Cross-relaxation rates were experimentally determined as a function of temperature in a wide temperature range from 10 to 1,000 K and used for modeling of the decays with standard rate equation technique. Influence of the forward and the backward CR processes, and their temperature dependence, on 3P0 decay shape was discussed. The present results give insight into factors involved in the CR quenching in materials activated with Pr3+ ions. Described approach is planned to be used to investigate Pr3+ 3P0 cross-relaxation in other hosts.
This information could be helpful in the development of praseodymium-based phosphors for fluorescence thermometry utilizing the intensity ratio method or the lifetime decay method.
References
A.A. Kaminskii, Advanced Solid-State Lasers (New Orleans, LA, 1993) vol 15, ed by A. Pinto, T.Y. Fan (Optical Society of America, Washington DC, 1993), p. 266
P. Boutinaud, E. Pinel, M. Oubaha, R. Mahiou, E. Cavalli, M. Bettinelli, Opt. Mater. 28, 9 (2006)
M. Nikl, P. Bohacek, A. Vedda, M. Fasoli, J. Pejchal, A. Beitlerova, M. Fraternali, M. Livan, J. Appl. Phys. 104, 093514 (2008)
B.M. van der Ende, L. Aartsa, A. Meijerink, Phys. Chem. Chem. Phys. 11, 11081 (2009)
T. Gun, P. Metz, G. Huber, Appl. Phys. Lett. 99, 181103 (2011)
Y. Rabinovitch, O.K. Moune, D. Tetard, M.D. Faucher, J. Phys. Chem. A 108, 8244 (2004)
Y. Huang, K. Jang, X. Wang, G. Xian, E. Cho, H.S. Lee, S.H. Ki, J. Non-Cryst, Solids 353, 4102 (2007)
I.R. Mitchell, P.M. Farrell, G.W. Baxter, S.F. Collins, K.T.V. Grattan, T. Su, Rev. Sci. Instrum. 71, 100 (2000)
I. Kamma, P. Kommidi, B.R. Reddy, Phys. Status Solidi C 6, S187 (2009)
J. Jordan, D.A. Rothamer, Thermographic phosphor based planar thermometry using the trivalent praseodymium ion (Pr3+) doped into a yttrium aluminum garnet (YAG) crystal, Spring Technical Meeting of the Central States Section of the Combustion Institute (April 22–24, 2012)
D.H. Yoon, I. Yonenaga, N. Ohnishi, T. Fukuda, J. Crystal Growth 142, 339 (1994)
D.A. Pawlak, Y. Kagamitani, A. Yoshikawa, K. Wozniak, H. Sato, H. Machida, T. Fukuda, J. Crystal Growth 226, 341 (2001)
C.D. Brandle, H. Steinfink, Inorg. Chem. 8, 1320 (1966)
M. Malinowski, M. Kaczkan, S. Turczynski, D. Pawlak, Opt. Mater. 33, 1004 (2011)
S. Mahlik, M. Malinowski, M. Grinberg, Opt. Mater. 33, 1525 (2011)
H. Chen, R. Lian, M. Yin, L. Lou, W. Zhang, S. Xia, J.C. Krupa, J. Phys. Condens. Matter 13, 1151 (2001)
A.A. Kaminskii, Laser crystals, their physics and properties (Springer, Berlin, 1981)
T.T. Basiev, YuV Orlovskii, K.K. Pukhov, F. Auzel, Laser Phys. 7, 1139 (1997)
L.A. Riseberg, H.W. Moos, Phys. Rev. 174, 429 (1968)
J. Hegarty, D.L. Huber, W.M. Yen, Phys. Rev. B 25, 5638 (1982)
J.C. Vial, R. Buisson, J. Phys. Lett.-Paris 43, L-745-753 (1982)
X. Wu, W.M. Dennis, W.M. Yen, Phys. Rev. B 50, 6589 (1994)
H. Yamane, M. Omori, T. Hirai, J. Mater. Sci. Lett. 14, 470 (1995)
S. Bhushan, M.V. Chukichev, J. Mater. Sci. Lett. 9, 319 (1988)
Y. An, P.S. May, J. Lumin. 118, 147 (2006)
Acknowledgments
This work was supported by the MNiSzW N N515 081537 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kaczkan, M., Boruc, Z., Fetlinski, B. et al. Temperature dependence of 3P0 Pr3+ fluorescence dynamics in Y4Al2O9 crystals. Appl. Phys. B 113, 277–283 (2013). https://doi.org/10.1007/s00340-013-5469-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5469-3