Abstract
This study presents findings related to the characterization of cubic SnS (π-SnS) thin films and p-SnS/n-Si heterojunction structures produced simultaneously using the ultrasonic spray pyrolysis technique. In this context, the impact of different spray solution flow rates on the morphological, structural, optical, and electrical characteristics of the films was examined. Morphological analyses revealed that higher flow rates resulted in films with denser and smoother surfaces, approximately 6 nm in roughness. Additionally, it was observed that both the thickness and the growth rate of the films could be adjusted through the modulation of the flow rate. Structural analyses determined that the crystallite size increased and micro-strain values decreased with increasing flow rates. Optical evaluations indicated a decline in the optical band gap of the thin films from about 1.8 eV to 1.7 eV as the flow rates increased. This trend was consistently observed in the data obtained using the Tauc method and the derivative of transmission with respect to wavelength versus photon energy graphs. Electrical analyses revealed that the resistivity values of the thin films increased from 5.24 × 105 Ωcm to 1.64 × 106 Ωcm with increasing flow rates. Furthermore, I-V analyses of the Au/p-SnS/n-Si/Ag heterojunction structures indicated significant variability in key electrical properties. The saturation currents displayed a broad range, suggesting varying efficiencies in charge carrier collection across different samples. Similarly, the change of ideality factors pointed to differences in charge transport mechanisms, while the shifts in barrier heights indicated changes in junction properties with different fabrication conditions. The results of this study offer valuable perspectives for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the search for energy-efficient technologies with sustainable and environmentally friendly approaches has made the discovery of new materials with exceptional properties a focal point of research [1]. This situation has driven researchers to innovate and create materials for thin film solar cells (TFSCs) that are characterized by enhanced efficiency [2]. Within the field of TFSCs, those employing Cu(In, Ga)(S, Se)2 (CIGS) and CdTe as absorber layers stand out. These materials have not only achieved commercial success but also power conversion efficiencies (η) exceeding 22% [3]. Yet, the toxicity of these absorber materials (like cadmium) and the scarcity of critical raw materials (like indium, gallium, and tellurium) compel researchers to explore alternative absorber materials like Cu2ZnSn(S, Se)4 (CZTS), which have environmentally friendly and more abundant elements [4,5,6]. Nonetheless, the complex crystal structure of the CZTS absorber layer, undesirable secondary phases, and defect mechanisms are factors that complicate achieving high efficiency in solar cells [7]. Considering these factors, binary compound thin film absorber layers, which are easier to control stoichiometry, low in cost, and contain elements abundantly available in nature, emerge as an ideal material option for solar cell applications [8, 9]. In this context, tin monosulfide (SnS), a member of the IV-VI group of semiconductors, is emerging as a viable candidate for solar cell applications. This is primarily due to its advantageous opto-electrical properties.
The crystalline structures are crucial in defining the physical characteristics of materials. SnS, a polymorphic material, crystallizes in the orthorhombic (SnS-ORT) phase as its ground state and also in a semi-stable cubic (SnS-CUB) phase, named π-SnS, discovered by Rabkin et al. in 2015 [10,11,12]. The π-SnS phase, compared to the SnS-ORT phase (Eg ∼1.1–1.3 eV), possesses a higher direct band gap (Eg ∼1.6–1.8 eV), strong absorption onset (α ∼104-105 cm− 1), and a larger dielectric constant, thus potentially offering higher solar cell efficiency [13,14,15]. The π-SnS phase is noted for its structural stability at room temperature and isotropic optical properties, which are considered valuable for various device applications as well [16, 17]. Research suggests that the π-SnS phase is well-suited for optoelectronic uses, including thermoelectric devices and near-infrared (NIR) photodetectors [18,19,20]. However, despite theoretical power conversion efficiencies of around 24% for SnS-based TFSCs, practical applications usually report efficiencies below 4%, with only a few studies achieving successes above 4% [21,22,23,24,25,26,27]. The reasons for this include challenges such as determining the appropriate buffer layer, producing quality materials, and the presence of secondary phases [6, 28]. Moreover, current research indicates that the influence of the π-SnS phase on the performance of photovoltaic devices remains underexplored. Nonetheless, depending on the synthesis method and conditions, thin films crystallizing in either the π-SnS or SnS-ORT phase can exhibit different physical properties. The optical band gap of SnS thin films can be adjusted without changing the structural phase, through appropriate doping or modifications in deposition parameters [29]. For these reasons, improving the physical properties of high-quality π-SnS thin films necessitates comprehensive research.
A variety of techniques have been explored for producing SnS thin films, as reported in existing studies, such as sputtering [30], atomic layer deposition (ALD) [31], thermal evaporation [32], electro-deposition [33], spin-coating [34], chemical bath deposition (CBD) [35], and spray pyrolysis [36]. Film thickness is one of the critical parameters affecting the structural, morphological, optical, and electrical attributes of SnS thin films produced through these techniques. In their research, Javed et al. [29] analyzed how varying film thicknesses (374 nm to 723 nm) impact the physical properties of SnS films prepared via chemical bath deposition. Their findings revealed that these films consistently formed in a cubic phase. Notably, as the film thickness increased, there was a corresponding rise in surface roughness and changes in the optical and electrical properties, with the optical band gap widening slightly from 1.70 eV to 1.74 eV and electrical resistivity increasing from 1.592 × 103 Ωcm to 1.785 × 104 Ωcm. These findings indicate that by varying film thickness, it is feasible to manipulate their optical band gap and electrical conductivity. In another study, Messaoudi et al. [37] observed notable variations in the physical characteristics of SnS films, which were fabricated using spray pyrolysis over spray durations of 15, 30, 45, and 60 min. The study highlighted how these different spraying times substantially influenced the physical properties of the films. The study revealed that as the duration of the spraying process was prolonged, the thickness of the films significantly increased, ranging approximately from 200 nm to 2000 nm. Despite all films exhibiting an orthorhombic crystalline structure, superior crystallization was observed when the spraying duration was minimized. Moreover, the formation of a secondary Sn2S3 phase was noted during extended spraying periods of 60 min. Furthermore, indicated that the optical band gap of the films was notably influenced by the duration of the spraying process, showing an increase from 0.69 eV to 2.10 eV as the spraying time was extended. Morphological analyses showed an increase in surface roughness (from 14.6 nm to 56.7 nm) and that the film surfaces had good coverage without pinholes or cracks. These findings suggest that these properties would have a positive effect on their application as absorber layer materials in efficient solar cells. Moreover, given the extensive uses of SnS materials, the fabrication of high-quality π-SnS thin films through economical and straightforward methods is crucial. The ultrasonic spray pyrolysis (USP) technique is particularly notable for its vacuum-free process, which allows for operation in open-air environments, making it more accessible and cost-effective than other chemical deposition approaches. This method involves the creation of thin films by spraying a solution, which contains various concentrations and volumes of chemical salts aligned with the materials composition, through an ultrasonic nozzle. The spray forms fine droplets that are then deposited on preheated substrates via a carrier gas, following specific flow rates and durations [38]. In this context, several key deposition variables including the concentration of the spray solution, the rate of solution flow (or spraying speed), the duration of spraying, the pressure of the carrier gas, the temperature of the substrate, and the distance between the nozzle and substrate significantly influence the quality of the films [39]. Notably, a higher solution flow rate often results in increased film thickness, making it a critical factor in determining the films growth dynamics and their physical characteristics. Both the studies mentioned above and the impact of the spray solution flow rate from the microstructure to the crystal quality/opto-electrical properties indicate that all physical properties are affected. However, to our knowledge, the effect of solution flow rate on π-SnS thin films produced by the USP technique has not been determined yet.
This research clarifies how variations in the flow rate of the spray solution impact the structural, morphological, optical, and electrical characteristics of π-SnS thin films, which hold substantial importance for technology applications. The thin films were deposited on glass substrates using the USP technique. Current findings suggest that more comprehensive research is required to thoroughly assess how the flow rate influences these physical properties. Consequently, the purpose of this study is to conduct a detailed examination of how varying the flow rate affects the physical properties and performance characteristics of π-SnS thin films applied on glass substrates using the USP method, as well as the p-SnS/n-Si heterostructures that form simultaneously. The findings provide valuable insights for future research, highlighting how the properties of π-SnS thin films can be enhanced and customized for specific applications through the use of suitable dopants.
2 Experimental methods
SnS thin films and p-SnS/n-Si heterojunction structures were simultaneously deposited on glass and n-Si substrates at different flow rates by preparing spray solutions. The cleanliness of the substrates, preparation of the spray solution, deposition recipe, and devices used in characterization are provided below.
2.1 Substrate cleaning
Before proceeding to the crucial cleaning stage for film quality, glass and n-Si substrates were cut into sizes of 10 × 10 mm and 20 × 20 mm. Initially, a four-stage cleaning procedure was applied to the glass substrates in an ultrasonic bath. These stages, each lasting 15 min and performed at room temperature, included washing with detergent deionized (DI) water, rinsing with DI water only, cleaning with acetone-methanol, and a final rinse with DI water. Additionally, between these stages, the glass substrates were rinsed under running DI water and subsequently dried inside an oven set at 100 °C. Secondly, the n-Si substrates were washed applying standard RCA cleaning and then dried with nitrogen (N2) gas. After completing the cutting and cleaning of the substrates, the preparation phase for the spray solution was initiated.
2.2 Preparation of Spray Solution
Initially, the chemical spray solution was prepared by dissolving tin chloride (SnCl2.2H2O) in deionized (DI) water at a concentration of 0.075 M, which did not result in any film formation on the substrate. Following this, a successful approach involved dissolving SnCl2.2H2O in HCl at 60 °C to achieve the same concentration, then extending it with DI water, leading to the emergence of crystallized films in subsequent trials. Consequently, the final spray solution was formulated by mixing 0.075 M SnCl2.2H2O in a solution of HCl and DI water at a 5:95 ratio and 0.075 M thiourea (CH4N2S) in DI water, combined equally by volume. After the spray solution was prepared, the deposition phase of the films commenced.
2.3 Deposition of SnS Thin films and p-SnS/n-Si heterostructures
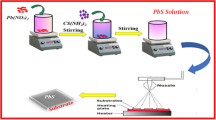
SnS thin films and p-SnS/n-Si heterojunction structures were simultaneously produced on glass and n-Si substrates via the ultrasonic spray pyrolysis technique. The spray solution was applied to substrates heated to 350 °C, utilizing flow rates of 4 ml/min, 6 ml/min, and 8 ml/min. An ultrasonic nozzle operating at a frequency of 100 kHz, paired with air pressured at 1 bar as the carrier gas, facilitated the atomization of the solution. The distance between the heating plate and the nozzle was kept at 30 cm, while the substrate temperature was regulated by a PID controller with a K-type thermocouple positioned on the heating element. The deposition process lasted for 25 min. Figure 1 presents the schematic diagram of the USP system employed in this study. Following the deposition, the next phase involved characterizing the SnS thin films and p-SnS/n-Si heterojunctions.
2.4 Characterization of SnS Thin films and p-SnS/n-Si heterostructures
The morphological analyses of the thin films were examined through surface images taken at different magnifications with a Carl Zeiss-Gemini 300 model scanning electron microscope (SEM). Additionally, cross-sectional SEM images were taken to determine the thickness of the thin films, which was calculated using the ImageJ software. Moreover, morphological analyses were supported by AFM images taken in non-contact mode with a Park Systems XE 100 model atomic force microscope (AFM). The average surface roughness values (Ra) were determined using XEI version 1.7.1 software. The structural properties of the thin films were analyzed using X-ray diffraction (XRD) patterns obtained from a PANalytical Empyrean system, which operates with CuKα radiation (λ = 1.5405 Å) at 40 kV and 30 mA. The 2θ data, spanning from 20° to 80°, were collected in continuous scan mode with a step size of 0.013°. The optical properties of the thin films were examined through transmittance and absorption spectra taken in the wavelength range of 300–1050 nm using a Rigol Ultra-3660 model spectrophotometer. In the phase of electrical analyses of the thin films, the type of electrical conductivity was initially determined using the hot probe technique. Subsequently, the electrical resistivity values of the thin films were detected using a Lucas Labs Pro4 four-probe system, utilizing 5 different points taken from the film surfaces. Additionally, the produced p-SnS/n-Si heterojunction structures were formed by depositing gold (Au) and silver (Ag) metal point contacts respectively on SnS and n-Si layers using an MTI brand DC sputter system. The device performances of such formed Au/p-SnS/n-Si/Ag heterojunctions were evaluated by analyzing current-voltage (I-V) measurements taken at room temperature and in a dark environment with a Keithley 2400 source meter. Following this, the effects of flow rate on the morphological, structural, optical, and electrical analyses as well as on the device performances of the deposited thin films and formed heterostructures were discussed in detail.
3 Results and discussions
3.1 Morphological analysis
SEM images of SnS thin films deposited on glass substrates are presented in Fig. 2, illustrating the films at magnifications of ×1 K and ×30 K for different flow rates: 4 ml/min, 6 ml/min, and 8 ml/min. In the ×1 K magnified SEM images shown in Fig. 2(top), no voids or crack-like formations were observed on the film surfaces. On the other hand, it was observed that the particle formation density on the film surfaces relatively increased as the flow rate increased. This situation suggests that the atomization of the solution is negatively affected by the increase in solution flow rate. However, the analysis of SEM images at ×30 K magnification, as depicted in Fig. 2(bottom), reveals that the structure of the films becomes denser and more compact with increasing solution flow rates. Additionally, cross-sectional images provided in Fig. 2(bottom-with inset) confirm a correlation between the flow rate and film thickness, which significantly increases across the flow rates of 4 ml/min (338 nm), 6 ml/min (686 nm), and 8 ml/min (1098 nm). This increase aligns with expectations since higher flow rates facilitate the deposition of more solution onto the substrate per unit of time, a finding supported by similar studies in the literature [37, 40]. Upon examining the AFM images given in Fig. 3, the films exhibit a smooth and homogeneous surface, devoid of any cracks. The bright and dark spots identified correspond to the stacking of grain peaks and the creation of valleys, respectively. Notably, the surface roughness measurements − 8.3 nm at 4 ml/min, 5.5 nm at 6 ml/min, and 6.1 nm at 8 ml/min- indicate low roughness levels. Such smoothness is particularly beneficial for applications in devices like solar cells, where low surface roughness is crucial for efficiency.
3.2 XRD Analysis
Figure 4 presents the XRD patterns (20° ≤ 2θ ≤ 80°) of SnS thin films deposited on glass substrates at varying flow rates: 4 ml/min, 6 ml/min, and 8 ml/min. The XRD patterns shown in Fig. 4 were compared with the reference cards for the SnS-ORT phase (JCPDS card no. 39–0354) and the SnS-CUB phase, also referred to as π-SnS (JCPDS card no. 86-9477). The JCPDS card no. 86-9477 for π-SnS was introduced in 2017 [10, 41]. The XRD patterns in Fig. 4, differing from the SnS-ORT crystal structure, matched the π-SnS crystal structure with a lattice parameter of a = 11.568 Å [41]. Additionally, the triple peak structure appearing in the 2θ = 30°-33° range corresponding to the (400), (410), and (411) crystallographic planes confirm the cubic phase formation. Thus, it was determined that the deposited thin films have a cubic crystal structure, and the obtained XRD patterns closely match those in studies related to π-SnS [10, 11, 13, 42]. Moreover, no secondary phases were detected in the XRD patterns. Upon examining Fig. 4, the diffraction peaks on the XRD patterns appeared sharper and more intense with increasing solution flow rate. This suggests an increase in crystallization level of the films with increasing solution flow rate, i.e., higher deposition rate. Furthermore, the crystallite size (D) and micro-strain (ε) of the samples were determined using the Debye-Scherrer formula, presented as Eq. (1), and a related strain equation, shown as Eq. (2) [43, 44];
In these equations, K (0.94) represents the shape factor, λ is the x-ray wavelength, β is the full-width at half-maximum in radians, and θ is the Bragg angle, also in radians. Additionally, the lattice constant -a of the cubic crystal structure was derived using the equation below [43];
where d stands for the interplanar distance, and h, k, and l are the Miller indices. Values for crystallite size (D), micro-strain (ε), and lattice constant (a) were determined through their respective formulas and are summarized in Table 1. Examining the values in Table 1, the films deposited at 8 ml/min flow rate have larger crystallite sizes compared to those deposited at 4 ml/min and 6 ml/min, with lower micro-strain values. This change in crystal structure may be related to the increase in thickness of the thin films [45]. Additionally, it is noticeable that micro-strain values decrease with increasing flow rate, suggesting lattice relaxation in the films. The lattice constant values of the π-SnS crystal structure vary depending on the deposition conditions [11]. Calculated lattice constant values are slightly lower than the reference value (a = 11.568 Å) but closely approximate it as the flow rate increases (Table 1). It is also thought that there is a relationship between the decrease in lattice strain with increasing flow rate and the approach of the lattice constant to the standard value. Based on all these structural analyses, it can be suggested that highly crystalline π-SnS thin films can be obtained by ultrasonically spraying the prepared spray solution at a flow rate of 8 ml/min onto glass substrates.
3.3 Optical analysis
Figure 5(a) and 5(b) illustrate the optical transmittance and absorption spectra of the SnS thin films, respectively. As depicted in Fig. 5(a), the films show low transmittance in the visible region, with a noted increase in transmittance at longer wavelengths. Additionally, transmittance decreases as the solution flow rate increases, which correlates with changes in film thickness, crystallization, and surface roughness [9, 46]. Despite a reduction in surface roughness and enhanced crystallization at higher flow rates, the augmented thickness tends to lower the transmittance. In the absorption spectrum presented in Fig. 5(b), the films demonstrate strong optical absorption within the visible range, which intensifies at shorter wavelengths. The absorption coefficients, derived using Eq. (4) shown below, are given as a function of wavelength in Fig. 5(c);
where α denotes the absorption coefficient, A is the absorbance, and d is the film thickness [47]. Table 2 outlines the absorption coefficient values of the SnS thin films at different wavelengths and flow rates. At a wavelength of 550 nm, the coefficients are as follows: for a flow rate of 4 ml/min, the value is approximately 1.2 × 105 cm− 1; at 6 ml/min, it increases slightly to 1.3 × 105 cm− 1; and at 8 ml/min, it decreases to 7.9 × 104 cm− 1. This suggests that while the absorption capability generally increases with flow rate, it shows a decline at the highest flow rate examined. Conversely, at 650 nm, the absorption coefficients are lower overall and exhibit a gradual increase with the flow rate, measured at approximately 4.4 × 104 cm− 1, 6.2 × 104 cm− 1, and 7.1 × 104 cm− 1 for the respective flow rates of 4, 6, and 8 ml/min. This trend indicates that the film’s ability to absorb light increases with flow rate at this longer wavelength. As known from the literature, the absorption characteristics of all films are strong enough in the visible region to facilitate the formation of electron-hole (e-h) pairs in deposited SnS films [29]. Therefore, it can be said that all films can be used as absorber layers in TFSC applications. On the other hand, as observed in Fig. 5(b), the absorption edge shifts to longer wavelengths with an increasing flow rate, and the absorption edge becomes sharper. This indicates that the optical band gap of SnS films narrows with an increasing flow rate, and the band edge tailings decrease. Two different methods were adopted for determining the band gap. One of these is the Tauc method, expressed by Eq. (5) below, based on the absorption and transmittance data. The optical band gap can be deduced from the plot of (αhv)2 versus hv, as shown in Fig. 6(a). This method involves locating the point where the linear segment of the plot intersects the hv axis. The relationship is defined by the equation;
where Eg represents the optical band gap, h is the Planck constant, A is an energy-independent constant, and ν is the frequency of the incident light. The exponent n is 2, which is typical for allowed direct transitions [48]. Alternatively, the band gap can also be determined by utilizing the changes in the transmission and absorption spectra with wavelength. As shown in Fig. 6(b), a plot of (dT/dλ) vs. λ, which identifies the rapid change in the transmission spectrum, peaks at the wavelength corresponding to the optical band gap, and the energy corresponding to this peak wavelength represents the optical band gap [49]. The optical band gap values detailed in Table 2 reveal a slight decrease with increasing flow rates. This trend correlates with the previously observed increase in film thickness across different flow rates, where thicker films typically exhibit a narrower band gap. This effect, a well-understood phenomenon in thin film physics, is due to the quantum confinement that diminishes as the film thickens [50]. Additionally, the decrease in lattice strain, often accompanying increased crystallization from higher flow rates, further contributes to the band gap narrowing. Furthermore, these variations in band gap can be attributed to differences in crystallization quality and synthesis techniques used for the films. The calculated optical band gap values for π-SnS films align well with existing literature values [51, 52]. Additionally, the Urbach energy (EU) values, indicative of band edge tailings, are derived from the slope of the linear segments of the lnα versus hν plots, as per Eq. (6) displayed in Fig. 6(c). These values are also listed in Table 2.
Here, α0 is a constant [47]. The Urbach energy values, which reflect the extent of band tailings, show a noticeable decline from 217 meV to 130 meV as the solution flow rate increases, as detailed in Table 2. This trend suggests an improvement in the quality of the thin films, characterized by a reduction in the density of states in the band tails. The decrease in Urbach energy signifies fewer localized states associated with structural disorder, indicating enhanced crystallization of the films at higher flow rates. This correlation between increasing flow rates and reduced Urbach energy highlights the relationship between deposition conditions and film crystallinity, suggesting that optimized flow rates can directly influence the structural and optical properties of the films.
3.4 Electrical analysis
The hot probe technique established that the SnS thin films exhibit p-type electrical conductivity. Electrical resistivity measurements conducted using the four-probe technique at five distinct points on each film surface are reported in Table 3. These measurements show an increase in resistivity from 5.24 × 105 Ωcm to 1.64 × 106 Ωcm with higher flow rates. Typically, factors like increased film thickness and surface roughness contribute to higher resistivity due to enhanced porosity, grain boundary scatterings, and interface effects [29, 53]. However, the findings in this study present an unexpected trend; despite the increased film thickness and denser film formation, which generally reduce resistivity by improving crystallite size and reducing surface roughness, the resistivity values unexpectedly rose. This anomaly could be attributed primarily to a reduction in carrier concentration at higher flow rates, despite potential increases in carrier mobility due to decreased scattering at crystal boundaries, which usually act as scattering centers. On the other hand, gold (Au) and silver (Ag) metal point contacts were applied to the front and back surfaces of p-SnS/n-Si heterojunctions simultaneously deposited with SnS thin films, forming Au/p-SnS/n-Si/Ag heterojunction structures. The device behaviors of the heterojunction structures created were analyzed by examining the linear I-V curves obtained under dark conditions at room temperature, as presented within Fig. 7. In Fig. 7, semi-logarithmic I-V graphs corresponding to the created p-SnS/n-Si heterojunction structures are presented. Accordingly, the charge transport processes in heterojunction structures were analyzed within the framework of thermionic emission (TE) theory. TE theory models the variation of current in response to voltage across a heterojunction through the equation shown below:
In this expression, I0 represents the reverse saturation current, indicative of the current that flows through the diode when it is in reverse bias, effectively with zero applied voltage. The parameter η, known as the diode ideality factor, characterizes how closely the diode behavior follows the ideal diode equation. k stands for Boltzmann constant, T is the absolute temperature in Kelvin, and V is the voltage applied across the diode, dictating the diode operating point. This formula is critical in predicting how current escalates with voltage in semiconductor devices [54,55,56]. In the condition V > 3kT/q, the term beside the exponential term in Eq. (7) can be neglected. Taking the natural logarithm, Eq. (8) can be written, and deriving with respect to V, Eq. (9) can be written [54,55,56]:
The ideality factor (η) and the saturation current (I0) of the diode are quantitatively assessed from the slope and intercept, respectively, of the linear part of the semi-logarithmic current-voltage (I-V) plots. The barrier height (ϕb) is then calculated from the saturation current using the following relation [54,55,56]:
where A represents the effective diode area, and A* is the Richardson constant, which is 120 A/K2cm2 for n-type silicon. Utilizing Eqs. (8), (9), and (10), the saturation current, ideality factor, barrier height, and rectification ratio (RR) were determined and the results are listed in Table 3. As given in Table 3, the rectification ratio of the heterojunction structures created at different flow rates varies between 226 and 393 at ± 1.5 V. Thus, as can be seen from Fig. 7, all heterojunction structures exhibit good rectifying behavior and rectification ratio. The ideality factor is known as a parameter indicating the quality of a p-n junction, ideally equal to one, and varies between one and two in a conventional diode [57]. Therefore, an ideality factor greater than one indicates the presence of other transport mechanisms besides pure thermionic emission [58]. In this study, for heterojunction structures created at flow rates of 4 ml/min, 6 ml/min, and 8 ml/min, it was found that the ideality factor (η) values were 3.19, 1.91, and 3.99, respectively; the saturation current (I0) values were 3.49 × 10− 7 A, 3.05 × 10− 9 A, and 7.04 × 10− 9 A, respectively; and the barrier height (ϕb) values were 0.71 eV, 0.84 eV, and 0.81 eV, respectively. Based on these data, it can be said that the p-SnS/n-Si heterojunction structure deposited at a flow rate of 6 ml/min exhibited better device properties with a significantly lower ideality factor (1.91) and lower saturation current (3.05 × 10− 9 A) compared to other structures. However, the large ideality factor values for p-SnS/n-Si heterojunction structures deposited at 4 ml/min and 8 ml/min could be due to factors such as interface defects, device series resistance, barrier inhomogeneity, recombination-generation processes in the depletion region, and lattice mismatch [59,60,61].
4 Conclusion
In this research, the physical properties and device behaviors of π-SnS thin films, as well as the created Au/p-SnS/n-Si/Ag heterostructures, were investigated using the ultrasonic spray pyrolysis (USP) technique. Improved π-SnS thin films were obtained at flow rates of 4 ml/min, 6 ml/min, and 8 ml/min. Morphological analysis showed that as the flow rate increased, thin films with both denser structures and significantly low surface roughness important for device applications were formed. It was also determined that the control of the solution flow rate allowed for the adjustment of film thickness and deposition rate. Structural analysis revealed that the deposited thin films were consistent with the π-SnS cubic crystal structure, and an increase in crystallite size and a decrease in micro-strain values were observed with increasing flow rates, indicating an enhancement in the crystallization level of π-SnS thin films. Optical analyses indicated that with the increase in flow rate, transmittance values decreased while absorption increased, and the optical band gap also showed a tendency to decrease. Additionally, it was found that Urbach energies decreased with increasing flow rate, correlating with the increase in crystallization levels. Electrical analyses demonstrated that resistivity values increased from 5.24 × 105 Ωcm to 1.64 × 106 Ωcm with increasing flow rate. p-SnS/n-Si heterostructures deposited at a flow rate of 6 ml/min exhibited better device characteristics with a lower ideality factor (1.91) and lower saturation current (3.05 × 10− 9 A) compared to those formed at other flow rates. Considering the experimental parameters, it is inferred that a solution flow rate between 6 and 8 ml/min is ideal for obtaining π-SnS materials for use as absorber layers in photovoltaic applications. The results of this research demonstrate that the ultrasonic spray pyrolysis (USP) method, along with the specific experimental conditions outlined in the study, is effective in depositing high-quality π-SnS thin films suitable for photovoltaic applications and other uses. Overall, this work provides significant contributions to the development and optimization of π-SnS thin films for various technological purposes.
Data availability
Data will be made available on request.
References
N.M. Philip, M.C.S. Kumar, Tuning of electronic properties of co-evaporated ag:SnS thin films for heterojunction devices. J. Mater. Sci. Mater. Electron. 35, 1–10 (2024). https://doi.org/10.1007/s10854-024-12078-6
D. Lim, H. Suh, M. Suryawanshi, G.Y. Song, J.Y. Cho, J.H. Kim, J.H. Jang, C.W. Jeon, A. Cho, S.J. Ahn, J. Heo, Kinetically controlled growth of phase-pure SnS absorbers for thin Film Solar cells: achieving Efficiency Near 3% with Long-Term Stability using an SnS/CdS heterojunction. Adv. Energy Mater. 8, 1–9 (2018). https://doi.org/10.1002/aenm.201702605
M.A. Green, E.D. Dunlop, M. Yoshita, N. Kopidakis, K. Bothe, G. Siefer, X. Hao, Solar cell efficiency tables (Version 63), Prog. Photovoltaics Res. Appl. 32, 3–13 (2024). https://doi.org/10.1002/pip.3750
A. Mavlonov, T. Razykov, F. Raziq, J. Gan, J. Chantana, Y. Kawano, T. Nishimura, H. Wei, A. Zakutayev, T. Minemoto, X. Zu, S. Li, L. Qiao, A review of Sb2Se3 photovoltaic absorber materials and thin-film solar cells. Sol Energy. 201, 227–246 (2020). https://doi.org/10.1016/j.solener.2020.03.009
A. Zakutayev, Brief review of emerging photovoltaic absorbers, Curr. Opin. Green. Sustain. Chem. 4, 8–15 (2017). https://doi.org/10.1016/j.cogsc.2017.01.002
R.E. Banai, M.W. Horn, J.R.S. Brownson, A review of tin (II) monosulfide and its potential as a photovoltaic absorber. Sol Energy Mater. Sol Cells 150 (2016) 112–129. https://doi.org/10.1016/j.solmat.2015.12.001
I. Gunes, V. Bilgin, E. Sarica, Non-stoichiometric effect and disorder in as-prepared Cu2ZnSnS4 films deposited at different temperatures by ultrasonic spray pyrolysis. Mater. Sci. Semicond. Process. 152, 107120 (2022). https://doi.org/10.1016/j.mssp.2022.107120
B. Pejjai, V.R. Minnam Reddy, S. Gedi, C. Park, Status review on earth-abundant and environmentally green Sn-X (X = Se, S) nanoparticle synthesis by solution methods for photovoltaic applications. Int. J. Hydrogen Energy. 42, 2790–2831 (2017). https://doi.org/10.1016/j.ijhydene.2016.11.084
E. Sarica, Investigation of spray pyrolyzed cubic structured Cu doped SnS films, Phosphorus, Sulfur Silicon Relat. Elem. 196, 1103–1108 (2021). https://doi.org/10.1080/10426507.2021.1986498
F.D.B. Sánchez, M.T.S. Nair, P.K. Nair, Insights to the production of SnS-cubic thin films by vacuum thermal evaporation for photovoltaics. Semicond. Sci. Technol. 39 (2024). https://doi.org/10.1088/1361-6641/ad0f4c
R.E. Abutbul, A.R. Garcia-Angelmo, Z. Burshtein, M.T.S. Nair, P.K. Nair, Y. Golan, Crystal structure of a large cubic tin monosulfide polymorph: an unraveled puzzle, CrystEngComm. 18 (2016) 5188–5194. https://doi.org/10.1039/c6ce00647g
A. Rabkin, S. Samuha, R.E. Abutbul, V. Ezersky, L. Meshi, Y. Golan, New nanocrystalline materials: a previously unknown simple cubic phase in the SnS binary system. Nano Lett. 15, 2174–2179 (2015). https://doi.org/10.1021/acs.nanolett.5b00209
P.K. Nair, A.R. Garcia-Angelmo, M.T.S. Nair, Cubic and orthorhombic SnS thin-film absorbers for tin sulfide solar cells. Phys. Status Solidi Appl. Mater. Sci. 213, 170–177 (2016). https://doi.org/10.1002/pssa.201532426
J.M. Skelton, L.A. Burton, F. Oba, A. Walsh, Metastable cubic tin sulfide: a novel phonon-stable chiral semiconductor. APL Mater. 5 (2017). https://doi.org/10.1063/1.4977868
V.E.G. Flores, M.T.S. Nair, P.K. Nair, Thermal stability of metastable cubic tin sulfide and its relevance to applications. Semicond. Sci. Technol. 33 (2018). https://doi.org/10.1088/1361-6641/aac524
D. Cabrera-German, J.A. García-Valenzuela, M. Cota-Leal, M. Martínez-Gil, R. Aceves, M. Sotelo-Lerma, Detailed characterization of good-quality SnS thin films obtained by chemical solution deposition at different reaction temperatures. Mater. Sci. Semicond. Process. 89, 131–142 (2019). https://doi.org/10.1016/j.mssp.2018.09.009
T.R. Kishore Bhat, A. Pramitha, K. Jeganath, Y. Raviprakash, The impact of silver incorporation on the structural, morphological, and optical properties of spray-pyrolyzed cubic SnS thin films. Mater. Res. Express. 11 (2024). https://doi.org/10.1088/2053-1591/ad1b05
S.U. Rehman, F.K. Butt, B. Ul Haq, S. AlFaify, W.S. Khan, C. Li, Exploring novel phase of tin sulfide for photon/energy harvesting materials. Sol Energy. 169, 648–657 (2018). https://doi.org/10.1016/j.solener.2018.05.006
M.S. Mahdi, N.M. Ahmed, A. Hmood, K. Ibrahim, M. Bououdina, Comprehensive photoresponse study on high performance and flexible π-SnS photodetector with near-infrared response. Mater. Sci. Semicond. Process. 100, 270–274 (2019). https://doi.org/10.1016/j.mssp.2019.05.019
E. Barrios-Salgado, L.A. Rodríguez-Guadarrama, A.R. Garcia-Angelmo, J. Campos Álvarez, M.T.S. Nair, P.K. Nair, Large cubic tin sulfide–tin selenide thin film stacks for energy conversion. Thin Solid Films. 615, 415–422 (2016). https://doi.org/10.1016/j.tsf.2016.07.048
J.Y. Cho, S.Y. Kim, R. Nandi, J. Jang, H.S. Yun, E. Enkhbayar, J.H. Kim, D.K. Lee, C.H. Chung, J.H. Kim, J. Heo, Achieving over 4% efficiency for SnS/CdS thin-film solar cells by improving the heterojunction interface quality. J. Mater. Chem. A 8, 20658–20665 (2020). https://doi.org/10.1039/d0ta06937j
P. Sinsermsuksakul, L. Sun, S.W. Lee, H.H. Park, S.B. Kim, C. Yang, R.G. Gordon, Overcoming Efficiency limitations of SnS-Based solar cells. Adv. Energy Mater. 4, 1–7 (2014). https://doi.org/10.1002/aenm.201400496
M. Cheraghizade, F. Jamali-Sheini, Photovoltaic behavior of SnS solar cells under temperature variations. Optik (Stuttg). 254, 168635 (2022). https://doi.org/10.1016/j.ijleo.2022.168635
M. Fathy, S. Elyamny, A.A. Bishara, G.D. Roston, A.E.H.B. Kashyout, SnS nanocrystalline thin films for n-CdS/p-SnS solar cell devices. J. Mater. Sci. Mater. Electron. 31, 18120–18134 (2020). https://doi.org/10.1007/s10854-020-04362-y
S. Sebastian, S. Vinoth, K.H. Prasad, M.S. Revathy, S. Gobalakrishnan, P.K. Praseetha, V. Ganesh, S. AlFaify, Quantitative analysis of Ag-doped SnS thin films for solar cell applications. Appl. Phys. Mater. Sci. Process. 126, 1–12 (2020). https://doi.org/10.1007/s00339-020-03959-8
V.E. González-Flores, R.N. Mohan, R. Ballinas-Morales, M.T.S. Nair, P.K. Nair, Thin film solar cells of chemically deposited SnS of cubic and orthorhombic structures. Thin Solid Films. 672, 62–65 (2019). https://doi.org/10.1016/j.tsf.2018.12.044
S. Gedi, V.R. Minnam Reddy, S. Alhammadi, P. Reddy Guddeti, T.R.R. Kotte, C. Park, W.K. Kim, Influence of deposition temperature on the efficiency of SnS solar cells. Sol Energy. 184, 305–314 (2019). https://doi.org/10.1016/j.solener.2019.04.010
V. Steinmann, R. Chakraborty, P.H. Rekemeyer, K. Hartman, R.E. Brandt, A. Polizzotti, C. Yang, T. Moriarty, S. Gradečak, R.G. Gordon, T. Buonassisi, A two-step Absorber Deposition Approach to overcome shunt losses in Thin-Film Solar cells: using tin sulfide as a proof-of-Concept Material System. ACS Appl. Mater. Interfaces. 8, 22664–22670 (2016). https://doi.org/10.1021/acsami.6b07198
A. Javed, N. Khan, S. Bashir, M. Ahmad, M. Bashir, Thickness dependent structural, electrical and optical properties of cubic SnS thin films. Mater. Chem. Phys. 246, 122831 (2020). https://doi.org/10.1016/j.matchemphys.2020.122831
S.I. Son, D. Shin, Y.G. Son, C.S. Son, D.R. Kim, J.H. Park, S. Kim, D. Hwang, P. Song, Effect of working pressure on the properties of RF sputtered SnS thin films and photovoltaic performance of SnS-based solar cells. J. Alloys Compd. 831, 154626 (2020). https://doi.org/10.1016/j.jallcom.2020.154626
X. Zhao, L.M. Davis, X. Lou, S.B. Kim, S. Uličná, A. Jayaraman, C. Yang, L.T. Schelhas, R. Gordon, Study of the crystal structure of SnS thin films by atomic layer deposition. AIP Adv. 11 (2021). https://doi.org/10.1063/5.0032782
Y. Kawano, J. Chantana, T. Minemoto, Impact of growth temperature on the properties of SnS film prepared by thermal evaporation and its photovoltaic performance. Curr. Appl. Phys. 15, 897–901 (2015). https://doi.org/10.1016/j.cap.2015.03.026
H. Kafashan, A. Rabiei, Baboukani, Electrochemically deposited nanostructured Cd-doped SnS thin films: structural and optical characterizations. Ceram. Int. 50, 5717–5727 (2024). https://doi.org/10.1016/j.ceramint.2023.11.354
T. Garmim, S. Chahib, L. Soussi, R. Mghaiouini, Z. El Jouad, A. Louardi, O. Karzazi, M.E. Jouad, E.K. Hlil, B. Hartiti, M. Monkade, Optical, electrical and electronic properties of SnS thin films deposited by sol gel spin coating technique for photovoltaic applications. J. Mater. Sci. Mater. Electron. 31, 20730–20741 (2020). https://doi.org/10.1007/s10854-020-04586-y
J.Y. Cho, S. Sinha, M.G. Gang, J. Heo, Controlled thickness of a chemical-bath-deposited CdS buffer layer for a SnS thin film solar cell with more than 3% efficiency. J. Alloys Compd. 796, 160–166 (2019). https://doi.org/10.1016/j.jallcom.2019.05.035
A.M.S. Arulanantham, S. Valanarasu, K. Jeyadheepan, V. Ganesh, M. Shkir, Development of SnS (FTO/CdS/SnS) thin films by nebulizer spray pyrolysis (NSP) for solar cell applications. J. Mol. Struct. 1152, 137–144 (2018). https://doi.org/10.1016/j.molstruc.2017.09.077
M. Messaoudi, S. Boudour, Extent of dependence of crystalline, morphological, optical and electrical properties on deposition time of sprayed SnS thin films. Microsc Res. Tech. 86, 342–350 (2023). https://doi.org/10.1002/jemt.24275
L. Filipovic, S. Selberherr, G.C. Mutinati, E. Brunet, S. Steinhauer, A. Kock, J. Teva, J. Kraft, J. Siegert, F. Schrank, C. Gspan, W. Grogger, Modeling the growth of tin dioxide using spray pyrolysis deposition for gas sensor applications. IEEE Trans. Semicond. Manuf. 27, 269–277 (2014). https://doi.org/10.1109/TSM.2014.2298883
E. Sarica, V. Bilgin, I. Akyuz, Phase transition of ultrasonically sprayed VOx thin films: the role of substrate temperature. Optik (Stuttg). 228, 1–8 (2021). https://doi.org/10.1016/j.ijleo.2020.166231
S. Dhanapandian, A.G. Manohari, C. Manoharan, K. Santhosh Kumar, T. Mahalingam, Optimization of spray deposition parameters for the formation of single-phase tin sulfide thin films. Mater. Sci. Semicond. Process. 18, 65–70 (2014). https://doi.org/10.1016/j.mssp.2013.11.001
J. Breternitz, R. Gunder, H. Hempel, S. Binet, I. Ahmet, S. Schorr, Facile bulk synthesis of π-Cubic SnS. Inorg. Chem. 56, 11455–11457 (2017). https://doi.org/10.1021/acs.inorgchem.7b01623
K. Jeganath, N.J. Choudhari, G. Shruthi Pai, A. Rao, Y. Raviprakash, Role of substrate temperature on spray pyrolysed metastable π-SnS thin films. Mater. Sci. Semicond. Process. 113 (2020). https://doi.org/10.1016/j.mssp.2020.105050
B.D. Cullity, Elements of X-Ray Diffraction (Addison-Wesley Publishing Company, Inc., 1956)
E. Sarica, V. Bilgin, Study of some physical properties of ultrasonically spray deposited silver doped lead sulphide thin films. Mater. Sci. Semicond. Process. 68, 288–294 (2017). https://doi.org/10.1016/j.mssp.2017.06.034
N. Khedmi, M. Ben Rabeh, M. Kanzari, Thickness dependent structural and optical properties of vacuum evaporated CuIn5S8 thin films. Energy Procedia. 44, 61–68 (2014). https://doi.org/10.1016/j.egypro.2013.12.010
B.P. Kafle, S. Acharya, S. Thapa, S. Poudel, Structural and optical properties of Fe-doped ZnO transparent thin films. Ceram. Int. 42, 1133–1139 (2016). https://doi.org/10.1016/j.ceramint.2015.09.042
J.I. Pankove, Optical Process in Semiconductors (Dover, New York, 1975)
J. Tauc, R. Grigorovici, A. Vancu, Optical properties and Electronic structure of Ge. Phys. Stat. Sol. 627, 627–637 (1966)
M. Wang, E.J. Kim, S. Kim, J.S. Chung, I.K. Yoo, E.W. Shin, S.H. Hahn, C. Park, Optical and structural properties of sol-gel prepared MgZnO alloy thin films. Thin Solid Films. 516, 1124–1129 (2008). https://doi.org/10.1016/j.tsf.2007.05.039
A. El-Shaer, S. Ezzat, M.A. Habib, O.K. Alduaij, T.M. Meaz, S.A. El-Attar, Influence of Deposition Time on Structural, Morphological, and Optical properties of CdS Thin films grown by low-cost chemical bath deposition. Crystals. 13 (2023). https://doi.org/10.3390/cryst13050788
N. Khan, A. Javed, M. Bashir, S. Bashir, Role of triethanolamine complexing agent in chemical bath deposition of tin sulfide thin films: Microstructural and optical properties. Results Opt. 14, 100610 (2024). https://doi.org/10.1016/j.rio.2024.100610
U. Chalapathi, B. Poornaprakash, S.H. Park, Chemically deposited cubic SnS thin films for solar cell applications. Sol Energy. 139, 238–248 (2016). https://doi.org/10.1016/j.solener.2016.09.046
K. Hartman, J.L. Johnson, M.I. Bertoni, D. Recht, M.J. Aziz, M.A. Scarpulla, T. Buonassisi, SnS thin-films by RF sputtering at room temperature. Thin Solid Films. 519, 7421–7424 (2011). https://doi.org/10.1016/j.tsf.2010.12.186
D.K. Schroder, S. Material, D. Characterization, T. Edit, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2006. https://doi.org/10.1002/0471749095
E. Coskun, H.H. Gullu, T. Colakoglu, C. Emir, E. Bozdogan, M. Parlak, Effects of Si nanowire on the device properties of n-ZnSe/p-Si heterostructure. J. Mater. Sci. Mater. Electron. 30, 4760–4765 (2019). https://doi.org/10.1007/s10854-019-00769-4
T. Tunç, Ş. Altindal, I. Uslu, L. Dökme, H. Uslu, Temperature dependent currentvoltage (IV) characteristics of Au/n-Si (1 1 1) Schottky barrier diodes with PVA(Ni,Zn-doped) interfacial layer. Mater. Sci. Semicond. Process. 14, 139–145 (2011). https://doi.org/10.1016/j.mssp.2011.01.018
J.H. Kim, S.J. Yun, H.S. Lee, J. Zhao, H. Bouzid, Y.H. Lee, Plasma-Induced Phase Transformation of SnS2 to SnS. Sci. Rep. 8, 1–7 (2018). https://doi.org/10.1038/s41598-018-28323-y
E. Coşkun, H.H. Güllü, I. Candan, O. Bayrakli, M. Parlak, C. Erçelebi, Device behavior of an In/p-Ag(Ga,In)Te2/n-Si/Ag heterojunction diode. Mater. Sci. Semicond. Process. 34, 138–145 (2015). https://doi.org/10.1016/j.mssp.2015.02.043
R. Touati, I. Trabelsi, M. Ben Rabeh, M. Kanzari, Structural and electrical properties of the Al/p-Cu2ZnSnS4 thin film schottky diode. J. Mater. Sci. Mater. Electron. 28, 5315–5322 (2017). https://doi.org/10.1007/s10854-016-6189-3
M. Yıldırım, A. Kocyigit, A. Sarılmaz, F. Ozel, The effect of the triangular and spherical shaped CuSbS2 structure on the electrical properties of Au/CuSbS2/p-Si photodiode. J. Mater. Sci. Mater. Electron. 30, 332–339 (2019). https://doi.org/10.1007/s10854-018-0297-1
E. Sarica, I. Gunes, I. Akyuz, V. Bilgin, K. Erturk, Sol-gel derived ZnO:Sn thin films and fabrication of n-ZnO:Sn/p-Si heterostructure. Opt. Mater. (Amst). 118, 111283 (2021). https://doi.org/10.1016/j.optmat.2021.111283
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The author declares that no funds, grants, or other support were received during the preparation of this paper.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Ibrahim Gunes: Writing - Original Draft, Methodology, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunes, I. Enhancing π-SnS thin films and fabrication of p-SnS/n-Si heterostructures through flow rate control in ultrasonic spray pyrolysis for improved photovoltaic performance. Appl. Phys. A 130, 574 (2024). https://doi.org/10.1007/s00339-024-07737-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07737-8