Abstract
A new mid-infrared sensor platform is described, which combines difference frequency generation (DFG)-based tunable laser sources with simple direct absorption spectroscopy. DFG lasers operating in the 3–5 micron window are tuned to access a variety of species in the C–H, N–O, and C–O stretch regions. The sensors are capable of sub-ppb detection of key greenhouse gas species as well as common pollutants and tracer species. Specific examples of sensor data obtained for methane, nitrous oxide, and carbon monoxide are presented, including relevant time series data and associated Allan Variances. The platform provides a cost-effective alternative to other laser-based approaches in some cases, performing at similar or superior levels. Emphasis on achieving key performance metrics driven by World Meteorological Organization guidelines for Global Air Watch program and other applications is highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Real-time, ultra-sensitive gas analyzers are required for a wide array of applications, including pollution and greenhouse gas (GHG) monitoring. Commercial instruments based on traditional approaches such as non-dispersed infrared absorption (NDIR) and chemiluminescence are being replaced with new, laser-based analyzers that are capable of providing real-time ppb level detection of key atmospheric species. Most commercially available laser-based analyzers are based on near-infrared telecom lasers, wherein vibrational overtones are measured. However, the detection of chemical species via these bands often requires that elaborate, ultra-sensitive approaches be used to increase overall system performance, including ultra-long path astigmatic Herriott cells [1], resonant photoacoustic approaches [2], and optical cavity-based methods such as cavity ring-down spectroscopy (CRDS) and integrated cavity output spectroscopy (ICOS) [3, 4]. Alternatively, monitoring of chemical species via their associated fundamental rovibrational bands in the mid-infrared is employed, and the requisite selectivity and sensitivity for monitoring greenhouse gases, industrial pollutants, and combustion precursors, intermediates, and products have been demonstrated in numerous studies. As the absorption intensity is typically 10–10,000 times stronger in the mid-infrared compared to that of the near-infrared, simple and robust direct absorption spectroscopy can in many cases be used to reliably determine the absolute concentrations or mole fractions of many targets without the need for high finesse optical cavities or other complicated approaches.
We have recently developed a new analyzer platform that is based on difference frequency generation (DFG) mid-infrared lasers, wherein two near-infrared wavelengths are mixed in periodically poled lithium niobate (PPLN) to produce tunable middle infrared light. This approach provides narrow linewidth (~2 MHz), excellent mode quality (TEM00), and high-frequency stability (<10 MHz drift). DFG-based absorption spectrometers have been developed for decades, and in the last decade extremely sensitive instruments have been developed and successfully deployed in numerous embodiments, in some cases achieving sensitivity levels on the order of 10−10/cm/Hz1/2 [5]. Although some research-grade DFG instruments have achieved these impressive performance levels, they have traditionally been based on relatively complex and expensive configurations that would be challenged to compete in the market with other approaches such as CRDS- or ICOS-based instruments, which have largely defined the acceptable price point for commercial analyzers. We have recently developed a relatively low-cost commercial DFG-based analyzer that, although not performing at the highest levels demonstrated for DFG-based instruments, provides a viable option to cavity-enhanced instruments at a competitive price. Moreover, our relatively simple DFG-based analyzer platform is relatively robust and general such that a wide array of species and applications are possible, including potentially open-path systems that are not practically feasible for cavity-enhanced approaches.
In this paper we demonstrate that this relatively simple DFG analyzer platform is capable of monitoring numerous important species with high sensitivity and selectivity, in some cases matching with or exceeding high performance cavity-enhanced approaches. Specifically, the O–H, N–H, and C–H stretching regions between 3 and 4 microns and the C–O and N–O stretching regions between 4 and 4.6 microns are used to measure the three primary greenhouse gases (CH4, CO2, and N2O) as well as carbon monoxide and water vapor. Results from the now commercially available versions as well as pre-market prototypes are shown here, with emphasis on demonstrating the generality, selectivity, precision, and accuracy of the systems for extended periods of time. Extension to other species that are accessible in the spectral regions can be directly inferred from these examples.
2 Instrumentation: mid-IR DFG-based analyzer platform
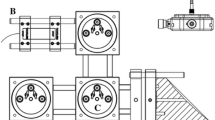
A photo of the analyzer platform together with a basic optical schematic of the apparatus is shown in Fig. 1, which details the DFG laser module, multipass Herriot cell, and detector. The DFG laser employs an all-fiber-coupled polarization-maintaining (PM) architecture, wherein two, optically isolated PM fiber-coupled semiconductor lasers are combined in a fiberoptic multiplexer and subsequently imaged into a 5-cm-long PPLN crystal using a single pre-focused collimation optic. The PPLN crystal is located on a single thermoelectric cooler, and the lasers are frequency mixed in the crystal in single pass geometry, negating the need to optically stabilize the platform as would be the case in a resonant cavity approach. The two residual pump and signal frequencies are blocked using a Ge filter and a single lens is then used to mode-match the mid-IR idler wave into the ~40 cm, 14-pass (~5 m total) Herriot cell. Two 1″-diameter metallized concave mirrors of 1 m focal length comprise the Herriot cell, wherein the input and output beams pass through the same hole in the input mirror, the latter exiting at a small angle (few degrees) with respect to the input beam. The return beam is subsequently picked off just below the input beam path and focused into the thermoelectrically cooled Mercury–Cadmium–Tellurium (MCT) detector (Vigo) using a 1″ focal length off-axis parabola. The signal is subsequently amplified and digitized with 16 bits of depth at a 1-MHz sampling rate.
Thermo Scientific IRIS 5500 Mid-IR laser-based methane analyzer and optical layout schematic: DFG-generated mid-IR laser light is imaged into a simple Herriot cell where it undergoes 14 passes for an effective absorption path length of approximately 5 m. A TE-cooled MCT detector measures the laser intensity as one of the semiconductor lasers is tuned in frequency, tuning the mid-IR difference frequency. Spectra are obtained at a 500-Hz repetition rate, and the entire spectrum is used to uniquely identify the target as well as stabilize the laser frequency over long timescales
To tune the mid-IR wavelength, one of the semiconductor lasers is current tuned at a constant temperature, using a pre-programmed waveform generated by the digitizer card. The laser is scanned at a repetition rate of 500 Hz, typically over a 1–2 cm−1 region that includes a few fully resolved absorption lines. The sloping baseline is due to the variation of mid-IR output power as one of the lasers is current tuned and is mathematically removed before normalization of the spectrum to obtain the absorption strength. The zero baseline (i.e., detector offset) is determined at the end of each scan (current ramp) when the laser is turned off. The entire lineshapes in the scanned region are measured, while the gas is maintained at a constant flow rate and pressure is held constant via a simple PID control loop, wherein the built-in vacuum pump speed is throttled. Typically, the pressure is kept low enough such that the absorption lines are primarily Doppler broadened (between 150 and 225 mbar for the various species), simplifying potential linewidth contributions from (moreover, variations in) pressure broadening. The hermetic optical bench houses the PPLN frequency conversion stage, and the combined near-infrared lasers are ported via PM fiber through a small, sealed hole in the optical bench, with no connectors (i.e., spliced after assembly).
The entire optical tub, multipass cell, DFG laser module, and DFG control electronics are housed in an insulated and temperature-controlled thermal enclosure, which is held at slightly elevated temperature (approximately 37–38 °C). A high precision pressure transducer is used to record pressure, and multiple thermistors are placed in the system for monitoring and control. The DFG laser provides approximately 10 Watts of heating inside the enclosure, while a thermoelectric heat exchanger is used to either warm or cool the enclosure as needed. A series of thin-film heaters with simple dead-band controllers are used to heat the outer shell of the thermal enclosure, reducing the temperature differential between the insulated inside and the outside skin of the enclosure. The interior temperature is controlled at the 10 mK level under common laboratory conditions. All temperatures and relevant active variables are recorded and accessible to the user.
Active temperature control one of the near-IR lasers is used to stabilize the frequency of the MIR laser to keep the absorption line center in the same part of the current ramp, effectively removing the slow, long-term drift commonly encountered in near-infrared semiconductor lasers. Stabilizing the laser in this way precludes the need for an external wavemeter and reduces anomalies from baseline variations in the fitted data, which would otherwise occur if the line moved to different parts of the ramp over time. Absorption spectra are fit using a fixed Voigt lineshape profile, which is a safe approximation in this case as the temperature and pressure are held constant. In some cases, target molecules are measured simultaneously with water vapor such that dry mole fractions can be determined as humidity varies. The analyzer includes an internal computer, diaphragm pump, and 4-inlet gas manifold with a calibration menu that allows routine time-stamped calibration of the system at user-defined intervals. The software is windows based, and supports remote log-in functions as well as other common remote connection capabilities via USB or Ethernet ports.
3 Measurements
3.1 Methane and water vapor
Methane and water vapor were measured in the 3.27 micron region, which includes a single well-resolved water line and three partially blended methane lines in each laser scan. This frequency was obtained by mixing single frequency semiconductor lasers operating in the 1,053 and 1,553 nm regions. All three methane lines are fit, leveraging the fixed relative line strengths, all of which are for the 12CH4 isotopologue. For methane in particular, monitoring concentration via the 3 micron band is highly desirable as the associated absorption intensity is approximately 180 times stronger than those of the more commonly used 1.6 micron overtone band. Figure 2 shows the analyzer concentration data for a calibration gas at approximately 2 ppm (ambient level) over a period of 24 h. Also shown in Fig. 2 is the Allan Variance for the above time series, a metric now commonly used to quantify precision and stability of laser-based analyzers [6]. Here the first data point indicates a precision level of approximately 250 ppt (10-s average/0.1 Hz) and a minimum of about 50 ppt after approximately 9 min of signal averaging. This level of performance satisfies the World Meteorological Organization (WMO) guidelines for ambient methane monitoring, which are highlighted in the green-shaded area of the plot.
3.2 Temperature cycling and system drift
Figure 3 shows the typical analyzer performance, with temperature cycling between 15–30 °C in an environmental chamber, using a 5 °C/hr ramp rate at 7.5 °C intervals. Data are shown both for the 10-s average and 60-min smoothed result. The associated temperature profile is also shown but is slightly offset as it is recorded inside the case, where the heat from the nearby power supplies systematically elevates the value by a few degrees. In this case, a peak-to-peak variation of 0.7 ppb is obtained for the 60-min averaged data, with little or no hysteresis evidenced. This level of performance is consistent with stringent ambient research requirements for greenhouse gases. Similar performance is evidenced below for other instruments operating in the 4–5 micron spectral region.
The drift apparent in these data are primarily due to the presence of residual optical fringes in the optical train, which in the case of a DFG laser can occur at three wavelengths: the pump, signal, and idler wavelengths in the 1, 1.5, and 3.3 micron regions, respectively, within different parts of the system. In this case, the 1 micron laser is fixed in frequency, and thus any fringes including and upstream of the PPLN crystal tend to be fixed and thus primarily lead to offsets that are easily calibrated. However, tuning of the 1.5 micron laser and the associated translation into the MIR can result in fast as well as slowly varying fringes that can occur throughout the optical system. Such etalons can in some cases be isolated and minimized, yet can be strongly influenced by minute temperature changes inside the optical core.
Thermal control of the lasers as well as optical core comprising the optical bench and multipass cell are key to minimizing drift due to optical fringes, which will typically have less of an effect on the extracted concentrations as long as their period is substantially either narrower or broader than the absorption linewidth. In addition to minimizing the magnitude of the fringes, the etalons should not drift appreciably over time, which means the lasers, associated drive electronics, and physical dimensions of the core must all be as stable as possible. When subjected to relatively rapid external temperature changes as in the above tests, transitory fringing is indeed the primary source of drift in the system as all of these systems reequilibrate to the new temperature, which typically takes tens of minutes. In some cases, temperature appears to correlate well with drift, but in others it appears to be anticorrelated. This phenomenon happens as the phase can change depending on whether riding up or down an associated fringe, and thus typically these drift sources cannot be removed via single or even multiple temperature measurements within the system. This phenomena appears to be prevalent at a significant level in nearly all high precision, narrow linewidth laser-based analyzers, regardless of the technique employed as, at the most basic level, all of these instruments comprise highly sensitive optical interferometers. Given that physical changes at the nm level translate into frequency scanning at the MHz level, temperature changes at the mK level are sufficient to produce a measurable effect on the spectrum when the fidelity required is at the 5,000:1 level or better. Similarly, electronics can in some cases produce measurable effects at nA (or nV) levels of drift. As such, in addition to careful design of the optical components, validation of those components performance, and careful optical alignment, thermal testing of the entire system is presently required to validate analyzer performance for ambient GHG monitoring applications.
3.3 Methane field data: Sutro tower
Field testing of a methane analyzer (Model IRIS 5500) at Sutro Tower in the San Francisco Bay Area was performed working in collaboration with Lawrence Berkeley National Lab, which has been operating tower measurements including collaborative U.S. NOAA flask analyses of methane and other long-lived greenhouse gases. The analyzer in this test was placed inside of an enclosure that enables the system to run in an environmentally uncontrolled shed, wherein it was subjected to significant temperature variation and high moisture, including condensing fog and some rain. The monitoring shed was located at the base of Sutro tower and was relatively open to the environment with partial sheltering from the elements. The enclosure was primarily used to shelter the instrument from rain and fog, while air was circulated through the enclosure for cooling. The sample air was provided from the downstream flow of an existing flask sampling system, which was connected to a draw tube that sampled air from a height of 232 m above ground level. The instrument sample flow rate was fixed at approximately 300 sccm, and the internal cell pressure was maintained at precisely 225 mbar by controlling the small internal diaphragm pump rotational speed. System calibration was achieved using the built-in gas manifold and calibration software, wherein the duration and period of the calibration cycle is set by the user. The instrument was installed in September 2011 and remained in service continuously until approximately mid-December 2011.
Figure 4 displays a typical time series for methane dry mole fraction (DMF) obtained at Sutro tower, in this plot comprising approximately 20 days of autonomous operation. In this case, regular calibration cycles were programmed to run every 6 h, wherein the instrument drift was corrected automatically. In these tests, a total variation of only 2 ppb was noted in the data, most of which occurred in the first week of operation. Typical calibrations at only the 100 ppt level were required after the first week of continuous operation. In either case, high fidelity methane DMF data were obtained at the site, with excursions at the 100 ppb level commonly occurring. The accuracy obtained with the analyzer was at the same level evidenced in the laboratory, across wider temperature ranges than those conducted in the environmental data above.
3.4 Carbon monoxide
The wavelength agility of the MIR laser-based analyzer platform enables numerous other important species to be measured, including carbon monoxide, carbon dioxide, and nitrous oxide. Carbon monoxide was measured via discrete lines in the 4.6 micron region, in this case, mixing semiconductor lasers operating in the 1,170- and 1,574-nm regions, the former comprising a novel quantum-dot-based laser. For species such as CO, mid-infrared absorption strength is more than three orders of magnitude times stronger than the associated near-infrared bands in the 1.5 micron region, in this case, directly resulting to superior sensitivity even compared with cavity-based NIR sensors. Figure 5 shows a 20-h time series measurement from a calibration tank of CO at the 475 ppb level, in this case with data points recorded every second. Also shown is the corresponding Allan Variance, indicating a sensitivity level of <1 ppb is achieved in approximately 10 s, with an ultimate sensitivity of 140 ppt achieved after approximately 6 min of averaging. In this case, the WMO ambient monitoring target is satisfied at 1 s.
Figure 6 shows the result of ambient CO measurement in the laboratory over a period of several days. Here, the nearby local commuter traffic is clearly evident during the weekdays as both the morning and afternoon commute (9 am and 5 pm, resp.), which is approximately 1 mile away (U.S. Route 101). Additionally, decreased CO is noted during rainy periods, reducing both the average as well as peak events significantly. During the night, the air conditioning is off, and air exchange limited, leading to relatively constant CO levels in the building. Shortly after 6 am on each day the air conditioning comes on and outside air is introduced. For the late night periods when the ventilation is off, the standard deviation is approximately 440 ppt for a 10-s measurement. This level of precision suitable for high fidelity source attribution, as correlations of CO with CO2 can be used to discriminate, for example, anthropogenic versus biogenic sources CO2.
Time series concentration data for ambient CO in the laboratory over several days. Most of the variation in the baseline values outside the commuter peaks is correlated with the building HVAC system turning on and off, noting that bringing in outside causes increases in CO levels. Here, a precision of 1 ppb is demonstrated for 10-s averages with drift on the order of 2 ppb without periodic calibration
3.5 Nitrous oxide
Nitrous oxide is the third most important greenhouse gas, and it is presently not possible to measure ambient levels accurately in the near-infrared using existing methods due to the combination of low overtone absorption with typical sub-ppm concentrations. Nitrous oxide is extremely well mixed in the atmosphere, evidenced by a pole-to-pole variation of only 1 ppb. WMO guidelines for nitrous oxide measurements thus desire accuracies in the 150–200 ppt regime, presenting a significant challenge for fielded instruments. There are presently three commercial laser-based N2O analyzers that operate in the middle infrared, which are based on relatively expensive QC-DFB lasers (Los Gatos Research, Aerodyne, and Campbell Scientific), and these analyzers are both relatively large and expensive as a result. Here, nitrous oxide was measured using the DFG-based IRIS Model 4600, which measures absorption in the 4.5 micron region. In this case, the aforementioned 1,170 nm quantum-dot laser is mixed with a suitable DFB diode laser in the 1.5 micron region. In addition to N2O absorption, this instrument also simultaneously measures water vapor absorption to determine DMF. Figure 7 shows a typical time series obtained on a calibration tank for a 10-s average, together with the associated Allan Variance. In this case, a precision of 180 ppt was achieved in 10 s, with an associated typical sensitivity of 50 ppt achieved at approximately 100 s. The Allan Variance here indicates a calibration interval on the order of an hour to realize this level of accuracy routinely in the field. Presently, N2O analyzers have been located at field sites and continue to demonstrate comparable performance. Long-term field testing for methane and nitrous oxide analyzers are ongoing at field sites in California, Colorado, and Illinois.
4 Conclusion
Three different trace gas analyzers based on the same mid-infrared difference frequency generation laser absorption platform were demonstrated, indicating selectivity, sensitivity, and accuracy levels commensurate with those sought for measuring key greenhouse gas and trace pollutant species. Methane, water vapor, carbon monoxide, and nitrous oxide were measured with instruments configured for operation in the 3.3 micron region for methane, and 4.5–4.6 micron region for CO and N2O. Sensitivity levels in the sub-ppb regime are all clearly indicated in the time series and associated Allan Variances, and low-drift was demonstrated across a temperature range likely to be encountered in most ambient field monitoring stations. Methane measurements were performed for an extended period of time at Sutro tower in the San Francisco Bay Area in an environmentally uncontrolled shed, and autonomous, ppb level performance was demonstrated for an extended period of time over a wide temperature and moisture range. Carbon monoxide was notably measured at 1 ppb levels in 10 s, providing a potentially new cost/performance level for laser-based sensors.
Field testing is presently underway for both N2O and CO instruments, and results to date indicate stable, autonomous operation in the field over a period of months since initial installation. In addition to these specific analyzer configurations, we are presently working on further developing the same core platform to provide instruments with unique multiple species capabilities that are not presently offered by competing approaches. Moreover, the generality and relatively low-cost of the core DFG laser engines used in these systems provide a potential high value proposition for multiple species embodiments compared with competing technologies. Finally, instruments capable of measuring isotopes of key species are also under development, further indicating an exciting future where this platform often exceeds the performance of commercially available optical cavity-enhanced instruments operating in the NIR region.
References
E.C. Richard, K.K. Kelly, R.H. Winkler, R. Wilson, T.L. Thompson, R.J. McLaughlin, A.L. Schmeltekopf, A.F. Tuck, A fast-response near-infrared tunable diode laser absorption spectrometer for in situ measurements of CH4 in the upper troposphere and lower stratosphere. Appl. Phys. B 75(2–3), 183–194 (2002)
J. Rey, C. Romer, M. Gianella, M.W. Sigrist, Near-infrared resonant photoacoustic gas measurement using simultaneous dual-frequency excitation. Appl. Phys. B 100, 189–194 (2010)
T.J. Morville, D. Romanini, A.A. Kachanov, M. Chenevier, Two schemes for trace detection using cavity ringdown spectroscopy. Appl. Phys. B 78(3–4), 465–476 (2004)
Joshua B. Paul, Larry Lapson, James G. Anderson, Ultrasensitive Absorption Spectroscopy with a High-Finesse Optical Cavity and Off-Axis Alignment. Appl. Opt 40(27), 4904–4910 (2001)
D. Richter, B.P. Wert, A. Fried, P. Weibring, J.G. Walega, J.W.C. White, B.H. Vaughn, F.K. Tittel, High precision carbon dioxide isotope spectrometer with a difference frequency generation laser source. Opt. Lett. 34(2), 172–174 (2009)
P. Werle, Accuracy and Precision of laser spectrometers for trace gas sensing in the presence of optical fringes and atmospheric turbulence. Appl. Phys. B 102, 313–329 (2011)
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Scherer, J.J., Paul, J.B., Jost, H.J. et al. Mid-IR difference frequency laser-based sensors for ambient CH4, CO, and N2O monitoring. Appl. Phys. B 110, 271–277 (2013). https://doi.org/10.1007/s00340-012-5244-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5244-x