Abstract
In this work, the Ni1-xMoxO, (x = 0.000, 0.025, 0.050, 0.075, 0.100, and 0.150) nanoparticles were prepared employing the coprecipitation method. The X-ray diffraction (XRD) confirmed that all the samples have a face-centered cubic (FCC) structure with no secondary phases by the effect of the Mo-doping. The Mo-dopants yielded smaller crystallites, reaching a size of 9 nm with x = 0.150. The transmission electron microscope (TEM) images revealed agglomerated NiO nanoparticles with nearly spherical shapes varied to elliptical-like shapes upon increasing Mo concentration. The energy dispersive X-ray (EDX) confirmed the purity of the synthesized samples. The XPS analysis confirmed the valence states of the presented elements in the samples as Ni2+, Ni3+, Mo6+, and O2− ions. The XPS detected the reduction of the nickel and oxygen vacancies, by studying the ratio of Ni2+/Ni3+ and lattice oxygen (OL) to vacant oxygen (OV) peaks. The Raman analysis demonstrated the active vibrational modes of NiO, for all the samples, along with stretching Mo = O bonds for the doped samples. The Photoluminescence (PL) spectroscopy was employed to study the near band edge and deep level emissions, giving insight to the defect levels within the band gap. The PL affirmed the decrease of the oxygen vacancies upon Mo-doping. Besides, the magnetic hysteresis measurements at room temperature revealed the superparamagnetic contribution embedded in the antiferromagnetic matrix of NiO. The magnetization was tuned by Mo doping concentration, where it affected the saturation magnetization, coercivity, and remnant magnetization. Mo dopant can modify the magnetic property of NiO nanoparticles and can be a potential candidate in biomedical field and data storage applications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Materials with at least one dimension between 1 and 100 nm are known as nanostructure materials. Because of their distinct size, shape, and large surface area, they have exceptional chemical and physical properties that enable them to display a wide range of characteristics [1,2,3]. The nanostructures, particularly transition metal oxide nanoparticles (NPs), have been known for numerous applications due to their optical, electrical, and magnetic properties. Pure NiO in the bulk form exhibits FCC structure and it is known to be an antiferromagnetic (AFM) below Neel temperature (523K) [4]. It is a highly transparent p-type semiconductor oxide due to its broad bandgap energy (3.6-4.0 eV) and the presence of Ni2+ vacancies [5]. At the nanoscale, due to the fact that the fraction of surface atoms increases, the number of defects and elastic stresses increases, NiO exhibits anomalous structural and distinctive optical properties as well as different types of magnetic ordering including weak ferromagnetic (FM), spin-glass (SG) and superparamagnetic (SPM) [6]. The recent ongoing research on nanoscale NiO has revealed a wide range of applications in spintronic devices, p-type transparent conducting films, photocatalysis, gas sensors, dye sensitized solar cells, battery electrodes, supercapacitors, etc [7, 8]. Numerous ways were intended to get newly improved characteristics of NiO NPs for specific applications such as the formation of nanocomposites, surface modifications, and doping processes. However, the synthesis method plays an important role in controlling the size, morphology of the obtained NiO nanoparticles and subsequently their applications. Additionally, doping NiO with transition metals is an effective way to modulate the structural, optical and magnetic properties of NiO NPs [9]. The suitable dopant not only alters the crystal symmetry and generates strains in the structure but also affects the overall magnetic ordering of NPs. For Fe-doped NiO NPs, synthesized using the cost effective coprecipitation method, the particles are agglomerated with nearly spherical and/or hexagonal shape up to 4% Fe doping and are of square like and well separated for 6% Fe doping. The disappearance of a 2M band at about 1550 cm-1 in the Raman spectra indicates the reduction in antiferromagnetic coupling strength in pure and Fe doped NiO NPs due to the smaller crystallite and disorder induced by defects [10]. Similar results were obtained for Zn doped and Cu doped NiO NPs, prepared using a simple sol-gel method [11, 12]. The presence of weak ferromagnetism in the hysteresis loop measured at room temperature and the absence of 2M band of Raman spectra indicates that the two-magnon (2M) excitations were reduced. Under these conditions, the magnetic properties of the AFM NiO nanoparticles are enhanced even at room temperature by doping, which enables these oxides for the application in spintronic devices. According to earlier recent studies, Mo has garnered attention because it effectively leads to minimization in crystallinity, large surface area, and alternation in the morphology and affected greatly the electronic, optical and magnetic properties of metal oxides NPs [13,14,15,16]. Moreover, Mo demonstrates multiple valence states between +6 and +2 and this enables the contribution of multiple carriers by a single Mo dopant atom. Recently, Nabi et al. [17] investigated band gap tuning through controlled growth of Mo-doped NiO spherical nanoparticles synthesized via sol-gel method. These nanoparticles were effectively utilized as a photocatalyst for water decontamination. Analysis using X-ray diffraction (XRD) indicated a decrease in crystallite size from 67 nm to 44 nm and a significant reduction in band gap from 3.5 eV to 2.9 eV with 3 wt. % Mo doping. Maheskuma et al. [18] studied Mo-doped Ni/NiO nanocomposite was developed for energy saving hydrogen production via boosting urea oxidation kinetics using a simple pulsed laser ablation (PLA) method. The exceptional performance of the Mo-doped Ni/NiO nanocomposite catalyst can be attributed to the high-valence Mo doping, which enhances the catalytic activity of the Ni atoms, facilitating the oxidation of Ni2+ into Ni3+, and providing numerous active sites for the electrochemical reaction. It is noteworthy that
PLA, in contrast to the traditional methods employing for the synthesis of nanomaterials, is described as directness, versatile, and environmentally friendly in generating small nanoparticles with a narrow size distribution.
Nanostructured pure and Mo doped NiO thin films were successfully sputtered on indium-doped tin oxide (ITO) substrates for application in ultraviolet (UV) photodetector sensors [19]. The results indicated that as the concentration of Mo dopants increased in the NiO thin films, there was a corresponding decrease in the optical band gap. Additionally, a significant enhancement in both the responsivity and external quantum efficiency (EQE) of the photodetectors was observed with increasing Mo concentration. Adewinbi et al. [20] conducted a study to investigate the impact of Mo dopant ions on the surface structural and electrochemical properties of NiO thin films. They employed a straightforward facile two-electrode electrochemical deposition process for the growth of the film. The findings revealed that the presence of Mo dopant ions enhances the suitability of the NiO thin film as a contact electrode for potential use in both optoelectronic devices and electrochemical supercapacitors.
It is noteworthy to mention that recent developments in chemical-based deposition methods have led to lower processing temperatures, and the ability to deposit at atmospheric pressure with higher throughput [21, 22]. In particular, these techniques often result in non-stoichiometric NiOx films and therefore a range of different film properties [23].
Alam et al. [24] studied the influence of Mo-doped NiO NPs for antioxidant and antibacterial applications, synthesized using sonochemical methodology. The crystallite size of pure NiO and Mo doped NiO was found to be 21 nm and 18 nm respectively. TEM micrographs revealed that the pure NiO and Mo doped NiO NPs exhibited rectangular and rod like morphology respectively. They concluded that the Mo-doped NiO NPs exhibited better results in comparison to the pure NiO NPs, owing to their smaller size. Ahmed et al. [25] studied the electrochemical properties of Mo-doped NiO nanowires, prepared using the hydrothermal method, for supercapacitor applications. The crystallite size was found equal to 32 nm and it get decreased with increasing Mo doping. They reported that Mo-doping changed the morphology of NiO nanomaterials and enlarged the active surface area and active sites, resulting the improved specific capacitance. TEM micrographs showed that a large number of distributions of nanowire, thus enhancing the active surface area and consequently better supercapacitive performance. Similarly, Shah et al. [26] studied Mo-doped NiO nanostructures prepared using the coprecipitation method calcined at 700 °C. The crystallite size of pure NiO was estimated as 33 nm and it decreased with increased Mo doping down to 27 nm. SEM micrographs depicted that the nanoparticles are observed to be hexagonal in shape with uniform size distribution. The smaller grain size and this type of morphology will offer a large surface area and hence both the magnetization and specific capacitance increased with Mo doping and as a result it can be used for spintronic devices and energy storage system like capacitors. Table 1 presents the comparative study of the structural properties and applications of pure and Mo doped NiO nanostructured synthesized via different methods. Among various synthetic methods, the co-precipitation method has been, up to now, the most commonly used because of the low cost of the required equipment, the mild reaction conditions, relatively high purity and excellent stoichiometry at the atomic level [27].
Hence because of this, in the present work, the Mo-doped NiO NPs with different concentrations were successfully fabricated using the co-precipitation method. Different techniques were applied to investigate the effects of doping concentration on the structure, surface morphology, optical, and magnetic properties of the samples. These approaches include XRD, TEM, XPS, Raman, and VSM techniques. Additionally, the magnetization was fitted using a Langevin model, modified with a linear function. It is worth mentioned that, so far there is no systematic study focused on the magnetic property modulation of Mo-doped NiO to be used in variety of magnetic applications.
2 Experimental methodology
2.1 Synthesis process
Pure and Mo-doped NiO NPs were synthesized using the co-precipitation method. Pure NiO was prepared starting from 1M of nickel chloride hexahydrate (NiCl2∙6H2O), molybdenum pentachloride (MoCl5∙5H2O), and 4M sodium hydroxide (NaOH). The acquired homogeneous solution was stirred at room temperature followed by adding NaOH dropwise up to pH=13, a highly basic condition, which is convenient for direct preparation of spherical NiO nanoparticles with good dispersity and uniform sizes [28]. Subsequently, the solution was stirred magnetically and heated at 60°C for 2 hours. Afterward, the obtained green solution was washed several times with deionized water, using two filter papers, until its pH dropped to 7. Then, the wet powder was dried using a furnace at 100°C for 16 hours. Finally, the obtained green powders were ground and calcined for four hours at 550°C. The same procedure was applied to the Mo-doped NiO samples with nominal composition Ni1-xMoxO with x=0.025, 0.050, 0.075, 0.100, and 0.150. This synthesis procedure is illustrated in Figure 1.
2.2 Characterization techniques
Structural properties were characterized by X-ray diffraction (XRD) using a Panalytical Xpert Pro diffractometer using Cu-Kα radiation with wavelength (λ=1.5406 Å) at 40 kV/30 mA; divergence slit=0.19 mm; receiving slit =0.1 mm; scanning 2θ range 20° to 80°; step size 0.02° and scan speed of 0.05 s-1. The surfaces and chemical composition were conducted on a JEOL, JCM 6000PLUS scanning electron microscope (SEM), with energy dispersive X-ray (EDX) capabilities. The accelerating voltage used for analysis was 15 keV. The transmission electron microscope (TEM) using JEOL JEM-200CX2100F, with an accelerating voltage of 200 kV was used to study morphology and confirm the nanostructure of the prepared samples. X-ray photoelectron spectroscopy (XPS) spectra were performed on a Thermo Fisher Scientific system equipped with a micro-focused, monochromatic Al-Kα source of X-ray (1486.6 eV) to study the elemental compositions and chemical states of the thin layer on sample surfaces. The resulting XPS spectrum of each element was calibrated first with carbon 1s at nearly 285 eV before data analyses began. Micro-Raman with a confocal microscope (Alpha 300R-WITEC) was utilized, using an excitation of 532 nm from an Nd-YAG laser. The laser worked at a power of 5 mW and 50x magnification objective. The Raman spectra were estimated at room temperature, after putting the samples on a silicon wafer. The photoluminescence (PL) spectroscopy was implemented to study the optical properties of the studied sample, using Jasco FP-8600 spectrofluorometer on 8 mg of the nanoparticles dispersed in 40 mL of ethanol and sonicated for 5 minutes. The magnetic characteristics were investigated at room temperature using a vibrating sample magnetometer (VSM, Lake Shore-7410) with field strengths up to ± 20 kOe.
3 Results and discussions
3.1 X-ray diffraction analysis (XRD)
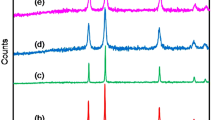
Figure. 2a depicts the XRD patterns of the pure and Ni1-xMoxO with x=0.025, 0.050, 0.075, 0.100, and 0.150. All patterns reveal five diffraction peaks (111), (200), (220), (311), and (222), corresponding to the face-centered cubic structure with space group Fm-3m [29], which is confirmed by the JCPDS (Card No. 04-083). No traces of metallic Mo or any secondary phases can be detected within XRD detection limit, which indicate that Mo has entered the NiO lattice without changing the crystal structure. Furthermore, as can be seen in Figure. 2b, the intensity along the (200) peak is narrow and intense for pure NiO, but as Mo concentrations increase, the intensity decreases, widens, and shifts toward a higher angle. This shift of (200) peak clearly indicates the lattice contraction with Mo doping in NiO lattice. Moreover, it suggests that Mo ions substituted into the periodic lattice of NiO, causing the strain at Ni sites. It can be concluded that as Mo-doping concentration increases, the crystalline quality of NiO NPs degrades. Similar results were reported for Mo-doped ZnO thin films synthesized using spray pyrolysis method [30]. Furthermore, the observed bottom broadening or a hump for x=0.15 indicate the smaller size of the synthesized NiO NPs [30, 31].
To further analyze the effects of Mo on the crystal structure, the Rietveld refinement of XRD data for Ni1-xMoxO samples was done using MAUD program as shown in Figure 3(a–f). The refinement factors (χ2, Rwp, Rb and Rexp) and structural parameters such as lattice parameters, the unit cell volume V, LNi-O bond length, etc. are listed in Table 2. The Rietveld refinement agrees well with the measured data and is reliable. The Rietveld analysis indicated the FCC structure of each sample with space group Fm-3m. It can be seen that, from Table 2 the values of lattice parameter and Ni-O bond length decrease with the increase in Mo concentration which results in unit cell volume contraction. In fact, by considering the oxidation state of Mo ions have a +6 oxidation state, the reduction of the lattice parameters is allocated to the total inclusion of the Mo6+ ions in the lattice, which have smaller ionic radii (0.059 nm) as compared to the nickel ions (0.069 nm) [32]. The +6 oxidation state of Mo ions was further confirmed from the chemical analysis performed via the X-ray photoelectron spectroscopy (XPS).
Additionally, the variations of the bond length are linked to the changes of the electronegativity between Ni2+ and Mo6+ ions. Since Ni2+ ions have electronegativity of 1.91 on Pauling scale, which is less than that of Mo6+ ions, having electronegativity of 2.16 on Pauling scale [33] Hence, upon doping NiO by Mo6+ ions, the structural environment is disturbed affecting the changes of the bond length. Thus, the bond length is reduced due to the greater electronegativity of the doped ion. Yousaf et al. [34] detected the increase of bond length in Ni-O bond by substituting Cu2+ and Zn2+ ions into NiO NPs that was justified by their smaller electronegativity.
The Rietveld refinement was performed by taken initially the Ni and O atoms at the 4a (0, 0, 0) and 4b (0.5, 0.5, 0.5) Wyckoff sites, respectively. The elemental occupation factor for the nanostructured Ni1-xMoxO samples obtained through Rietveld refinement are given in Table 3. The Ni and Mo occupy the 4a Wyckoff positions with different site occupation factors depending on the Ni/Mo ratio. The occupation of oxygen at 4b Wyckoff position increases with the increase in Mo concentration, which indicates less number of oxygen vacancies.
The average crystalline size D, for Ni1-xMoxO, is calculated using the well known Scherrer equation:
where 2θ is the Bragg diffraction angle and β is full width at half-maximum (FWHM). In the Scherrer equation, the β parameter must be corrected to eliminate the so-called instrumental effects. The best expression for this instrumental effect correction is:
where βexp is the measured width and βstandared the width due to standard sample. The values of average crystallite size are listed in Table 4. It is observed that the crystallite size decreases from 22 nm for pure NiO to 9 nm for Mo-doped NiO with x = 0.150, indicating that doping with Mo6+ ions inhibits the growth of crystallites. However, the contribution of both the crystallite size and lattice micro-strain ε, can be calculated using Williamson–Hall (W–H) equation [35]:
The average micro-strain is obtained from the slope of the straight line fit as shown in Figure 4. The average crystallite size is calculated from y –intercept. The crystallite size and microstrain obtained from W-H plot were also given in Table 4. The negative slope in the plots of the synthesized samples suggests that the crystal lattice contraction results in compressive strain in the nanocrystal. The tendency of variation in crystallite size found in the Scherrer formula and the W-H plot are comparable. However, the W-H method gives smaller crystallite size than Scherrer formula due to the inclusion of a strain correction factor in W-H plot. It may be noted that the micro-strain induced in the samples with smaller crystallite size is higher and vise –verse, which is understandable.
The dislocation density δ was also calculated using the following equation:
The dislocation density was also included given in Table 2.
The changes in the value of δ arises from differences in ionic radii or valency charges between the substituted Mo element and the host Ni element leading to increased bulk deformation of crystals [36]. Consequently, the \(\delta\) value increases. At high doping concentrations, as Mo replaces Ni in the lattice, it intensifies lattice distortion, increasing compressive strain within NiO nanoparticles as seen in Table 4. This strain disrupts the normal growth of NiO crystals, potentially causing them to contract and thereby reducing their crystalline quality. A similar behavior was reported for Mo doped NiO thin films grown by spray pyrolysis where XRD graphs demonstrates a reduction in crystalline quality as the concentration of Mo increases [37].
3.2 Transmission electron microscope (TEM)
TEM micrographs and their corresponding particle size histograms of pure and Ni1-xMoxO, with x=0.000, 0.025, 0.050, 0.075, 0.100, and 0.150, are shown in Figure 5(a–f). The pure NiO NPs demonstrated almost quasi spherical shaped particles. Some particles may aggregate together due to strong inter-particle interactions. On the other hand, as the Mo dopant concentration increases, the morphology of quasi-spherical shaped particles was modified to an agglomerated elliptical-like nanostructure (x=0.150). The variation in the morphology with the incorporation of Mo6+ ions, is mainly associated with the ionic radii differences between Mo6+ and Ni2+ ions that impose the introduction of structural defects, as it is seen from the increase of the dislocation density, estimated in the XRD section. Image J was used for estimating the particle size distribution. The size of the particles in pure NiO was found to be in the range of 10 and 50 nm with a mean particle size of 27.66 nm. This is consistent with the size (27.79 nm) reported by Al Boukhari et al. [38] for NiO NPs prepared by coprecipitation method. As the Mo concentration increases, the particle size gets smaller and ranges between 6 nm and 30 nm for x=0.050 and keeps decreasing till it achieves a minimum range of 3 nm to 15 nm for x=0.150, with a mean particle size of 7.41 nm. However, the average crystallite size estimated from W-H plot are nearly comparable to the results of TEM within the error margin, as shown in Figure 6.
3.3 Energy dispersive X-ray (EDX)
Figure 7a–c depicts the EDX analysis of Ni1-xMoxO, with x=0.000, 0.025 and 0.150 nanostructures and their corresponding SEM micrographs in the inset. SEM micrographs show that the grains adhere to each other to form large irregular agglomerates of NiO which contain many nanospheres and small nanoparticles indicating the successful synthesis of NiO nanoparticles. Figure 7a shows the EDX spectrum of the NiO nanoparticles, which mostly consists of Ni and O peaks, reflecting the high purity of the sample. Upon doping, new peaks linked to Mo element appeared in Figure 7b, c, confirming the effective doping of Mo ions in NiO lattice. The experimental atomic percentages of Ni1-xMoxO with x =0.000, 0.025 and 0.150 nanoparticles are in a good agreement with the calculated elemental composition and are listed in Table 5
3.4 X-ray photoelectron spectroscopy (XPS)
To confirm the incorporation of the Mo ions into NiO NPs and to explore the changes in chemical states of different elements upon Mo doping, XPS was performed. The full XPS survey spectra of pure NiO and Ni1-xMoxO (for x=0.025 and x=0.150), are shown in Figure 8, which affirms the existence and valence state of the fundamental elements Mo, Ni and O. The electron binding energies of all elements were calibrated against the contaminated C 1s level (284.6 eV). XPS spectral lines are assigned to the excited photoelectrons from nickel Ni (2s, 2p, 3s, and 3p), oxygen (O 1s), and carbon (C 1s) energy levels, with signs of the Auger electrons transition from nickel (Ni-LMM) and oxygen (O-KLL). The small peak of C1s at 284.6 eV may be due to adventitious carbon contamination on samples forming due to air exposure [39].
For Mo-doped samples, some additional photoemission peaks are observed, related to Mo 3p and Mo 3d, confirming the incorporation of Mo in the host structure of NiO. Furthermore, the absence of spectral lines assigned to elements other than Ni, Mo and O validates their purity in a good agreement with XRD results.
To study the core energy levels, the XPS spectra were deconvoluted for Ni 2p, O 1 s, and Mo 3d as shown in Figs. 9, 10. The spin–orbit coupling \(\left(j=l+s\right)\) induces the doublet of the Ni-2p core energy level, revealing the two regions for Ni 2p3/2 and Ni 2p1/2 [40]. Both can be separated into four components, as shown in the Fig. 9, indicating the presence of the mixed Ni valence states (Ni2+ and Ni3+) in addition to satellite peaks due to the shakeup process [41, 42]. The detection of Ni3+ ions is justified based on the quasi-localized holes that are induced by Ni2+ vacancies [43]. These nickel vacancies leave two holes that are transformed to the nearest Ni2+ ions to transform it into Ni3+ ions, and by this Ni3+ ions are detected [44]. Moreover, the Ni2+/Ni3+ area ratio is estimated to be 0.32, 0.42, and 0.46 for x = 0.000, x = 0.025, and x = 0.150, respectively. This indicates that Ni2+/Ni3+ area ratio increased with increasing concentration of Mo6+ dopant ions. Hence, the Ni3+ ion concentration is reduced in the lattice. Consequently, the concentration of nickel vacancies is decreased by the effect of Mo6+ dopant ions, aligning with the lattice parameters variations. Similar results were obtained with Abdallah et al. [45] for studying the effect of ruthenium element on the properties of NiO NPs.
The O 1s photoemission peak of the Ni1-xMoxO NPs is deconvoluted into two peaks, as shown in Figure 6. They were observed at (529.47 eV and 531.06 eV), (529.83 eV and 531.32 eV), and (530.30 eV and 532.39 eV), for x=0.000, x=0.025, and x=0.150, respectively, where they were assigned to O2- in NiO NPs [4]. The first sharp peak represents the lattice oxygen atoms (OL) linked with metal oxygen bond such as Ni-O and Mo-O bonds [46]. However, the other shoulder peak is assigned to the oxygen vacancies (OV) on the surface of the samples [47]. Furthermore, Mo may strongly interact with oxygen atoms or oxygen vacancies via the formation of chemical bonds in the NiO crystal lattice, suggesting the formation of structural defects such as Ni-O-Mo and Mo-Ni-O bonds by Mo doping. Interestingly, the area ratio of OL/OV decrease from 1.97 to 1.95 from x=0.000 to x=0.025, indicating the increase of oxygen vacancies. However, this ratio increased significantly to 5.05 with x=0.150, representing the decrease of the oxygen vacancies with increasing the Mo-dopant’s concentration. These variations of the oxygen vacancies with Mo-dopants may affect the magnetic properties of the doped samples.
Figure 10 shows the deconvolution to Gaussian function of the curve related Mo-3d of x=0.025 and x=0.150, respectively. Two observable peaks appear at 232.5 eV (Mo3d5/2) and 235.43 eV (Mo3d3/2) which are characteristics of typical values of 3d doublet Mo6+, confirming that the Mo is present in the 6+ valence state.
3.5 Raman spectroscopy
Raman spectra can be used to study phonon vibration modes and identify lattice defects. Figure 11a shows the room-temperature Raman spectra for the pure and Mo-doped NiO NPs. The Raman spectra are de-convoluted by Voigt peaks, as shown in Figure 11a and the peak positions and assignments are listed in Table 6. In pure NiO, five Raman peaks were observed owing to longitudinal (LO), transverse (TO), and surface (SO) optical phonons scattering. However, the Mo-doped NiO NPs exhibited nine phonon modes, where 4 new phonon modes emerged, as compared to the pure NiO sample. Along the five observed phonon modes of pure NiO nanoparticles, two-phonon optical modes of LO+TO peak and two-magnon (2M) mode appeared in the doped samples. In addition, two peaks show up in the range of 810-830 cm-1 (strong) and 946-970 cm-1 (weak), which correspond to the asymmetric and symmetric stretching vibration modes of the Mo=O terminal bond [48].
The obtained Raman active modes are in good accordance with the reported characteristic phonon mode of vibrations of intrinsic NiO nanostructures. The appearance of the one-phonon modes is ascribed to the presence of defects and surface effects [49]. The missing of the two phonon (TO+LO) mode at 906 cm-1 for the pure sample may be a sign of the nanocrystalline nature of the synthesized NPs, and its appearance for Mo-doped samples indicates the deterioration of this crystallinity nature [50]. This is assisted by the broadened reduced peak intensity of the XRD patterns that indicated the reduction of the crystallinity with increasing the concentration of the Mo-dopants in the NiO matrix. Moreover, the presence of the (1SO) phonon mode is ascribed to the distortion of the transitional symmetry of the surface potential at the interface [51]. However, the SO modes usually overlap with the LO mode and maybe undistinguishable with the presence of one magnon modes. Hence, the theoretical and experimental frequencies of the SO modes vary. To find the effect of the background magnon mode, if present, and its overlap with 1LO mode, the DC model is implemented, as in the following equation [52]:
where, \({\omega }_{SO}\) and \({\omega }_{TO}\) are the wavenumbers of the SO and TO modes. The dielectric constants in the static, host medium, and the high-frequency range are represented by the following symbols and values, as, \({\varepsilon }_{0}=11.9\), \({\varepsilon }_{M}=1\), and \({\varepsilon }_{\infty }=5.7\), respectively. The experimental and calculated values are listed in Table 5. It is noticed that the values are close to each other and the deviation increased with increasing the doping concentration. This may be due to the reduced intensity of the 1SO peak with higher Mo-concentrations, due to the induced smaller particle sizes and their effect in suppressing the distortion of the surface potential at the interface.
Further, the peak broadening and intensity of 1LO mode become more pronounced on increasing the concentration of the Mo dopants (x>0.05). On the other hand, the width and intensity of the 2LO mode are highly reduced with the Mo dopants. These changes are linked to the structural modifications that arose in the crystal upon Mo-doping, reflecting the non-stoichiometric characteristic of the lattice. Subsequently, the enhanced 1LO mode reflected the increased structural defects, which resulted from particle size reduction [53, 54]. This agrees with the XRD, TEM, and XPS results. Additionally, the peak shift of the prominent 1LO phonon mode towards higher wavenumbers is explained by different competitive mechanisms such as the size-induced phonon confinement effect, defects, and surface relaxation [55].
The intensity changes of the strongest peak of Mo = O phonon mode is compared to that of the 1LO phonon mode, and their ratios is plotted in Fig. 11b. The ratio variation shows an increase with the incorporation of Mo into the lattice till x = 0.050, followed by a continuous decrease with the increased concentration of Mo. The enhancement assures the incorporation of the Mo ions into the NiO lattice, however, the decrease at higher concentrations (x \(\ge\) 0.075) suggests the Mo dopant promotes an increase in the number of induced structural disorder as supported from the XRD results.
Usually, bulk NiO exhibits a two-magnon (2M) vibrational mode, which is associated with Ni2+-O2--Ni2+ super-exchange interaction between the nearest neighboring Ni ions through O [56]. This supports the existence of the antiferromagnetic phase at room temperature. From Figure 11a, the reduction or the complete vanishing of 2M mode in pure NiO and Mo-doped samples suggests a different magnetic behavior from the one expected in the bulk NiO (antiferromagnetic behavior) due to the finite size effect, smaller crystallite size, and the disorder induced by the defects [57]. It is previously reported that the minimum size needed for magnon excitation in NiO nanoparticles is between 10-12 nm [52], this proves its weak presence in the samples and complete vanishing at higher doping concentrations.
3.6 Photoluminescence (PL) spectroscopy
Figure 12 shows the room temperature PL emission spectra of pure and Mo-doped NiO nanoparticles at 250 nm excitation wavelength. The exact position of the peaks is assigned by deconvoluting the PL spectra by Voigt fitting that is shown in Figure 13. All spectra revealed an intense peak in the 365-329 nm range. This notable improvement in the intensity of this peak with a slight shift in its center towards a lower wavelength is remarked with the increase in the doping concentration. All the nanoparticles present bands in the ultraviolet region (270 – 385 nm), as well as in the visible region, with violet, blue and green emissions at 403 nm, 460 nm, and 540 nm, respectively. The band in the UV region is a feature of near-band edge emission [58, 59]. At 362 nm, the band is known for the recombination of excitons [58]. The enhancement of UV emission intensity and the peak shift towards blue region in NiO with Mo doping may be because of the decrease in particle size affirmed by XRD and TEM investigations. When the particle size decreases, the effective band gap increases, therefore, the emitted photon has comparatively higher energy giving photoluminescence peak at shorter wavelength. Furthermore, the sharp PL spectra also indicate that the nanoparticles are monodispersed.
In the visible region, deep level emission peaks are noticeable. The peaks could be related to several structural defects, such as oxygen interstitial defects and nickel vacancies present in the nanoparticles as a result of heat treatment and deviation from NiO stoichiometry. The presence of shoulder peaks at 403 (\(\pm\) 2) nm, 424 nm, and 434 nm are attributed to violet emission in the photoluminescence spectra. It proposes the presence of electrons trapped at Ni interstitial to the valence band [58].
Peaks at 450 nm and 460 nm correspond to the blue emission peaks that confirm the existence of surface oxygen vacancy in the samples [60, 61]. Also, they could be related to the recombination of electrons from Ni2+ vacancy to the holes in the valence band. The green emission band is observed at 534 nm, 540nm, and 562 nm in Ni1-xMoxO nanoparticles, with x = 0.000, 0.025, and 0.075, owing to the presence of oxygen vacancies [62]. Hence, the oxygen vacancies are suppressed as the intensity if this peak has diminished with increasing the concentration of Mo-dopants in NiO nanoparticles. The variation in the intensity of PL signals at different wavelengths demonstrates that the change in defect state density and shifting in the emission band position is due to the variation in the position of the defect levels in the bandgap [61].
3.7 Analysis of VSM data
To gain more information about the magnetic nature of the prepared pure and Mo-doped NiO NPs, the VSM was employed. Figure 14 shows the M-H hysteresis curves of for Ni1-xMoxO NPs, with x = 0.000, 0.025, 0.050, 0.075, 0.100, and 0.150 were measured at room temperature in the field ± 20 kOe.
Indeed, NiO in bulk form is basically antiferromagnetic below Neel temperature (523K), in despite of this magnetic order, may present a net magnetic moment and thus distinct magnetic properties as the particle size is reduced to the nano-regime [63, 64]. As shown in Figure 14 All the samples exhibited non-saturation in the high field regime up to a maximum applied field of 20kOe as is expected for antiferromagnetic materials. Additionally, a minor hysteresis loop in the low field regime corresponding to ineffective ferromagnetic characteristics of NiO. However the existence of small ferromagnetic domains was the origin of superparamagnetic behaviour.
To elucidate the magnetic nature of the prepared nanoparticles, fittings by combining the different magnetic contributions were applied, based on the following equation [65]:
where the first term refers to the ferromagnetic (FM) contribution, the second term allocates to the superparamagnetic (SPM) contribution and the third term indicates a linear magnetization (antiferromagnetic AFM or paramagnetic PM contribution). Trials were made on the M-H experimental loops by considering each contribution separately, then by combining FM + SPM, FM + AFM, and SPM + AFM. The best fitting results were achieved for a combined superparamagnetic and antiferromagnetic contribution. The fit with the experimental data is represented in Figure 14.
The superparamagnetic behavior observed in Mo-doped NiO nanoparticles may be influenced by several factors: (i) finite size effects due to reduced coordination of surface spins in NiO nanoparticles, (ii) presence of secondary Mo-related impurity phases, and (iii) the Mo+6 ions occupying the Ni. We investigates the presence and impact of these factors in our samples.
As mentioned before, the antiferromagnetic structure of NiO is determined by the dominant super exchange interactions in the linear Ni-O-Ni atomic chains [66]. The super exchange interaction is an indirect exchange mechanism where electrons are shared between the 3d orbitals of Ni+2 metal ions and the 2p orbitals of intervening O-2 ions. The strength of this interaction depends on the bond length and angle between the metal and oxygen ions [67].
The finite size and surface effects emerge from the reduced in the number of exchanges coupled surface spins within NiO nanoparticles due to insufficient compensation between antiferromagnetic sub-lattices [68]. With Mo doping, as the crystallite size decreases as supported from XRD results, the high surface to volume ratio gives noticeable surface effect through the distorted surface structure under the influence of the strain of surface atoms. This distortion in surface structure may be responsible for the creation of higher oxygen and Ni vacancy defects with low coordination numbers. Consequently, these factors could induce spin disorders by the broken exchange bonds for surface atoms. The disordered surface spin increases with decrease in crystallite size, and hence leads to reduce the exchange coupled spins at the surface [69].
We now consider the effect of Mo occupying Ni site on the magnetic properties of Mo doped NiO. The translation symmetry of NiO is broken on doping with Mo, which randomly occupies Ni sites. This leads to the formation of Ni-O-Ni-O-Mo-O-Ni-O-Ni quasi-paramagnetic coupling with a net magnetization per particle in the background of the antiferromagnetic correlation of the host NiO. Absence of signature of any secondary phases up to x=0.15 Mo doped NiO nanoparticles in the XRD pattern refers to that Mo occupies Ni Site in NiO matrix.
As a result, the superparamagnetic response arising from the broken antiferromagnetic symmetry of NiO prevails at low fields whereas at high fields the antiferromagnetic response of the host dominates [70, 71]. This is validated in Figure 15, where the magnetic contribution for each of the superparamagnetic (SPM) and antiferromagnetic (AFM) nature are estimated from the fit and plotted versus the applied magnetic field. It is noticed that at high fields, the AFM behavior dominates, however at low fields, the SPM behavior governs the magnetic nature. Mala et al. [72] showed that the hysteresis loops of Mn-doped NiO NPs prepared by the coprecipitation route exhibited superparamagnetic behaviour. Further, they reported that Mn doping increases the net magnetization due to the magnetic moment of Mn+2 is higher as compared Ni+2 ions.
The fraction of surface to total spins in antiferromagnetic nanoparticles should vary with the crystallite size, coercivity and magnetic moment. The values of coercivity Hc and remnant magnetization Mr are extracted from the M-H loops and listed in Table 7. Both Hc and Mr recorded small values, further assuring the SPM dominance at low fields. Besides, the coercivity decreases with Mo doping, indicating that, it is a function of the particle size. The change in coercivity with particle size can be understood by considering the domain structure, critical size, and the influence of surface and interface anisotropy within the crystal. A crystallite will spontaneously break up into a few domains to reduce the large magnetization energy if it is a single domain. Consequently, as particle size decreases, this energy decreases as well, indicating that even at very small sizes, crystallites tend to maintain a preference for single-domain behavior. [73]. Therefore, Mo doping has the potential to increase the superparamagnetic properties by minimizing the loop size. This makes it suitable for applications in ferro fluids and as a contrast agent in magnetic resonance imaging (MRI) [74].
The pure NiO and Mo-doped NiO show lower comparable magnetization values of Ms. For Mo-doped NiO nanoparticles, the lower values of Ms can be attributed to the non-magnetic nature of Mo6+ (d0) ions, with zero magnetic moment. Additionally, the compositional inhomogeneities and presence of oxygen vacancies, which may be induced by the substitution of higher valent Mo6+ (0.059 nm) ions at Ni2+ (0.069 nm) -sites are also responsible for the observed smaller Ms values.
The squareness ratio (SR) \(S={M}_{r}/{M}_{s}\) characterizes the squareness of the of hysteresis loop where SR ≪ 0.01 for all the samples, is a typical value of superparamagnetic particles [75]. In our results, the low value of Hc and Mr is found for x = 0.075, which is desirable for storage data applications such as RAM and ROM [72, 76].
4 Conclusion
Pure and Mo-doped NiO NPs were successfully prepared by chemical co-precipitation method. The XRD pattern revealed the formation of FCC phase of pure and Mo-doped NiO nanoparticles. As the concentration of Mo increases, the strain increases due to lattice distortion caused by the difference in size between Ni2+ and Mo6+. This results in a decrease in the size of the crystallites. The TEM micrographs showed the formation of nearly spherical morphology that changed to elliptical shaped particles upon Mo-doping. The XPS spectra of NiO and Mo-doped NiO NPs revealed the oxidation states of the presented elements as Ni2+, Ni3+, Mo6+, and O2- ions. In smaller NiO nanoparticles, the lack of second-order phonon and magnon modes in the Raman spectra suggests that the uncompensated surface spins are present and there is less spin correlation between the next nearest neighbor Ni2+ ions in the crystal lattice. Moreover, as the particle size decreases, the intensity of the UV PL peak rises and the peak shifts towards the blue region. The M-H loops were resolved into superparamagnetic and antiferromagnetic nature. The magnetic study illustrated the superparamagnetic nature imposed over the antiferromagnetic contribution of pure and Mo-doped NiO nanoparticles, implying the possibility of using these materials in biomedical field and data storage applications.
Data availability
The datasets during this study will be available on reasonable request.
References
P.M. Ponnusamy, S. Agilan, N. Muthukumarasamy, T.S. Senthil, G. Rajesh, M.R. Venkatraman, D. Velauthapillai, Structural, optical and magnetic properties of undoped NiO and Fe-doped NiO nanoparticles synthesized by wet-chemical process. Mater Charact 114, 166–171 (2016)
B. Bashir, M.U. Khalid, M. Aadil, S. Zulfiqar, M.F. Warsi, P.O. Agboola, I. Shakir, CuxNi1-xO nanostructures and their nanocomposites with reduced graphene oxide: synthesis, characterization, and photocatalytic applications. Ceram. Int. 47(3), 3603–3613 (2021)
B.R. Reddy, G.S. Harish, C.S. Reddy, P.S. Reddy, Synthesis and characterization of Cu doped NiO nanoparticles. Int. J. Mod. Eng. Res. 4(6), 62–66 (2014)
A.C. Gandhi, H.H. Chiu, K.T. Wu, C.L. Cheng, S.Y. Wu, Surface-spin driven room temperature magnetic memory effect in Fe-substituted NiO nanoparticles. Appl. Surf. Sci. 536, 147856 (2021)
G. Bharathy, P. Raji, Room temperature ferromagnetic behavior of Mn doped NiO nanoparticles: a suitable electrode material for supercapacitors. J. Mater. Sci. Mater. Electron. 28(23), 17889–17895 (2017)
A. Khan, M.D. Saad, T. Kamal, F. Rahman, Structural, morphological, optical and UV-light driven enhanced photocatalytic properties of Fe-doped NiO nanoparticles. Mater. Chem. Phys. 305, 127923 (2023)
M.A. Rahman, R. Radhakrishnan, R. Gopalakrishnan, Structural, optical, magnetic and antibacterial properties of Nd doped NiO nanoparticles prepared by co-precipitation method. J. Alloy. Compd. 742, 421–429 (2018)
K. Sathishkumar, N. Shanmugam, N. Kannadasan, S. Cholan, G. Viruthagiri, Influence of Zn2+ ions incorporation on the magnetic and pseudo capacitance behaviors of NiO nanoparticles. Mater. Sci. Semicond. Process. 27, 846–853 (2014)
K. Varunkumar, A.S. Ethiraj, A. Kechiantz, Optical absorption and thermal stability study of Cu doped NiO nanoparticles, AIP Conference Proceedings, Woodbury, 2018
K.N. Patel, M.P. Deshpande, V.P. Gujarati, S. Pandya, V. Sathe, S.H. Chaki, Structural and optical analysis of Fe doped NiO nanoparticles synthesized by chemical precipitation route. Mater. Res. Bull. 106, 187–196 (2018)
B. Kisan, J. Kumar, P. Alagarsamy, Room temperature ferromagnetism in Zn-doped NiO nanoparticles: an experimental and DFTU approach. J. Alloys Compd. 868, 159176 (2021)
A. Sunny, K. Balasubramanian, Phonon, magnon and magnetic properties of Cu-doped NiO nanostructures. J. Raman Spectrosc. 53(7), 1249 (2022)
S. Nandanwar, M.W. Lee, S. Borkar, J.H. Cho, N.H. Tarte, H.J. Kim, Synthesis characterization and anti-algal activity of molybdenum-doped metal oxides. Catalysts 10(7), 805 (2020)
S.E. Balaghi, C.A. Triana, G.R. Patzke, Molybdenum-doped manganese oxide as a highly efficient and economical water oxidation catalyst. ACS Catal. 10(3), 2074–2087 (2020)
C. Yu, K. Yang, Q. Shu, J.C. Yu, F. Cao, X. Li, X. Zhou, Preparation, characterization and photocatalytic performance of Mo-doped ZnO photocatalysts. Sci. Chin. Chem. 55(9), 1802–1810 (2012)
S. Bhanuchandar, G. Vinothkumar, P. Arunkumar, M. Sribalaji, A.K. Keshri, K.S. Babu, Unravelling the role of cationic Ni2+ Vacancies and Ni3+ ions in non-stoichiometric NiO: breakdown of anti-ferromagnetic ordering and large exchange bias. J. Mater. Sci. 58, 13136–13153 (2023)
G. Nabi, B. Atiq, H.I. Elsaeedy, M. Tanveer, W. Ali, A. Riaz, Bandgap tuning by controlled growth of Mo doped NiO nanoparticles and their functional role as excellent photocatalytic degradation agent. Inorg. Chem. Commun. 157, 111448 (2023)
V. Maheskumar, A. Min, C.J. Moon, R.A. Senthil, M.Y. Cho, Modulating the electronic structure of Ni/NiO nanocomposite with high-valence Mo doping for energy-saving hydrogen production via boosting urea oxidation kinetics. Small Struct. 4, 230021 (2023)
H.H. Abdelhalium, M.S. Abdel-wahab, M.T. Tamm, W.Z. Tawfik, Highly efficient ultraviolet photodetector based on molybdenum-doped nanostructured NiO/ITO thin film. Appl. Phys. A 129, 459 (2023)
S. Adewinbi, V. Maphiri, R. Marnadu, M. Shkir, N.M.H. Alsdran, H. Algarni, G. Sujithkumar, B. Taleatu, T. Niyitanga, H. Kim, Transparent, photosensitive and highly efficient pseudocapacitive binder-free Mo-modified NiO thin film electrode for bifunctional optoelectronic and energy storage applications. J. Alloy. Compd. 937, 168304 (2023)
A. Goktas, S. Modanlı, A. Tumbul, A. Kilic, Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO:Co nano rod-like homojunction thin films: role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 893, 162334 (2022)
A. Goktas, F. Aslan, A. Tumbul, Nanostructured Cu-doped ZnS polycrystalline thin films produced by a wet chemical route: the influences of Cu doping and film thickness on the structural, optical and electrical properties. J. Sol-Gel Sci. Technol. 75, 45–53 (2015)
M. Napari, T.N. Huq, R.L.Z. Hoye, J.L. MacManus-Driscoll, Nickel oxide thin films grown by chemical deposition techniques: potential and challenges in next-generation rigid and flexible device applications. InfoMat 3(5), 536–576 (2021)
M.W. Alam, A. BaQais, T.A. Mir, I. Nahvi, N. Zaidi, A. Yasin, Effect of Mo doping in NiO nanoparticles for structural modification and its efficiency for antioxidant, antibacterial applications. Sci.Rep. 13, 1328 (2023)
R. Ahmed, G. Nabi, F. Ali, F. Naseem, M.I. Khan, T. Iqbal, M. Tanveer, Q. Aain, W. Ali, N.S. Arshad, A. Naseem, M. Maraj, M. Shakil, Controlled growth of Mo-Doped NiO nanowires with enhanced electrochemical performance for supercapacitor applications. Mater. Sci. Eng. B 28, 115881 (2022)
M. Shah, M. Fatema, D.A. Ansari, D.K. Gupta, M.D. Rather, Tuning the structural, magnetic, and electrochemical properties of Mo-doped NiO nanostructures prepared by coprecipitation method. Inorg. Chem. Commun. 151, 110641 (2023)
S. Goktas, A. Goktas, A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: a review. J. Alloy. Compd. 863(2021), 158734 (2021)
A.M. Abdallah, M. Noun, R. Awad, Dielectric, impedance and conductivity properties of pristine and (Gd, Ru)-dual doped NiO nanoparticles. J. Alloy. Compd. 910, 164952 (2022)
P. Devi, S. Rohilla, J. Saini, R. Patwa, Structural characterization of nanocomposites of NiO/H2O4Sn3 prepared by coprecipitation method. ECS Trans. 107, 15291 (2022)
R. Swapna, M.C.S. Kumar, Growth and characterization of molybdenum doped ZnO thin films by spray pyrolysis. J. Phys. Chem. Solids 74, 418–425 (2013)
S. Panja, I. Chaudhuri, K. Khanra, N. Bhattacharyya, Biological application of green silver nanoparticle synthesized from leaf extract of Rauvolfia serpentina Benth. Asian Pac. J. Trop. Dis. 6(7), 549–556 (2016)
R.D. Shannon, IUCr, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides Acta Crystallogr. Sect. A. 32, 751–767 (1976)
P. Enghag, Encyclopedia of the elements: technical data–history–processing–applications. Foreword Bengt Nordén (2007). https://doi.org/10.1002/9783527612338.ch17
S. Yousaf, S. Zulfiqar, M.N. Shahi, M.F. Warsi, N.F. Alkhalli, M.F.A. Aboud, I. Shakir, Tuning the structural optical and electrical properties of NiO nanoparticles prepared by wet chemical route. Ceram. Int. 46, 3750–3758 (2019)
G. Sedaghati-Jamalabad, M.M. Bagheri-Mohagheghi, Influence of synthesis route on structural properties of SnFe2O4 spinel phase via methods of co-precipitation, sol–gel and solvothermal: morphology, phase analysis, crystallite size and lattice strain. Discov. Appl. Sci. 6, 202 (2024)
K.F. Qasim, M.A. Mousa, Physicochemical properties of oriented crystalline assembled polyaniline/metal doped Li4Ti5O12 composites for li-ion storage. J. Inorg. Organomet. Polym. 33, 2601–2617 (2023)
R.S. Kate, S.A. Khalate, R.J. Deokate, Physical and electrochemical properties of molybdenum doped nickel oxide thin film electrodes. IRJSE A1, 199–202 (2017)
J. Boukhari, A. Khalaf, R. Awad, Structural analysis and dielectric investigations of pure and rare earth elements (Y and Gd) doped NiO nanoparticles. J. Alloys Compd. 820, 153381 (2020)
D. Xue, J. Luo, Z. Li, Y. Yin, J. Shen, Enhanced photo electrochemical properties from Mo-doped TiO2 nanotube arrays film. Coatings 10, 75 (2020)
T. Ivanova, A. Harizanova, M. Shipochka, P. Vitanov, Nickel oxide films deposited by sol-gel method: effect of annealing temperature on structural, optical, and electrical properties. Mater. (Basel) 15(5), 1742 (2022)
M. Cheng, H. Fan, Y. Song, Y. Cui, R. Wang, Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors. Dalton Trans. 46, 9201 (2017)
X. Xu, H. Zhang, Y. Tong, Y. Sun, X. Fang, J. Xu, X. Wang, Tuning Ni3+ quantity of NiO via doping of cations with varied valence states: the key role of Ni3+ on the reactivity. Appl. Surf. Sci. 550, 149316 (2021)
S.G. Jo, C.S. Kim, S.J. Kim, J.W. Lee, Phase-controlled NiO nanoparticles on reduced graphene oxide as electrocatalysts for overall water splitting. Nanomater. (Basel) 11(12), 3379 (2021)
U. Kwon, B.G. Kim, D.C. Nguyen, J.H. Park, N.Y. Ha, S.J. Kim, S.H. Ko, S. Lee, D. Lee, H.J. Park, Solution processible crystalline NiO Nanoparticles for high-performance planar perovskite photovoltaic cells. Sci. Rep. 6, 30759 (2016)
A.M. Abdallah, R. Awad, Influence of Ru dopants on the structural, optical, and magnetic properties of nickel oxide nanoparticles. Physica B 629, 413651 (2022)
X. Li, Y. Wang, J. Wang, Y. Da, J. Zhang, L. Li, C. Zhong, Y. Deng, X. Han, W. Hu, Sequential electrodeposition of bifunctional catalytically active structures in MoO3/Ni–NiO composite electrocatalysts for selective hydrogen and oxygen evolution. Adv. Mater. 32, 2003414 (2020)
T.R. Silva, V.D. Silva, L.S. Ferreira, A.J.M. Araújo, M.A. Morales, T.A. Simões, C.A. Paskocimas, D.A. Macedo, Role of oxygen vacancies on the energy storage performance of battery-type NiO electrodes. Ceram. Int. 46, 9233–9239 (2020)
H. Hu, I.E. Wachs, Surface structures of supported molybdenum oxide catalysts. Characterization by Raman and Mo L3-Edge XANES. J. Phys. Chem. 99(27), 10897–10910 (1995)
N.A. Sagui, P. Ström, T. Edvinsson, I.B. Pehlivan, Nickel site modification by high-valence doping effect of tantalum impurities on the alkaline water electro-oxidation by NiO probed by operando Raman spectroscopy. ACS Catal. 12, 6506–6516 (2022)
K. Habanjar, M. Almoussawi, A.M. Abdallah, R. Awad, Magneto-optical effect of (Sm, Co) co-doping in ZnO semiconductor. Physica B 598, 412444 (2020)
J.A. Boukhari, Z. Bitar, A.A. Azab, R. Awad, Structural optical and magnetic properties of Ni1–2xMgxRuxO nanoparticles. Phys. Scr. 98, 075934 (2023)
A. Sunny, A. Sunny, Raman spectral probe on size-dependent surface optical phonon modes and magnon properties of NiO nanoparticles. J. Phys. Chem. C 124(23), 12636–12644 (2020)
K.N. Patel, M.P. Deshpande, K. Chauhan, P. Rajput, V. Sathe, S. Pandya, S.H. Chaki, Synthesis, structural and photoluminescence properties of nano-crystalline Cu doped NiO. Mater Res. Express 4, 105027 (2017)
D. Liu, D. Li, D. Yang, Size-dependent magnetic properties of branchlike nickel oxide nanocrystals. AIP Adv. 7, 015028 (2017)
S. Sahoo, S. Dhara, V. Sivasubramanian, S. Kalavathi, A.K. Arora, Phonon confinement and substitutional disorder in Cd1− xZnxS nanocrystals. J. Raman Spectrosc. 40(8), 1050–1105 (2009)
P. Ravikumar, B. Kisan, A. Perumal, Enhanced room temperature ferromagnetism in antiferromagnetic NiO nanoparticles. AIP Adv. 5, 087116 (2015)
W. Duan, S. Lu, Z. Wu, Y. Wang, Size effects on properties of NiO nanoparticles grown in alkali salts. J. Phys. Chem. C 116, 26043–26051 (2012)
U.K. Panigrahi, V. Sathe, P.D. Babu, A. Mitra, P. Mallick, Effect of Mg doping on the improvement of photoluminescence and magnetic properties of NiO nanoparticles. Nano Express 1(2), 020009 (2020)
C. Thangamani, P.V. Kumar, K. Gurushankar, K. Pushpanathan, Structural and size dependence magnetic properties of Mn-doped NiO nanoparticles prepared by wet chemical method. J. Mater. Sci. Mater. Electr. 31(14), 11101–11112 (2020)
U.K. Panigrahi, P.K. Das, R. Biswal, V. Sathe, P.D. Babu, A. Mitra, P. Mallick, P, Zn doping induced enhancement of multifunctional properties in NiO nanoparticles. J. Alloy. Compd. 833, 155050 (2020)
M. Shkir, K.V. Chandekar, A. Khan, T. Alshahrani, A.M. El-Toni, M.A. Sayed, S. AlFaify, Tailoring the structure-morphology-vibrational-optical-dielectric and electrical characteristics of Ce@NiO NPs produced by facile combustion route for optoelectronics. Mater. Sci. Semicond. Process. 126, 105647 (2021)
K.N. Patel, M.P. Deshpande, K. Chauhan, P. Rajput, V.P. Gujarati, S. Pandya, S.H. Chaki, Effect of Mn doping concentration on structural, vibrational and magnetic properties of NiO nanoparticles. Adv. Powder Technol. 29(10), 2394–2403 (2018)
A.M. Abdallah, R. Awad, Mixed magnetic behavior in gadolinium and ruthenium co-doped nickel oxide nanoparticles. Phys. Scr. 97, 015802 (2022)
J.A. Boukhari, A.A. Azab, Z. Bitar, R. Awad, Influence of (Mg, Cu) co-doping on the structural, optical and magnetic properties of NiO nanoparticles synthesized by coprecipitation method. Phys. B Condens. Matter 663, 415004 (2023)
S.P. John, J. Mathew, Determination of ferromagnetic, superparamagnetic and paramagnetic components of magnetization and the effect of magnesium substitution on structural, magnetic and hyperfine properties of zinc ferrite nanoparticles. J. Magn. Magn. Mater. 475, 160–170 (2019)
A. Bajpai, A. Banerjee, Super paramagnetism in polycrystalline Li0.5Ni0.5O samples: low-field susceptibility measurements. J. Phys. Rev. B. 62, 8996 (2000)
N. Srivastava, P.C. Srivastava, Realizing NiO nanocrystals from a simple chemical method. Bull. Mater. Sci. 33, 653–656 (2010)
R.H. Kodama, S.A. Makhlouf, A.E. Berkowitz, Finite size effects in antiferromagnetic NiO nanoparticles. Phys. Rev. Lett. 79, 1393–1396 (1997)
V. Sharma, S. Pal, D. Sharma, D.K. Shukla, R.J. Chaudhary, G.S. Okram, Size-induced exchange bias in single-phase co nanoparticles. Nanotechnology 35, 275702 (2024)
F. Zhao, H.M. Qiu, L.Q. Pan, H. Zhu, Y.P. Zhang, Z.G. Guo, Ferromagnetism analysis of Mn-doped CuO thin films. J. Phys. Condens. Matter 20, 425208 (2008)
P. Mallick, C. Rath, A. Rath, A. Banerjee, N.C. Mishra, Antiferro to superparamagnetic transition on Mn doping in NiO. Solid State Commun. 150, 1342–1345 (2010)
N.A. Mala, M.A. Dar, M.D. Rather, B.A. Reshi, S. Sivakumar, K.M. Batoo, Z. Ahmad, Supercapacitor and magnetic properties of NiO and manganese-doped NiO nanoparticles synthesized by chemical precipitation method. J. Mater. Sci. Mater. Electr. 34, 505 (2023)
S.P. John, J.M. Mathew, Determination of ferrimagnetic and superparamagnetic components of magnetization and the effect of particle size on structural, magnetic and hyperfine properties of Mg0.5Zn0.5Fe2O4 nanoparticles. J. Alloys Compd. 869, 159242 (2021)
C. Kartikeyan, R. Aepuru, K. Varaprasad, N. Tiwari, P.K. Sahoo, T. Jayaramudu, F. Sanhueza, M.R. Viswanathan, K. Kim, Superparamagnetic and antimicrobial biosynthesis of Ce/NiO nanomaterials for biomedical applications. Nano-Structures Nano-Objects 35, 101018 (2023)
J.S. Jung, L. Malkinski, J.H. Lim, M. Yu, C.J. O’Connor, H.O. Lee, E.M. Kim, Fabrication and magnetic properties of Co nanostructures in AAO membranes. Bull. Korean Chem. Soc. 29(4), 758 (2008)
S. Sivakumar, N.A. Mala, K.M. Batoo, E.H. Raslan, Efficient, highly stable Zn2+ doped NiO nanoparticles with enhanced magnetic and supercapacitor applications. Mater. Technol. 36, 1–13 (2021)
Acknowledgements
The authors would like to thank Beirut Arab University for encouraging and supporting their research. Additionally, they express their gratitude to the Lebanese Atomic Energy Commission, National Council for Scientific Research, Beirut, Lebanon, and the Novel Materials Lab, Faculty of Science, Alexandria University, Alexandria, Egypt for their expert assistance with the measurements and analysis.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. Khalaf: Formal analysis, Data curation, Writing- original draft. Rayane Saghir: Investigation, Data curation, Formal analysis, Writing- original draft. A. M. Abdallah: Formal analysis, Data curation, Writing- original draft. M. Noun: Validation, Data Curation, Writing- review and editing. R. Awad: Conceptualization, Writing- review and editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The author declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalaf, A., Saghir, R., Abdallah, A.M. et al. Influence of Mo doping on the structural, Raman scattering, and magnetic properties of NiO nanostructures. Appl. Phys. A 130, 691 (2024). https://doi.org/10.1007/s00339-024-07816-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07816-w