Abstract
Nanostructured pure and molybdenum (Mo)-doped nickel oxide (NiO) thin films with various concentrations of Mo dopants were successfully sputtered on indium-doped tin oxide (ITO) substrates to apply in the ultraviolet (UV) photodetector sensors. The influence of Mo concentration on the thin films' structural, morphological, and optical properties was studied using X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) equipped with energy-dispersive X-ray, and UV–Vis spectrophotometer. The XRD studies confirm that all the prepared films are polycrystalline and possess a cubic phase structure. The FE-SEM images suggest that the distribution of the prepared samples on the substrate is homogeneous and free from any cracks. Spectrophotometry studies reveal the decrement of the optical band gap with the increase of Mo concentration in NiO thin films. All thin-film current–voltage curves measured under dark conditions and UV illumination of 390 nm showed Ohmic contacts. A noticeable improvement in the responsivity and external quantum efficiency (EQE) with the increased Mo concentration was detected. The metal–semiconductor–metal (MSM) employed in NiO thin films showed maximum responsivity and EQE of 539 mA/W and 171.4% at 390 nm, respectively, for the sample with 1.73 at% Mo doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photodetectors (PDs) are optoelectronic devices that can transform a light signal into an electrical signal. This mechanism can occur when light incident on a material with energy greater than the band gap of the used material results in the formation of electron–hole pairs as electrons accept energy, exit to the conduction band, and leave a hole in the valance band, so photogenerated current increases. It is well known that the sun emits ultraviolet (UV) radiation in addition to visible and infrared light. Although the majority of UV-C (100–280 nm) and UV-B (280–315 nm) radiation can be absorbed by the ozone layer, UV-A (315–400 nm) light can reach the earth, causing several diseases, such as skin cancer and skin burn. To avoid these problems, wide-band-gap semiconductors can employ in PDs to detect harmful UV radiation [1]. UV PDs have attracted the attention of researchers around the world due to their superior performance in a wide range of areas, such as video imaging, spatial optical communication, chemical/biological sensing, environmental monitoring, space exploration, security, night vision, motion detection, and sterilization [2,3,4,5,6,7]. Previously, the UV PDs were made from nanostructured materials such as GaN, InGaAs, SiC, AlGaN, and CdS due to their large energy band gap, high photosensitivity, and excellent optical and electrical properties [2, 4, 6, 8]. Unfortunately, these materials have various issues, including high cost, and require complex manufacturing techniques [9,10,11,12,13]. Wide band-gap metal oxide semiconductors PDs are favored to overcome these issues. Recently, metal oxides have received much attention because of their excellent electrical and optical properties, low cost, easy manufacture, higher sensitivity, and responsivity in the UV detection area [2, 6]. Various metal oxides are used in UV PDs due to their photodetection capabilities, such as TiO2, ZnO, WO3, and NiO [14,15,16,17,18]. Among these metal–oxide semiconductors, nickel oxide (NiO) is classified as one of the most promising p-type materials with unique physical characteristics such as a direct and wide energy band gap ranging from 3.15 to 4 eV, a large exciton binding energy of 110 meV, poor absorption bands in the visible wavelength region, high hole concentration and mobility, and remarkable chemical stability and durability. Moreover, NiO has more numerous advantages, such as inexpensive, non-toxic, earth abundant, and transparent to visible light which makes it an excellent option for UV-based optoelectronic devices, including UV photodetectors, UV lasers, and UV LEDs [3, 4, 6, 19,20,21]. The doping with transition metal (TM) elements, such as Co, Cu, Mn, Ag, Cr, and Mo [2, 22,23,24,25,26,27,28,29,30], can improve the optical and electrical properties of NiO, so that increase the photocurrent and responsivity of NiO-based PDs. Bala et al. study UV photodetection of Cu-doped NiO which show high response time with rise time of 2.9 s, and fall time of 3.3 s, high photocurrent of 25.4 μA, and high responsivity of 0.202 AW−1 [31]. Raj et al. demonstrate Ag doped NiO-PD film which enhance the optical and electrical properties of NiO (photocurrent 4 μA, EQE 49%, and responsivity ~ 0.209 AW−1) [30]. Among these transition metal elements, the Mo element was selected as a dopant in the NiO matrix. Nevertheless, very few reports on UV PDs based on Mo-doped NiO thin films are available. Therefore, it is important to investigate the effects of Mo doping on the performance of UV PDs-based NiO thin film. NiO thin films could prepared using various techniques, including RF sputtering, reactive gas deposition, pulsed laser deposition, and electron beam evaporation as well as low cost wet chemical methods, such as successive ionic layer adsorption and reaction (SILAR), sol–gel, chemical bath deposition (CBD), spin coating, and spray pyrolysis [24, 26, 32,33,34,35]. In comparison to other techniques, RF sputtering is an appropriate method for fabricating NiO films on large substrate areas with great homogeneity, high deposition rate, and minimal defects [6]. In this paper, the Mo-doped NiO films were successfully fabricated on ITO semiconductor substrate using DC/RF sputtering with different Mo concentrations. Characterization, including the structure, composition, morphology, optical, and UV photodetection properties, were investigated systematically. The ITO semiconductor substrate improves the opto-electrical properties of NiO thin film as it increases light trapping, carriers’ collection efficiency, and inhibited reflection [4]. When the light incident on p-NiO/n-ITO films with a suitable wavelength (NiO λ ≤ 390 nm, approximately ≤ 3.1 eV), the generation of electron–hole pairs occurs from p–n junction, electrons (e−) in conduction band, and holes (h+) in valance band. The electrical nature of NiO is p-type conductivity; this nature was confirmed for all samples with Hall measurements’ analysis (see Table 1); so conduction will be mainly by holes beside electrons. The photogenerated holes migrate toward ITO electrode, and electrons migrate to the Ag electrode, reducing recombination process through applied voltage which accelerates movement of electrons and holes. The maximum value of responsivity, EQE, that obtained from our NiO/ITO UV PDs was 539 mA/W and 171.4%, respectively, under a UV irradiation of 390 nm at a power of 100 mW and applied voltage of -1 V.
2 Materials and methods
2.1 Thin films’ preparation
The pure and Mo-doped NiO thin films were deposited on a commercial pre-cleaned ITO on glass substrate (Sigma-Aldrich) using DC/RF magnetron sputtering system (Syskey Technologies, Taiwan) from a Ni target (3 × 0.6 inch) and molybdenum (Mo) (3 × 0.6 inches) metals with high purity (99.99%) at room temperature with different concentrations of Mo. Before deposition, the ITO on glass substrate was cleaned with acetone, ethanol, and distilled water and then dried with pressurized nitrogen gas. The distance between the target and the substrate was approximately 14 cm. After that, the chamber was evacuated to 9 × 10–6 Torr base pressure and filled with the ultra-pure Ar gas as plasma source with a constant flow rate of 20 sccm and oxygen flow rate of 10 sccm. NiO was sputtered on the ITO substrate under 5 × 10−3 Torr working pressure with DC sputtering power of Ni target was 200 W, and RF power for Mo target was changed from 0, 30, and 40, to 50 W, and the prepared samples were labeled as NiO, NMO-1, NMO-2, and NMO-3, respectively. The time of deposition was fixed for all samples at 600 s.
2.2 Thin films’ characterization
The samples were collected and analyzed using various systems. The structural characteristics of the deposited films were examined via X-ray diffraction data that were collected using an X'Pert Pro X-ray diffractometer with Cu Kα radiation (λ = 1.542 Å). Field emission scanning electron microscopy (FE-SEM) (JSM—7600F; JEOL—Japan) was utilized to examine the surface morphology of the prepared thin films and the film's elemental composition, and mapping its elements was carried out using energy-dispersive spectroscopy (EDS). To record transmittance for the prepared films, a UV–Vis spectrophotometer (Lambda 750, Perkin Elmer) was used within wavelengths ranging from 200 to 1100 nm and calculating the optical band gap. To measure the thicknesses of deposited thin films, a part of the pre-cleaned ITO on glass substrate was covered with a capton tape before deposition process to get a proper step to be measured with a surface profiler (DektakXT, Bruker, Germany). Ecopia HMS-3000 Hall measurement system was utilized to record the carrier concentrations of all samples.
2.3 Photodetection experiment
The device structure of the photodetector under UV illumination is shown in Fig. 1a. The current–voltage (I–V) curves of all films were measured at room temperature using a digital source-meter analysis system (Keithley 2450) under applied bias voltage ranging between − 1 and + 1 V in the presence of the light source of a Xe lamp (Newport) with a power density of 100 mW/cm2. Using a highly conducting silver paste, two electrodes were attached at the two opposite edges above the 1 cm2 sample. The effect of light wavelengths on the photodetectors was investigated using a monochromator with a grating of 390–636 nm. The photoresponsivity was measured by illuminating the samples on the NiO side. In the dark and under 390 nm UV illumination, the device's properties, such as responsivity, detectivity, photosensitivity, and external quantum efficiency (EQE) values, were evaluated.
3 Results and discussion
3.1 Structural studies
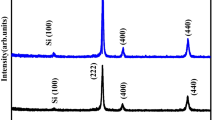
To investigate the crystal structural properties of pure NiO and Mo-doped NiO films, the PANalytical X'Pert Pro X-ray diffractometer was employed with monochromatic Cu Kα radiation (λ = 1.542 Å), a scan rate 0.02°s−1, and a 2-theta ranging from 20 to 80° at room temperature. Figure 2a shows X-ray diffraction patterns measured from pure NiO and Mo-doped NiO thin films. The figure reveals that all samples are polycrystalline with cubic phase structure and space group of Fm-3 m. According to XRD patterns of all prepared films, only two peaks are observed at 2θ ~ 36.1 and 41.7, corresponding to (111) and (200), respectively. All observed peaks agree with the standard spectrum for the NiO phase (JCPDS card No. 00-001-1239). No peaks related to other phases, such as Mo, Mo2O3, and MoNiO3, are detected in the patterns, indicating that Mo atoms were incorporated at Ni sites of the NiO lattice. This confirms that all films are purely grown successfully without any phase change. The other observed peaks in the XRD patterns are related to the ITO substrate. Understanding the structural characteristics of the prepared films also requires the computation of structural parameters, including crystallite size, dislocation density, and micro-strain.
The widely used Scherer's formula was used to determine the crystallite size (D) for the (111) plane of the pure NiO and Mo-doped NiO thin films [36,37,38]
where β represents the full width at half maximum for the (111) plane, λ is the X-ray wavelength (λ = 1.5406 Å), and θ is the Bragg's angle. The calculated crystallite size of all films is tabulated in Table 1. From Table 1, the crystallite size was initially increased from 29 to 40 nm by increasing the Mo concentrations up to 1.73% (NMO-2 sample) and then decreased to 36 nm for 2.02% Mo concentration (NMO-3 sample). The crystallite size increase from 29 to 40 nm as the sputtering power increases from 0 to 40 W, which increases the number of ejected atoms from the target and causes the increment in the thickness of the samples [34, 35]. The measured thickness values of all samples are tabulated in Table 1 and shown in Fig. 1(b). After that, the crystallite size decreases with increasing the sputtering power up to 50 W (NMO-3 sample). The reduction in crystallite size may be attributed to the Zener pinning effect that is causing increasing the compactness and reduction of the film thickness for the NMO-3 sample compared to the NMO-2 sample, as shown in Table 1. The Mo atoms may work as barriers that prevent grain boundary movement and lead to grain growth reduction [31]. The dislocation density (δ), micro-strain (ε), and constant lattice value (a) of all the prepared films were calculated using the following formulae [39, 40]:
where D is the crystallite size of the film, β is the (111) full-width half maxima, θ is the diffraction angle, hkl is Miller indices, and dhkl represents the interplanar spacing. The calculated values are listed in Table 1. Figure 2b shows the relation between micro-strain and crystallite size as a function of Mo concentration. Contrary to crystallite size, as the Mo concentration is increased up to 1.73% of Mo concentration (NMO-2 sample), the dislocation density and lattice strain reduces, and then, they increase for the NMO-3 sample with 2.02% Mo concentration. Initially, the decrement in the dislocation density and micro-strain indicates fewer defects in the lattice. This could be ascribed to the ionic radius of Mo being smaller than the ionic radius of Ni+2. Then, the increment in the micro-strain and dislocation density of NMO-3 sample could be mainly attributed to the high concentration of Mo, as when a large number of Mo replaces Ni ions, the distortion is produced in the lattice and therefore, the strain and dislocation density increased [39]. Furthermore, the lattice constant of cubic NiO lattice has the same micro-strain and dislocation density behavior. Its values decreased with increasing the Mo concentration up to 1.73% (NMO-2 sample) and then increased for the NMO-3 sample with a Mo concentration of 2.02%. This could be attributed to the smaller ionic radius of Mo (0.7 Ǻ) compared to the ionic radius of Ni+2 (0.72 Ǻ).
3.2 Surface morphology features
The optical and electrical properties of transparent conducting oxide films, which are important parameters in optoelectronic devices, are influenced by their surface properties. Thus, examining the surface morphology of the produced films is essential. The surface morphology of pure and Mo-doped NiO films was studied using FE-SEM equipped with an EDS part to analyze the elemental composition of all samples. Figure 3 depicts the FE-SEM images and the particle-size distribution of all deposited films on the ITO substrate. All films have a tiny grain size, are moderately dense, and the surface is free from cracks. The SEM images also revealed a granular texture, and the nanograins nearly covered the substrate surface. Furthermore, all sputtered particles are distributed uniformly over the ITO substrate. The average grain size has been calculated using Image J software [41]. The average grain size increased from 17.7 nm to 30.8 nm with an increase in Mo concentration up to 1.73% (NMO-2) sample and then decreased for the NMO-3 sample with a Mo concentration of 2.02%. These concentration ratios of Mo were estimated via energy-dispersive X-ray spectroscopy (EDX) analysis. The increment in the average grain size is related to the increase in the samples' thickness, ascribed to the increase in the sputtering power from 0 to 50 W [34, 35, 39]. These results agree with the XRD date; see Table 1. To determine the elemental composition of the as-deposited films, the EDX analysis was carried out, as shown in Fig. 4a–d. The results confirmed that Mo-doped NiO films contain both Ni, O, and Mo; those weights in pure and Mo-doped NiO films are listed in Table 1. The homogeneity of the Mo-doped NiO film is confirmed via the mapping analysis, as shown in Fig. 4g, which shows that the Ni, O, and Mo components are dispersed throughout the sample.
3.3 UV–Vis spectroscopy analysis
The optical transmittance spectra of NiO and Mo-doped NiO thin films deposited on ITO substrate were measured using a UV–visible spectrophotometer in wavelength varying from 200 to 1100 nm (covers the UV–visible–near-IR spectrum), as shown in Fig. 5a. From the figure, the transmittance of all samples increased in the visible region from 550 to 800 nm and reduced near the UV spectral region (< 400 nm), which makes NiO films a good proposal for the fabrication of UV detectors. Generally, the influence of crystallite size and thickness of the samples is responsible for the change in the transmittance spectra. The average transmittance reduced from 62% for pure NiO to 45.4% for the NMO-2 sample with a Mo concentration of 1.73% and then increased to 47.6% for the NMO-3 sample with a Mo concentration of 2.02%. This could be attributed to the increment in the Mo concentration accompanied by an increment in the samples' crystallite size and thickness, increasing the light's absorption and scattering [34, 35]. The increase in the absorption will lead to an increase in the electrical conductivity of the samples.
The optical band gap (Eg) for all deposited thin films was estimated from Tauc's equation associated with the energy of incident light with the absorption coefficient [42,43,44,45,46,47]
where A is a constant, hѵ is the energy of incident light, α is the absorption coefficient, Eg is the energy optical band gap, and n depends on the transition type (n = 2 for indirect allowed transitions and n = ½ for direct allowed transitions). Transition in pure NiO and MNO thin films is direct, so n equals ½. The absorption coefficient can be calculated using the following equation for all deposited thin film [48]:
The absorption coefficient depends on the thickness of film d and transmittance T. The energy of the optical band gap (Eg) is obtained by plotting direct transition (αhѵ)2 versus the energy of incident light (hѵ). Extrapolation of the curve's linear area cut the energy axis. Figure 5b shows the tauc plots for all NMO deposited samples. It is observed that, as the Mo concentration rose, the band-gap value changed (inset Fig. 5b). The band-gap change may result from Mo levels in the NiO lattice. The obtained energy band gap is shown in Table. 1. The energy band gap decreased with an increase in Mo concentration up to 1.73% (NMO-2) sample and then increased for the (NMO-3) sample with a Mo concentration of 2.02%. The decrement could be attributed to the increased crystallite size of prepared samples with increased Mo concentration, as shown in XRD results. Furthermore, the convergence between the valance band and conduction band in the NiO lattice could be ascribed to the Mo atoms increasing the number of holes in the valance band (bonding level) of NiO, shifting the VB upward and the conduction band (antibonding level) lower in comparison to pure NiO causing narrowing in the band gap of NiO [29, 49].
3.4 Photodetection measurements
Figure 6a shows the I–V characteristics of NiO and NMO photodetector samples with Ag electrodes on the coated ITO substrates in the dark. The I–V characteristics of the prepared photodetector samples show linear behavior, and the dark current and photocurrent increase with an increased applied voltage. This behavior indicates that the contact between Ag and NiO is Ohmic contact. The I–V characteristics of the dark current show that the maximum current value is (2.8 mA) for the MNO-2 sample. As a result of the NMO-2 sample demonstrating the optimum device performance, the I–V characteristics of the NMO-2 sample are studied under several laser illumination filters (390 nm, 405 nm, 460 nm, 508 nm, and 636 nm) and power density (100 mW/cm2), as shown in Fig. 6b. From this figure, the maximum photocurrent is (57 mA) at 390 nm; this wavelength matched with Eg of NiO (3.15–4 eV). Because of NMO-2 sample gives the maximum value of current at 390 nm, the I–V characteristic curves of all samples are studied at 390 nm, as shown in Fig. 6c. The increment in the current of the NMO-2 sample under UV light than in the dark ascribed to the higher absorption of light of the NMO-2 sample due to their high crystallite size, causing a high concentration of photogenerated electron–hole pairs, hence the photoconductivity increased [31]. The responsivity (R), external quantum efficiency (EQE), and detectivity (D*) are essential three parameters as they indicate the performance of the photodetector. These parameters are measured under different illumination wavelengths from 390 to 636 nm and applied power 100 mW/cm2. The responsivity is the ratio of the photocurrent to the area of the active layer and the incident light power calculated by the following relation [47, 50, 51]:
where Ip is photocurrent, Pin is the power of the incident light, and A is the effective area of the active layer. Figure 7a shows the responsivity of NMO-2 at different wavelengths, and the maximum value is 539 mA/W at 390 nm. Figure 8a shows the responsivity of the NiO and NMO photodetector samples at the applied voltage of 1 V at 390 nm; the R values are showed in Table 2. The responsivity increased from 215 to 539 mA/W with an increase in the Mo concentration up to 1.73% (NMO-2) sample and then decreased to 384 mA/W in the NMO-3 sample with a concentration of 2.02%. The increment in the responsivity is related to the increment of crystallite size with increased Mo concentration. Therefore, light absorption in the samples will increase, and the produced photocurrent will increase [52]. The detectivity is the ability of the photodetector to detect weak optical signals calculated by the following relation [47, 51]:
where R is responsivity, A is effective area, e is the electron charge, and Id is dark current. The detectivity is mainly dependent on "R" values. Figure 7c shows the detectivity of NMO-2 at different wavelengths. The maximum value is 1.78 × 108 Jones at 390 nm. Figure 8c represents the detectivity of the NiO and NMO photodetector samples at the applied voltage of 1 V at 390 nm. The D* values are 1.1 × 108, 1.6 × 108, 1.78 × 108, and 1.48 × 108 Jones for the NiO, NMO-1, NMO-2, and NMO-3 samples, respectively. The maximum value of detectivity is observed for the NMO-2 sample (1.78 × 108 Jones). This indicates that the NMO-2 sample has the most effective signal-detecting capacity among all the samples. The EQE is the ratio of the electrons harvested per unit of time to the absorbed photons and is calculated by the following relation [6, 47, 50]:
where λ is the wavelength of the laser light, C is velocity of light (3 × 108 m/s), h is Planck's constant (6.62 × 10–34 kg/s), e is electron charge (1.6 × 10−19coulombs), and R is responsivity. Figure 7b shows the EQE of NMO-2 at different wavelengths, and the maximum value is 171.4% at 390 nm. Figure 8b shows the external quantum efficiency of the NiO and NMO photodetector samples at the applied voltage of 1 V at 390 nm. The calculated EQE values are 68.5%, 143.6%, 171.4%, and 122.1% for the NiO, NMO-1, NMO-2, and NMO-3 samples. The maximum value of photodetection EQE observed for the NMO-2 sample (171.4%) might be due to the increase of the crystallite size of the NMO-2 sample, improving the absorption of light, and hence, carriers’ concentration increases which indicates that the quality of the samples is correlated with XRD and other characterizations [7]. Photosensitivity is also an important parameter; it determines the quality of performance of the photodetector. It is defined as the ratio of the change in current to the dark current calculated by the following relation [4, 53, 54]:
where Iph and Id are the photocurrent and dark current, respectively. Figure 7d shows the photosensitivity of NMO-2 at different wavelengths, and the maximum value is 1896.4% at 390 nm. Figure 8d shows the photosensitivity of the NiO and NMO photodetector samples at the applied voltage of 1 V at 390 nm. The calculated photosensitivity values are 1795.5%, 1882%, 1896.4%, and 1830% for the NiO, NMO-1, NMO-2, and NMO-3 samples. The maximum photosensitivity value was also observed for the NMO-2 sample (1896.4%). This is attributed to the large crystallite size and high light absorption leading to a high photocurrent of NMO-2. From the previous results, the best sample is NMO-2. Figure 9 shows the spectral responsivity and external quantum efficiency of NiO and NMO photodetector samples at the different applied voltages. At 390 nm, as the applied voltage increases, the responsivity and EQE of all samples increase with the voltage and then decrease for the NMO-3 sample. This could be attributed to the increment of photocurrent with increased voltage and then decrease for the NMO-3 sample, which ascribed to the decrement in the band gap and then increase for the NMO-3 sample.
3.5 Mechanism of NiO/ITO photodetector
In general, the performance of the photodetector under both dark and light conditions is explained in several ways. In the dark, the oxygen molecules in atmospheric air adsorbed on the surface of p-type NiO and trapped free electrons, forming negatively charged electron traps, which can be expressed as (O2 + e− → O2−); this results in the formation of a depletion layer with low conductivity close the NiO film surface. When NiO samples are exposed to UV light, the energy of the incident photons is higher than the energy gap of NiO. A large number of photogenerated electron–hole pairs produced on NiO photosensitive surface can be expressed as (NiO: Mo + UV light → e− + h+). The photogenerated holes migrate toward the NiO surface and neutralize the oxygen ions through recombination with holes and liberate oxygen from the NiO surface, and this can be expressed as (O2 − + h+ → O2) [31], thereby the depletion layer reduced and also the photocurrent reduced. The dark current and photocurrent of pure NiO are low. However, when Mo has introduced the crystal lattice of NiO, the number of electrons on the surface will decrease; therefore, the number of recombined holes will decrease, and the photocurrent and conductivity will increase. When electrons absorb incident photons in the valence band (VB), the electrons migrate to the conduction band (CB) [31]. Then, the electrons and holes from the Conduction band and Valance band diffused in the direction of the Ag electrodes due to the low energy of Ag. Under the applied external electric field, the photocurrent increase, and the chance of recombination is reduced. After the lighting is off., the oxygen molecules are trapped again on the NiO surface, reducing the current value. Generally, as the applied bias voltage increases, the recombination rate decreases, and the produced current increases. The performance of the photodetector depends on the interaction between light and prepared samples influenced by the band gap, crystallite size, and thickness of the samples. The NMO-2 sample shows a high absorbance, large crystallite size, and low band gap compared to other samples; hence, this sample gives the best performance of photo-sensing. The mechanism of the interaction of synthesized samples in dark and light is explained in Fig. 10. In the dark, small charge carriers are produced, and a small dark current is produced under the applied voltage. When the light incident on the samples, large amount of photogenerated charge carriers are produced, and the photocurrent increase due to increasing the absorbance and decrement of the band gap. Table 2 shows the final R, EQE, and D* values, including comparisons to earlier investigations. These results confirm that the NMO-2 sample shows excellent UV sensitivity, indicating that it is more suitable for optoelectronic applications.
4 Conclusion
Nanostructured pure (NiO) and molybdenum (Mo)-doped NiO were deposited using DC/RF sputtering on the ITO substrate. The impact of doping concentrations on the structural, optical, and electrical properties was investigated. The surface morphology of the NiO films was described using FE-SEM and EDS mapping. The surface of the film is homogeneous and free from any cracks. XRD analysis of the prepared films revealed that the cubic phase structure of NiO was unchanged with the doping of molybdenum. The crystallite size increased from 28.8 nm to 40 nm with increasing the Mo concentrations up to 1.73% (NMO-2 sample) and then decreased to 36 nm for 2.02% Mo concentration (NMO-3 sample). The optical measurements revealed a decrement in band gap and the transmission with increased doping concentration; hence, the absorption of UV light increased. The I–V characteristics of NiO films show that the photocurrent increased from 22.7 to 57 mA with an increase in Mo concentration up to 1.73%(NMO-2) sample and then decreased to 40.5 mA for the NMO-3 sample with Mo concentration 2.02%. The maximum photocurrent is 57 mA for the NMO-2 sample. The device's responsivity, detectivity, and EQE were evaluated for pure and NiO:Mo films, and the maximum values were 539 mA/W, 1.78 × 108 Jones, and 171%, respectively, for the NMO-2 sample. These results proved that the NMO-2 photodetector sample will produce a high photocurrent and device performance.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
References
L. Sang, M. Liao, M. Sumiya, A comprehensive review of semiconductor ultraviolet photodetectors: from thin film to one-dimensional nanostructures. Sensors 13, 10482–10518 (2013)
L. Huang, Y. Wang, X. Zhu, X. Zhao, G. Li, L. Li, W. Sun, Mg-doped nickel oxide as efficient hole-transport layer for perovskite photodetectors. J. Phys. Chem. C 125, 16066–16074 (2021)
M.D. Devi, A.V. Juliet, K. Hariprasad, V. Ganesh, H.E. Ali, H. Algarni, I. Yahia, Improved UV photodetection of terbium-doped NiO thin films prepared by cost-effective nebulizer spray technique. Mater. Sci. Semicond. Process. 127, 105673 (2021)
H. Kim, M.D. Kumar, M. Patel, J. Kim, ITO nanowires-embedding transparent NiO/ZnO photodetector. Mater. Res. Bull. 83, 35–40 (2016)
A.T. Nomaan, N.M. Ahmed, N.H. Al-Hardan, N.S. Shiong, A.A. Aziz, UV photodetector based on p-NiO film/n-Si heterojunction prepared by thermal oxidation. J. Phys: Conf. Ser. 1535, 012001 (2020)
A.A. Ahmed, M. Devarajan, N. Afzal, Fabrication and characterization of high performance MSM UV photodetector based on NiO film. Sens. Actuators, A 262, 78–86 (2017)
N. Fathima, N. Pradeep, J. Balakrishnan, Enhanced optical and electrical properties of antimony doped ZnO nanostructures based MSM UV photodetector fabricated on a flexible substrate. Mater. Sci. Semicond. Process. 90, 26–31 (2019)
X. Chang, Y.-F. Wang, X. Zhang, Z. Liu, J. Fu, S. Fan, R. Bu, J. Zhang, W. Wang, H.-X. Wang, UV-photodetector based on NiO/diamond film. Appl. Phys. Lett. 112, 032103 (2018)
P. Pramanik, S. Sen, C. Singha, A.S. Roy, A. Das, S. Sen, A. Bhattacharyya, Compositional inhomogeneities in AlGaN thin films grown by molecular beam epitaxy: Effect on MSM UV photodetectors. J. Appl. Phys. 120, 144502 (2016)
A. Müller, G. Konstantinidis, M. Dragoman, D. Neculoiu, A. Dinescu, M. Androulidaki, M. Kayambaki, A. Stavrinidis, D. Vasilache, C. Buiculescu, GaN membrane-supported UV photodetectors manufactured using nanolithographic processes. Microelectron. J. 40, 319–321 (2009)
W. Yang, F. Zhang, Z. Liu, Z. Wu, Effects of annealing on the performance of 4H-SiC metal–semiconductor–metal ultraviolet photodetectors. Mater. Sci. Semicond. Process. 11, 59–62 (2008)
H. Haugan, S. Elhamri, F. Szmulowicz, B. Ullrich, G. Brown, W. Mitchel, Study of residual background carriers in midinfrared In As∕GaSb superlattices for uncooled detector operation. Appl. Phys. Lett. 92, 071102 (2008)
Q. An, X. Meng, P. Sun, High-performance fully nanostructured photodetector with single-crystalline CdS nanotubes as active layer and very long Ag nanowires as transparent electrodes. ACS Appl. Mater. Interfaces. 7, 22941–22952 (2015)
H. Zhang, G. Chen, D.W. Bahnemann, Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 19, 5089–5121 (2009)
A.I. Hochbaum, P. Yang, Semiconductor nanowires for energy conversion. Chem. Rev. 110, 527–546 (2010)
J.-I. Hahm, Biomedical detection via macro-and nano-sensors fabricated with metallic and semiconducting oxides. J. Biomed. Nanotechnol. 9, 1 (2013)
S.K. Shim, W.Z. Tawfik, C.M. Manoj Kumar, X. Wang, N. Lee, J.K. Lee, Nanopatterned sapphire substrate to enhance the efficiency of AlGaN-based UVC light source tube with CNT electron-beam. J. Mater. Chem. C 8, 17336–17341 (2020)
T. Zhai, L. Li, X. Wang, X. Fang, Y. Bando, D. Golberg, Recent developments in one-dimensional inorganic nanostructures for photodetectors. Adv. Func. Mater. 20, 4233–4248 (2010)
K. Ukoba, A. Eloka-Eboka, F. Inambao, Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew. Sustain. Energy Rev. 82, 2900–2915 (2018)
M. Jlassi, I. Sta, M. Hajji, H. Ezzaouia, Optical and electrical properties of nickel oxide thin films synthesized by sol–gel spin coating. Mater. Sci. Semicond. Process. 21, 7–13 (2014)
V. Biju, M.A. Khadar, DC conductivity of consolidated nanoparticles of NiO. Mater. Res. Bull. 36, 21–33 (2001)
M. Yang, H. Pu, Q. Zhou, Q. Zhang, Transparent p-type conducting K-doped NiO films deposited by pulsed plasma deposition. Thin Solid Films 520, 5884–5888 (2012)
W. Chen, F.Z. Liu, X.Y. Feng, A.B. Djurišić, W.K. Chan, Z.B. He, Cesium doped NiOx as an efficient hole extraction layer for inverted planar perovskite solar cells. Adv. Energy Mater. 7, 1700722 (2017)
K. Sato, S. Kim, S. Komuro, X. Zhao, Characteristics of Cu-doped amorphous NiO thin films formed by RF magnetron sputtering. Jpn. J. Appl. Phys. 55, 0610 (2016)
E. Gagaoudakis, G. Michail, D. Katerinopoulou, K. Moschovis, E. Iliopoulos, G. Kiriakidis, V. Binas, E. Aperathitis, Transparent p-type NiO: Al thin films as room temperature hydrogen and methane gas sensors. Mater. Sci. Semicond. Process. 109, 104922 (2020)
S. Zargouni, S. El Whibi, E. Tessarolo, M. Rigon, A. Martucci, H. Ezzaouia, Structural properties and defect related luminescence of Yb-doped NiO sol-gel thin films. Superlatt. Microstruct. 138, 106361 (2020)
W. Guo, K. Hui, K.S. Hui, High conductivity nickel oxide thin films by a facile sol–gel method. Mater. Lett. 92, 291–295 (2013)
S.-H. Wang, S.-R. Jian, G.-J. Chen, H.-Z. Cheng, J.-Y. Juang, Annealing-driven microstructural evolution and its effects on the surface and nanomechanical properties of Cu-doped NiO thin films. Coatings 9, 107 (2019)
M.S. Abdel-Wahab, A. Hammad, Impact of heat treatment on the physical properties of sputtered nickel oxide thin films containing molybdenum. J. Ovon. Res. 18, 1–10 (2022)
I.L.P. Raj, S. Valanarasu, R.R. Isaac, M. Ramudu, Y. Bitla, V. Ganesh, I. Yahia, The role of silver doping in tuning the optical absorption, energy gap, photoluminescence properties of NiO thin films for UV photosensor applications. Optik 254, 168634 (2022)
R. Balakarthikeyan, A. Santhanam, R. Anandhi, S. Vinoth, A.M. Al-Baradi, Z. Alrowaili, M. Al-Buriahi, K.D.A. Kumar, Fabrication of nanostructured NiO and NiO: Cu thin films for high-performance ultraviolet photodetector. Opt. Mater. 120, 111387 (2021)
I. Hotovy, J. Liday, L. Spiess, H. Sitter, P. Vogrincic, Study of annealed NiO thin films sputtered on unheated substrate. Jpn. J. Appl. Phys. 42, L1178 (2003)
X. Xia, J. Tu, J. Zhang, X. Wang, W. Zhang, H. Huang, Electrochromic properties of porous NiO thin films prepared by a chemical bath deposition. Sol. Energy Mater. Sol. Cells 92, 628–633 (2008)
V.H. López-Lugo, M. García-Hipólito, A. Rodríguez-Gómez, J.C. Alonso-Huitrón, Fabrication of Li-doped NiO thin films by ultrasonic spray pyrolysis and its application in light-emitting diodes. Nanomaterials 13, 197 (2023)
L. Abdelghani, L. Said, B. Said, B. Okba, Spin coating method deposited nickel oxide thin films with various film thicknesses. J. Chem. Res. 46, 1–5 (2022)
U. Holzwarth, N. Gibson, The Scherrer equation versus the “Debye-Scherrer equation.” Nat. Nanotechnol. 6, 534–534 (2011)
A. Monshi, M.R. Foroughi, M.R. Monshi, Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2, 154–160 (2012)
P. Scherrer, Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Math.-Phys. Klasse 2, 98–100 (1918)
P. Salunkhe, D. Kekuda, Structural, spectroscopic and electrical properties of dc magnetron sputtered NiO thin films and an insight into different defect states. Appl. Phys. A 127, 1–12 (2021)
K.R. Devi, G. Selvan, M. Karunakaran, I.L.P. Raj, V. Ganesh, S. AlFaify, Enhanced room temperature ammonia gas sensing properties of strontium doped ZnO thin films by cost-effective SILAR method. Mater. Sci. Semicond. Process. 119, 105117 (2020)
A.S.S. Sharifah, J.J. Mohamed, Z.A. Ahmad, Dielectric properties of ZrO2 doped on NiO at high frequency. Adv. Mater. Res. Trans. Tech. Publ. Ltd 895, 216–220 (2014)
M. Shkir, S. AlFaify, Tailoring the structural, morphological, optical and dielectric properties of lead iodide through Nd3+ doping. Sci. Rep. 7, 1–9 (2017)
S. AlFaify, M. Shkir, A facile one pot synthesis of novel pure and Cd doped PbI2 nanostructures for electro-optic and radiation detection applications. Opt. Mater. 88, 417–423 (2019)
K.N. Manjunatha, S. Paul, Investigation of optical properties of nickel oxide thin films deposited on different substrates. Appl. Surf. Sci. 352, 10–15 (2015)
A.M. Ibrahim, M.S. Abdel-wahab, M. Elfayoumi, W.Z. Tawfik, Highly efficient sputtered Ni-doped Cu2O photoelectrodes for solar hydrogen generation from water-splitting. Int. J. Hydrogen Energy 48, 1863–1876 (2023)
W.Z. Tawfik, Z.S. Khalifa, M.S. Abdel-Wahab, A.H. Hammad, Sputtered cobalt doped CuO nano-structured thin films for photoconductive sensors. J. Mater. Sci.: Mater. Electron. 30, 1275–1281 (2019)
I.L. Poul Raj, S. Valanarasu, K. Hariprasad, J.S. Ponraj, N. Chidhambaram, V. Ganesh, H.E. Ali, Y. Khairy, Enhancement of optoelectronic parameters of Nd-doped ZnO nanowires for photodetector applications. Opt. Mater. 109, 110396 (2020)
M.S. Abdel-wahab, Substrate temperature impact on the structural, optical and photo-catalytic activity of sputtered Cu-Doped ZnO thin films. J. Electron. Mater. 50, 4364–4372 (2021)
Z.S. Shakir, S.M. Al-Jawad, D.S. Ahmed, Influence of cobalt doping concentration on ZnO/MWCNTs hybrid prepared by sol-gel method for antibacterial activity. J. Sol-Gel Sci. Technol. 100, 115–131 (2021)
M. Shkir, I. Ashraf, A. Khan, M.T. Khan, A.M. El-Toni, S. AlFaify, A facile spray pyrolysis fabrication of Sm:CdS thin films for high-performance photodetector applications. Sens. Actuators, A 306, 111952 (2020)
M. Shkir, M.T. Khan, I. Ashraf, A. Almohammedi, E. Dieguez, S. AlFaify, High-performance visible light photodetectors based on inorganic CZT and InCZT single crystals. Sci. Rep. 9, 12436 (2019)
P. Jubu, F. Yam, Development and characterization of MSM UV photodetector based on gallium oxide nanostructures. Sens. Actuators, A 312, 112141 (2020)
X. Zheng, Y. Chen, T. Zhang, B. Yang, C. Jiang, B. Yuan, Z. Zhu, Photoconductive semiconductor switch based on ZnS nanobelts film. Sens. Actuators, B Chem. 147, 442–446 (2010)
M. Husham, Z. Hassan, A.M. Selman, Synthesis and characterization of nanocrystalline CdS thin films for highly photosensitive self-powered photodetector. Eur. Phys. J. Appl. Phys. 74, 10101 (2016)
J. Mallows, M. Planells, V. Thakare, R. Bhosale, S. Ogale, N. Robertson, p-type NiO hybrid visible photodetector. ACS Appl. Mater. Interfaces. 7, 27597–27601 (2015)
B. Parida, S. Kim, M. Oh, S. Jung, M. Baek, J.-H. Ryou, H. Kim, Nanostructured-NiO/Si heterojunction photodetector. Mater. Sci. Semicond. Process. 71, 29–34 (2017)
P.K. Yadav, B. Ajitha, V. Annapureddy, Y.A.K. Reddy, A. Sreedhar, Improved UV photodetector performance of NiO films by substitutional incorporation of Li. Mater. Lett. 301, 130296 (2021)
J.-M. Choi, S. Im, Ultraviolet enhanced Si-photodetector using p-NiO films. Appl. Surf. Sci. 244, 435–438 (2005)
S. Ch, K. Reddy, F.J. Willars-Rodríguez, R.R. Bon, Self-powered broadband photodetector based on a solution-processed p-NiO/n-CdS: Al heterojunction. Nanotechnology 32, 095202 (2021)
D. Yang, F. Du, Y. Ren, T. Kang, P. Hu, F. Teng, H. Fan, A high-performance NiO/TiO2 UV photodetector: The influence of the NiO layer position. J. Mater. Chem. C 9, 14146–14153 (2021)
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds were received.
Author information
Authors and Affiliations
Contributions
HHA: methodology, software, validation, and writing—original draft. MSA-W: conceptualization, data curation, visualization, investigation, and writing—reviewing & editing. MTT: supervision, and writing—reviewing & editing. WZT: conceptualization, methodology, supervision, visualization, and writing—reviewing & editing. All authors have contributed to the revisions of the manuscript in its final form and agreed to the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All authors approved the submission.
Consent for publication
All authors approved the submission.
Article processing charges
We publish open access in your journals with fees covered under a Transformative Agreement plus fully OA agreement. This agreement covers all the public, private, and national universities, as well as the research centers related to the ministry of higher education and scientific research and all other governmental ministries in Egypt. APC will be covered by the institutional agreement between STDF, Egyptian Universities, and Springer Nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhalium, H.H., Abdel-wahab, M.S., Tamm, M.T. et al. Highly efficient ultraviolet photodetector based on molybdenum-doped nanostructured NiO/ITO thin film. Appl. Phys. A 129, 459 (2023). https://doi.org/10.1007/s00339-023-06721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06721-y