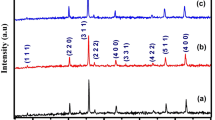
Abstract
NiFe2O4 nanoparticles have been synthesized using the flash auto-combustion technique, while TiO2 nanoparticles were prepared via sol–gel method. NiFe2O4/TiO2 nanocomposite was obtained through the sonochemical method. Investigated samples have been studied through XRD, FTIR, and FESEM. The crystallite sizes of NiFe2O4, TiO2, and NiFe2O4/TiO2 are 48.37, 25.56, and 32.46 nm, respectively. FTIR analysis was used to look into the materials’ functional groups. The images of FESEM illustrated that the particles were prepared in nanoscale. All samples were good absorbers in the UV spectrum through the wavelengths of 200–350 nm. The energy band gap values of TiO2, NiFe2O4, and NiFe2O4/TiO2 nanocomposite are 3.4, 2.3, and 2.5 eV, respectively, for the indirect transition. The samples’ dielectric characteristics have been investigated and they have a semiconductor like behavior. VSM and the Faraday technique were used to test the materials’ magnetic characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MeFe2O4 nanostructures (Me = Fe, Ni) are considered to be one of the most important nano ferrites due to their potential for various applications. One of the key aspects of spinel ferrite class is nickel ferrite [1], which has generated a lot of research interest. Nichel ferrite [2] adopted inverse spinel structure and possesses a saturation magnetization (Ms) of 23.604 eV/g and a coercivity (Hc) of 207.92Oe [1]. Experimentally, the direct transition optical band gap (Eg) is reported to be 4 eV, while for indirect transition, it was 2 eV [2].
Due to its exceptional properties, it is used in a widespread range of applications, such as: electrocatalytic, non-volatile memory, nanofibrillate, nano-biomass, low dielectric losses power applications [3,4,5,6,7]. As a result, different types of nano-structures based on Ni ferrite have been developed in the last few years using several techniques [8,9,10,11].
TiO2 is a non-toxic oxide, chemically stable against photo- corrosion with high oxidizing power [9]. However, TiO2 has some limitations for use in photocatalytic applications. It has a wide band gap Eg (3.0 eV for rutile, 3.2 eV for anatase), responding only to ultraviolet (UV) irradiation that corresponds to the wavelength of 390 nm for photoactivation. Some modifications for the solar irradiation response of TiO2 should be enhanced to reduce its Eg. Lately, the formation of a composite of TiO2 with magnetic MFe2O4 is a gorgeous field of study because MeFe2O4 (Me: Fe, Ni) nanoparticles have too narrow Eg to be a good sensitizer for wide Eg semiconductors (CoFe2O4: 1.10 eV, ZnFe2O4: 1.9 eV, NiFe2O4: 1.56 eV) [12,13,14]. They revealed astonishing trends such as suitable tunable band positions, numerous redox states, low cost, thermal stability in addition to the easiness of preparation [15,16,17,18,19].
Depending on the external magnetic field intensity, composites made of ferromagnetic materials display faster rates of pollutant degradation [20]. It has also been renowned that easy electron migration through the interface arises when the alignment of magnetic moments produced by the composite is the same for all constituent parts [21]. Additionally, it has been anticipated that charge-carrier recombination can be repressed by modifying the electron spin polarization states of the photocatalysts through doping, morphology changes and modifying the structure. Using spin-orbital or hyperfine coupling, such magnetic semiconductors allow for the flipping of the electron's spin state [22]. Most crucially, the photocatalytic composite that is formed of magnetic material is easy to recover once the photocatalytic process is terminated. A magnetic photocatalyst is effortlessly acquired where it can be reused for water treatment procedures [23, 24] since the gained material can easily be contaminated. MeFe2O4/TiO2 is essential and commonly employed in photocatalytic applications due to its ability to degrade organic hazardous chemicals [11, 13, 20,21,22].
Resistive switching (RS)-based memristor devices have been used in many different applications because of their superior switching performance [25,26,27,28]. Given that TiO2 is a potential model material to explore several nameless meristive phenomena [29, 30], the simple RS device structure makes them appropriate for use in the development of advanced computers and future technologies (metal–insulator-metal) and high efficiency with low power consumption [31, 32]. Positive exceptional electrical features that are not reachable with conventional oxide-oxide or ferrite-ferrite nanocomposites can be attained by merging two distinct material classes into a nanocomposite [33]. The RS phenomenon is presently present in many materials, such as transition metal oxides [34, 35] and perovskite oxides [36, 37].
In this piece of work, NiFe2O4, TiO2 nanoparticles and their nanocomposite were prepared using simple, fast and low cost technique. We also aimed to better understand their physical, optical, and electronic structures to estimate their proper applications.
2 Experimental techniques
2.1 Constituents
Nickel nitrate, iron nitrate, Ti isopropoxide, ethanol, and urea with chemical formula (Ni(NO)20.6H2O, 99%), (Fe(NO3)3·9H2O, 99%), (Ti{OCH(CH3)2}4), (C2H5OH, 99%) and (CH4N2O, 98%), were brought from Sigma Aldrich.
2.2 Sample preparation
2.2.1 Fabrication of NiFe2O4
Flash auto-combustion was used to fabricate nickel ferrite nanoparticles. The starting concentrations of the precursors, such as nickel (0.1 M), and iron (0.2 M) nitrates, matched the specific ferrite’s stoichiometric ratio. This precursor was combined with urea (0.7 M) through a powder phase; after that, the resultant mixture was stirred vigorously while judicious drops of deionized water were incorporated to get the dissolved mixture. The nitrates: urea mixture was heated to 250 °C in order to start the process of breaking down the urea into ammonia, where ferrites could form. This equation could potentially be used to explain the following steps for the composition of NiFe2O4:
After the precipitation, the final powder has been calcined for 2 h at 500 °C at a rate of 5 °C/min.
2.2.2 Preparation of TiO2
TiO2 nanoparticles were created by the sol–gel technique [38] by mixing 50 ml of an aqueous solution (25 ml deionized water, 25 ml ethanol) with 5 ml of Ti isopropoxide. After that, the mixture was continuously disturbed for thirty minutes. The final solution was then allowed to age for a full day. Subsequently, the precipitate was collected using a centrifuge and allowed to dry at 50 °C. Last but not least, the powder was calcined for two hours at 550 °C at a rate of 5 °C per minute.
2.2.3 Synthesis of NiFe2O4/TiO2 nanocomposite
Following these steps, a NiFe2O4/TiO2 nanocomposite was made with the same ratio. A 30-min ultrasonication process was used to combine 0.2 g of NiFe2O4 with 50 ml of ethanol and water. Next, the mixture was subjected to a dropwise addition of 50 ml of Ti isopropoxide, followed by two hours of ultrasonication. and then aging for 24 h. Centrifugation was then used to separate the final product from the precipitate. NiFe2O4/TiO2 nanocomposite was obtained by heating the powder in a furnace for two hours at 550 °C.
2.3 Samples’ analysis
Structural study has been achieved via X-ray diffractometer (using the Proker D8 advanced X-ray diffractometer, which has CuKα radiation). Fourier-transform infrared spectroscopy (FT-IR) (Perkin Elmer) has been utilized to identify the function groups in range 400–4000 cm−1. The samples shape has been inspected through FESEM (field emission scanning electron microscopy) (Sigma 300VP operated at 15 kV) with Energy-dispersive X-ray (EDX). A UV–visible spectrophotometer (Jasco (V-630) spectrometer) has been utilized to investigate the optical properties of the obtained samples. Magnetic behaviors have been examined through VSM (Vibrating Sample Magnetometer, Lakeshore 7410S), in addition to a homemade device that measures the magnetic susceptibility to various intensities of field. For the dielectric sample measurements, the powder was pressed in a uniaxial press at 5*108 N/m2 and coated with conducting silver paint at both surfaces to assure Ohmic contacts. Furthermore, dielectric properties related to the investigated samples were measured using the LCR bridge (HIOKI, 3532-50).
3 Results and discussion
Figure 1 shows the XRD of the investigated nanoparticles NiFe2O4, TiO2 and their composite NiFe2O4/TiO2. According to ICDD card number 01-080-0072, NiFe2O4 was prepared in a single phase cubic spinel structure. The diffraction peaks of NiFe2O4 were observed at 2θ values 30.26º, 35.66º, 37.28º, 43.33º, 53.82º, 57.36º, 62.98º, 71.53º, 74.58º, and 75.53º, according to (220), (311), (222), (400), (422), (511), (440), (320), (533), and (622), respectively. The data for TiO2 was indexed with the ICDD card number 01-076-1937. The main diffraction peak was observed at 2θ ≈ 25.307º corresponding to (210). The TiO2 exhibited orthorhombic structure. The XRD pattern of the NiFe2O4 /TiO2 composite is shown in Fig. 1 and indicates the presence of two phases of NiFe2O4 and TiO2 simultaneously. The absence of a third phase approves the good physical mixing of compounds to form the nanocomposite without any chemical reactions. The lattice parameters of Ni ferrite were calculated according to the following equation based on the cubic structure:
The lattice parameters of the orthorhombic TiO2 were calculated from Eq. (2).
The lattice parameters of the investigated samples were reported in Table 1.
The crystallite sizes of NiFe2O4, TiO2, and NiFe2O4/TiO2 were calculated using the well-known Scherer’s equation: [39]
where L refers to the average crystallite size, λ denotes the wave length of the X-ray radiation (λ = 1.5406 Å), β is the corrected full width at half maximum intensity of the peak, and θ is the Bragg angle. Table 1 contains the values of the crystallite size of the investigated samples. Equation (4) was used to calculate the theoretical density (Dx) of the investigated samples.
where V is the unit cell volume, M denotes the molecular weight, Z is the number of molecules in a unit cell, and NA is Avogadro’s number. The values of Dx were reported in Table 1.
The dislocation density (δ) is related to the total defects in the samples and was calculated using Eq. (5) and listed in Table 1.
FTIR spectroscopy of TiO2, NiFe2O4 and NiFe2O4/TiO2 nanocomposite was investigated. As revealed in Fig. 2, for the FTIR spectrum related to NiFe2O4, there are two noticeable absorption bands familiar to the spinel ferrite structure at 433 and 620 cm−1 which are associated with the stretching vibration mode of the M–O bond at the octahedral in addition to tetrahedral sites correspondingly [40]. The band of absorption around 1074 cm−1 was associated with a Fe3+ and O2 stretching vibration bond owing to existence of Fe3+ions on the tetrahedral site according to the inverse spinel structure of NiFe2O4 [41]. Furthermore, the band around 1400 cm−1 was credited with -CH2 asymmetric band. In the TiO2 spectrum, the bands around 568 and 901 cm−1 were corresponding to stretching vibration mode associated with Ti-O-Ti [42, 43]. For the FTIR spectra of NiFe2O4/TiO2 nanocomposite the distinctive bands of NiFe2O4 and TiO2 approving the attendance of NiFe2O4 and TiO2 NPs in nanocomposite. The absorption band around 1630 and 3500 cm−1 was also associated with the O–H band, which is assigned to O–H bending as well as stretching vibrational modes owing to the adsorbed water molecule [44].
FESEM of the investigated samples is shown in Fig. 3. The FESEM image of the NiFe2O4 is illustrated in Fig. 3a, and the particles have irregular shapes. TiO2 particles have spherical shapes, as illustrated in Fig. 3b. The shapes of the particles NiFe2O4 and TiO2 are presented in Fig. 3c for the NiFe2O4/TiO2 nanocomposite.
Figure 4 illustrates the EDX of the investigated samples. The atomic % and weight % of the elements of the samples were illustrated in the inset tables in Fig. 4. The data obtained from the EDX illustrates that the investigated samples, NiFe2O4, TiO2, and NiFe2O4/TiO2, were prepared with the same chemical formula. The presence of carbon elements in Fig. 4b is a result of the carbon tape where TiO2 was put on it inside the FESEM.
Figure 5 shows the absorbance of the UV–Vis. spectrum of TiO2, NiFe2O4, and NiFe2O4/TiO2 nanocomposite in the interior of 200–1000 nm. According to this spectrum, all the samples absorb intensely the wavelengths of 200–350 nm (ultraviolet light). By comparing the absorbance spectra of pure TiO2 and NiFe2O4 NPs with NiFe2O4/TiO2 nanocomposite, there is a red shift. This indicates the complexation between TiO2 and NiFe2O4 NPs. Figure 6a and b investigated the absorption coefficient (α) and extinction coefficient (K) related to the prepared specimen and given through the ensuing equations [40].
where A is the absorbance, λ is wavelength, and l represents the specimen thickness.
Figure 6a. shows a clear peak around λ ≈ 240 nm for all the prepared samples, which is related to π–π* transitions [41]. Figure 6b shows a notable rise in the extinction coefficient (K) of the investigated samples. The extinction coefficient gives information about the light portion that has been absorbed, scattered, or otherwise lost for every unit length of the dispersion medium.
Figure 7a and b displays Tauc plot for the direct and indirect transitions of TiO2, NiFe2O4, and NiFe2O4/TiO2 nanocomposite. The values of energy related to band gap were assumed from the Tauc relations, that have been calculated through these equations [42, 43]:
where A, α in addition to hν signify constants, absorption coefficient, and photon energy, respectively.
The energy band values of TiO2, NiFe2O4, and NiFe2O4/TiO2 nanocomposite are approximately 3.4, 2.3, and 2.5, respectively, for the indirect transition, which obviously reveals that the presence of NiFe2O4 makes the NiFe2O4/TiO2 nanocomposite further sensitive to light than pure TiO2. In light of the outcomes shown in Table 2, for values related to direct and indirect energy band gap transitions, we noticed that the transition between valence and conduction bands associated with prepared samples follows indirect transitions.
Finally, based on the above argument, NiFe2O4 nanoparticles have the capability of reducing the absorption edge related to TiO2 in the visible light range, thus enhancing the photocatalytic performance of TiO2 nanoparticles in the visible region.
The value of the real dielectric constant (ε/) was calculated according to the following equation:
where d refers to the thickness of the pellet, C is the capacitance of the sample, A is the pellet cross-sectional area and εo is the permittivity in free space (εo = 8.86 × 10–12 F/m). Figure 8 illustrates the dependence of the dielectric constant (ε/) on the absolute temperature for NiFe2O4/TiO2. In the first temperature region (300–500 K), ε/ is nearly independent on the temperature. While in the second temperature region (500–600 K), ε/ increases with raising the temperature due to the sufficient thermal energy to liberate the dipoles to be aligned with the applied field, thereby the polarization and ε/ increased. Figure 8a shows that the correlation between the ε/ values and the frequency, ε/ decreases with increasing frequency. At very high frequencies, the electric dipoles are not able to follow the frequency of the applied field, so ε/ decreases. The dependence of the dielectric constant on the frequency originates from the presence of different types of polarization, such as ionic, space charge, electronic, and dipolar polarizations [45]. At low frequencies, ε/ values are high due to the participation of dipolar and space charge polarization. At high frequencies, the electronic and ionic polarization participate in the low dielectric constant. Space charge polarization is the result of charges being deposited at grain boundaries by an electric field applied at low frequencies. This results in a large number of capacitances, which raise the dielectric constant. Because the charge exchange quickens in higher frequency regions, less polarization is obtained [46]. As a result, the dielectric constant values are reduced and almost constant. The comparative study of ε/ and T(K) for the samples NiFe2O4 and NiFe2O4 /TiO2 is shown in Fig. 8b. It was observed that the ε/ values of NiFe2O4 /TiO2 is larger than that of pure NiFe2O4. The presence of TiO2 in the NiFe2O4 /TiO2 increases the dielectric constant due to enhancement of the polarization from the conduction mechanism as a result of the charges hopping between various ions leading to the increase in the space charge polarization [45]. Moreover, the interfacial polarization is initiated the interfaces between grain of different compositions of the nanocomposite.
Figure 9 shows the dependence of ac conductivity on the reciprocal of T for the composite NiFe2O4/TiO2. The investigated samples have a semiconductor like behavior due to the ac conductivity increases with raising the temperature. The values of σac obey the Arrhenius equation [44]:
where T denotes the absolute temperature, E refers to the activation energy, and k is the Boltzmann’s constant. The values of the activation energies were calculated and reported in Table 3. The values of activation energies indicate the semiconductor like behavior. The values of E of NiFe2O4/TiO2 composite are less than that of Ni ferrite due to the presence of semiconductor TiO2 particles. The NiFe2O4/TiO2 sample exhibits an increase in ac conductivity due to an increase in space charge carriers and their mobility. The decrease in the activation energy of the NiFe2O4/TiO2 composite recommends the use of these materials in heavy metal photodegradation.
The variation of the molar magnetic susceptibility (χM) with T at different magnetic field intensities (H = 1010 Oe, 1340 Oe, and 1660 Oe) for NiFe2O4 and NiFe2O4/TiO2 composite is shown in Fig. 10a and b. A sensible decrease in (χM) with increasing temperature is detected until a certain temperature (Tc) after which it reaches its minimum value. The inset of the figure shows δχM/δT from which the Curie temperature Tc is depicted.
From Fig. 10C, it was obvious that the molar magnetic susceptibility (χM) of nanocomposite is smaller than that of Ni ferrite. On the other hand, both effective moments and Curie temperatures for the nanocomposite were larger than for the pure nano Ni ferrite.
The Curie–Weiss law [40] was for compliance at the high-temperature region. Accordingly, the Curie temperature (Tc), Curie–Weiss constant (θ), and the effective magnetic moment (μB) are detected from the paramagnetic region of the relation between χ−1M and absolute temperature (not present here) by applying the subsequent formulas [47]. Table 4 tabulates the computed data.
The tabulated data shows that the Curie temperature Tc decreases as TiO2 is added to the pure Ni ferrite, but the effective magnetic moment (μB) increases.
The value of Tc represents the strength of characteristic magnetic interactions, i.e., A-B exchange interactions, between the cations present at the tetrahedral and octahedral lattice sites in Ni ferrite, so the thermal energy required to regulate the spin alignment decreases, thus decreasing the Curie temperature [48, 49] due to weakness in the A-B exchange interaction as TiO2 was added.
The composite has a ferrimagnetic nature at room temperature, it can be described by a model of indirect double exchange, which is explained by oxygen vacancies and the exchange interaction between the 3d orbitals of Ti3+ and Ti4+ at the interface [42].
The magnetic hysteresis curves at 300 K are typical of ferrimagnetic materials. In general, the shape and width of the hysteresis loop depend on some factors, such as chemical composition, cation distribution, porosity, grain size, etc. Figure 11a and b illustrates the magnetization versus external magnetic field (M–H) hysteresis loop which is determined with an external applied magnetic field ranging between − 20 and 20 kOe. the Ms value that corresponds to 20 kOe is 23.604 emu/g.
From the hysteresis loop, Ms, remnant magnetization (Mr), Hc, squareness (Mr/Ms), experimental magnetic moments (nB), and anisotropy constant (K) are illustrated in Table 5.
Stoner–Wohlfarth relation were used to calculate the anisotropy constant ‘K’ as follows [50]:
At room temperature, the experimental magnetic moment nB is calculated from the saturation magnetization (MS) value as mentioned in the previous work [47, 51] as follows:
The table makes it evident that the magnetic parameters of the NiFe2O4/TiO2 composite have lower values compared with the pure nanoferrite sample. The lower value of saturation magnetization could result from the existence of spin canting, which was reported in several nanometer-sized [52] in addition to the frustrated spin structure at the interfaces. Since the squareness ratio < 0.5 is mostly ascribed to the formation of a multi-domain MD structure, then (Mr/Ms) values refer to the particles interact by magneto static interactions (Mr/Ms < 0.5), comparison with the reported literature will be an added value, especially for the room temperature parameters.
4 Conclusion
Flash combustion synthesis was used to prepare nanoparticles of NiFe2O4, while TiO2 nanoparticles were synthesized using sol–gel method. The nanocomposite magnetic catalyst obtained from a combination between both NiFe2O4 and TiO2 nanoparticles. Results from FESEM, FTIR, and XRD show that TiO2 and NiFe2O4 NPs were successfully synthesized. The NiFe2O4/TiO2 composite's XRD pattern is depicted and demonstrates the simultaneous existence of two phases, NiFe2O4 and TiO2. From the optical study, we concluded that the addition of NiFe2O4 makes the nanocomposite more sensitive to light than pure TiO2. As the value of Eg decreased from 3.4 to 2.5 eV, and the activation energy of the NiFe2O4/TiO2 nanocomposite reached 0.85 and 0.69 at 400 kHz, we recommended the use of these materials in heavy metal photodegradation. The magnetic hysteresis curves at 300 K are typical of ferrimagnetic materials for NiFe2O4 and its nanocomposite with TiO2. This pointed to the facility of reuse this catalyst after magnetic decantation of treated water.
Data availability
The data are available upon request.
References
A. M. El-Sayed, Mater. Chem. Phys. 82, 583–587(2003)
S.N.A. Hussain, Opt. Mater. 139, 113797 (2023)
A. Azizi, J. Saien, Optimization of Cr(VI) photocatalytic reduction by UV/TiO2: influence of inorganic and organic species and kinetic study. Arch Hyg Sci 7(2), 81–90 (2018)
S. Bakardjieva, J. Subrt, V. Stengl, M.J. Dianez, M.J. Sayagues, Photoactivity of anatase–rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase. Appl. Catal. B 58, 193–202 (2005)
S. Chandrasekaran, C. Bowen, P. Zhang, Z. Li, Q. Yuan, X. Ren, L. Deng, Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J. Mater. Chem. A 6, 11078 (2018)
C.J. Chang, Z. Lee, M.D. Wei, C.C. Chang, K.W. Chu, Photocatalytic hydrogen production by magnetically separable Fe3O4@ ZnS and NiCo2O4@ ZnS core-shell nanoparticles. Int. J. Hydrogen Energy 40(35), 11436–11443 (2015)
C.J. Chang, Z. Lee, K.W. Chu, Y.H. Wei, Chang, CoFe2O4@ ZnS core–shell spheres as magnetically recyclable photocatalysts for hydrogen production. J. Taiwan Inst. Chem. Eng. 66, 386–393 (2016)
Z. Duan, Y. Zhu, Z. Hub, J. Zhang, D. Liu, X. Luo, M. Gao, L. Lei, X. Wanga, G. Zhao, Micro-patterned NiFe2O4/Fe–TiO2 composite films: fabrication, hydrophilicity and application in visible-light-driven photocatalysis. Ceram. Int. 46, 27080–27091 (2020)
P. Laokul, V. Amornkitbamrung, S. Seraphin, S. Maensiri, Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr. Appl. Phys. 11(1), 101–108 (2011)
O. Kerkez-Kuyumcu, E. Kibar, K. Dayıoğlu, F. Gedik, A.N. Akın, Ş Özkara-Aydınoğlu, A comparative study for removal of different dyes over M/TiO2 (M = Cu, Ni Co, Fe, Mn and Cr) photocatalysts under visible light irradiation. J. Photochem. Photobiol. A 311, 176–185 (2015)
X. Zhu, F. Zhang, M. Wang, J. Ding, S. Sun, J. Bao, C. Gao, Facile synthesis, structure and visible light photocatalytic activityof recyclable ZnFe2O4/TiO2. Appl. Surf. Sci. 319, 83–89 (2014)
G. Rekhila, Y. Bessekhouad, M. Trari, Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrog. Energy 38, 6335–6343 (2013)
P. Sathishkumar, R.V. Mangalaraja, S. Anandan, M. Ashokkumar, CoFe2O4/TiO2 nanocatalysts for the photocatalytic degradation of Reactive Red 120 in aqueous solutions in the presence and absence of electron acceptors. Chem. Eng. J. 220, 302–310 (2013)
P.A.K. Reddy, S. Basavaraju, D.K. Valluri, V.S. Muthukonda, S. Machiraju, J.S. Lee, CaFe2O4 sensitized hierarchical TiO2 photo composite for hydrogen production under solar light irradiation. Chem. Eng. J. 247, 152–160 (2014)
C. Peng, W. Fan, Q. Li, W. Han, X. Chen, G. Zhang, Y. Yan, Q. Gu, C. Wang, H. Zhang et al., Boosting photocatalytic activity through tuning electron spin states and external magnetic fields. J. Mater. Sci. Technol. 115, 208–220 (2022). https://doi.org/10.1016/j.jmst.2021.11.031
N. Li, M. He, X. Lu, L. Liang, R. Li, B. Yan, G. Chen, Enhanced norfloxacin degradation by visible-light-driven Mn3O4/γ-MnOOH photocatalysis under weak magnetic field. Sci. Total. Environ. 761, 143268 (2021). https://doi.org/10.1016/j.scitotenv.2020.143268
J. Li, Q. Pei, R. Wang, Y. Zhou, Z. Zhang, Q. Cao, D. Wang, W. Mi, Y. Du, Enhanced photocatalytic performance through magnetic field boosting carrier transport. ACS Nano 12, 3351–3359 (2018). https://doi.org/10.1021/acsnano.7b08770
Y. Wang, S. Wang, Y. Wu, Z. Wang, H. Zhang, Z. Cao, J. He, W. Li, Z. Yang, L. Zheng et al., A α-Fe2O3/rGO magnetic photocatalyst: enhanced photocatalytic performance regulated by magnetic field. J. Alloy. Compd. 851, 156733 (2021). https://doi.org/10.1016/j.jallcom.2020.156733
Y.M. Hao, C. Man, Z.B. Hu, Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 184, 392–399 (2010). https://doi.org/10.1016/j.jhazmat.2010.08.048
L. Zhang, Y. He, Y. Wu, T. Wu, Photocatalytic degradation of RhB over MgFe2O4/TiO2 composite materials. Mater. Sci. Eng. B 176, 1497–1504 (2011)
Y. Lu, H.-C. Wang, X. She, D. Huang, Y. Yang, X. Gao, Z. Zhu, X. Liu, Z. Xie, A novel preparation of GO/NiFe2O4/TiO2 nanorod arrays with enhanced photocatalytic activity for removing unsymmetrical dimethylhydrazine from water. Mater. Sci. Semicond. Process. 121, 105448 (2021)
P. Cheng, C. Deng, M. Gu, W. Shangguan, Visible-light responsive zinc ferrite doped titania photocatalyst for methyl orange degradation. J. Mater. Sci. 42, 9239–9244 (2007)
M.M. Arman, Preparation, characterization and magnetic properties of Sm0.95Ho0.05FeO3 nanoparticles and their application in the purification of water. Appl. Phys. A 129(1), 38 (2023)
Lu. Ya-bo, H.-C. Wang, X.-Y. She, D. Huang, Y.-X. Yang, X. Gao, Z.-M. Zhu, X.-X. Liu, Z. Xie, A novel preparation of GO/NiFe2O4/TiO2 nanorod arrays with enhanced photocatalytic activity for removing unsymmetrical dimethylhydrazine from water. Mater. Sci. Semicond. Process. 121, 105448 (2021)
Y. Abbas, I.S. Han, A.S. Sokolov, Y.R. Jeon, C. Choi, Rapid thermal annealing on the atomic layer-deposited zirconia thin film to enhance resistive switching characteristics. J. Mater. Sci. Mater. Electron. 31, 903–909 (2020)
H. Abbas, M.R. Park, Y. Abbas, Q. Hu, T.S. Kang, T.S. Yoon, C.J. Kang, Resistive switching characteristics of manganese oxide thin film and nanoparticle assembly hybrid devices. Jpn. J. Appl. Phys.. J. Appl. Phys. 57, 06HC03 (2018)
Y. Abbas, S.M. Ansari, I. Taha, H. Abunahla, M.U. Khan, M.D. Rezeq, H.M. Aldosari, B. Mohammad, Stopping voltage-dependent PCM and RRAM-based neuromorphic characteristics of germanium telluride Adv. Funct. Mater. 33, 2214615 (2023)
Y. Abbas, R.B. Ambade, S.B. Ambade, T.H. Han, C. Choi, Tailored nanoplateau and nanochannel structures using solution-processed rutile TiO2 thin films for complementary and bipolar switching characteristics. Nanoscale 11, 13815–13823 (2019)
N.A. Ahir, A.V. Takaloo, K.A. Nirmal, S.S. Kundale, M.Y. Chougale, J. Bae, D. Kim, T.D. Dongale, Mater. Sci. Semicond. Process.Semicond. Process. 125, 105646 (2021)
C. Hu, M. McDaniel, A. Posadas, A. Demkov, J. Ekerdt, E. Yu, Highly controllable and stable quantized conductance and resistive switching mechanism in single-crystal TiO2 resistive memory on silicon. Nano Lett. 14, 4360–4367 (2014)
O. Krestinskaya, A. Irmanova and A. P. James, Memristors: properties, models, materials. Deep learning classifiers with memristive Networks. Modeling and Optimization in Science and Technologies, vol. 14 (Switzerland: Springer, Cham) pp. 13–40 (2020)
F. Gul, H. Efeoglu, Bipolar resistive switching and conduction mechanism of an Al/ZnO/Al-based memristor. Superlattices Microstruct.Microstruct. 101, 172–179 (2017)
A.S. Nikam, G.U. Kamble, A.R. Patil, S.B. Patil, A.D. Sheikh, A.V. Takaloo, P.K. Gaikwad, R.K. Kamat, J.H. Kim, T.D. Dongale, Nanotechnology 34, 425201 (2023)
Y.S. Lin, F. Zeng, S.G. Tang, H.Y. Liu, C. Chen, S. Gao, Y.G. Wang, F. Pan, Resistive switching mechanisms relating to oxygen vacancies migration in both interfaces in Ti/HfOx/Pt memory devices. J. Appl. Phys. 113, 064510 (2013)
Y. Abbas, Y.R. Jeon, A.S. Sokolov, S. Kim, B. Ku, C. Choi, Compliance-free, digital SET and analog RESET synaptic characteristics of sub-tantalum oxide based neuromorphic device. Sci. Rep. 8, 1228 (2018)
H.L. Park, T.W. Lee, Organic and perovskite memristors for neuromorphic computing. Org. Electron. 98, 106301 (2021)
R.P. Patil, A.S. Nikam, S.B. Teli, A.V. Takaloo, R.K. Kamat, T.D. Dongale, P.D. Kamble, K.M. Garadkar, Semicond. Sci. Technol.. Sci. Technol. 38, 125002 (2023)
M.V. Arularasu, Effect of organic capping agents on the optical and photocatalytic activity of mesoporous TiO2 nanoparticles by sol–gel method. SN Appl. Sci. 1, 393 (2019)
E.E. Ateia, D. Gawad, M.M. Arman, Ab-initio study of structural, morphological and optical properties of multiferroic La2FeCrO6. J. Alloy. Compd. 976, 173017 (2024)
A.Z. Saravani, M. Nadimi, M.A. Aroon, A.E. Pirbazari, Magnetic TiO2/NiFe2O4/reduced graphene oxide nanocomposite as a recyclable photocatalyst for photocatalytic removal of methylene blue under visible light. J. Alloy. Compd. 803, 291–306 (2019)
A.M. Ismail, R. Ramadan, M.M. El-Masry, The role of nanoparticles inclusion in monitoring the physical properties of PVDF. J. Aust. Ceramic Soc. (2023). https://doi.org/10.1007/s41779-023-00836-4
M.M. El-Masry, R. Ramadan, The effect of CoFe2O4, CuFe2O4 and Cu/CoFe2O4 nanoparticles on the optical properties and piezoelectric response of the PVDF polymer. Appl. Phys. A 128, 110 (2022). https://doi.org/10.1007/s00339-021-05238-6
M.M. Arman, M.K. Ahmed, M.M. El-Masry, Cellulose Acetate polymer spectroscopic study comprised LaFeO3 perovskite and graphene as a UV-to-visible light converter used in several applications. J. Mol. Struct. 1281, 135153 (2023)
L.J. Berchmans, R. Sindhu, S. Angappan, C.O. Augustin, Effect of antimony substitution on structural and electrical properties of LaFeO3. J. Mater. Process. Technol. 207, 301 (2008)
I. M. Aniz, M. Maisnam, H. S. Singh & C. Chaliha, Frequency dependence of dielectric and impedance properties of ZnO and TiO2 added K0.5Na0.5NbO3 ceramics. Mater. Today: Proc. (2023). https://doi.org/10.1016/j.matpr.2023.02.299
X. Vendrell, J.E. García, X. Bril, D.A. Ochoa, L. Mestres, G. Dezanneau, Improving the functional properties of (K0.5Na0.5)NbO3 piezoceramics by acceptor doping. J. Eur. Ceramic Soc. 35(1), 125–130 (2015)
E.E. Ateia, A.T. Mohamed, K. Elsayed, J. Magn. Magn. Mater.Magn. Magn. Mater. 452, 169–178 (2018)
M.A. Gilleo, J. Phys. Chem. Solids Phys. Chem. Solids. 13, 33–39 (1960). https://doi.org/10.1016/0022-3697(60)90124-4
M. Chen, Y. Liu, W. Zhou, P. Wu, Ceramics Int. 49, 7676–7682 (2023)
E.C. Stoner, E.P. Wohlforth, Philos. Trans. R. Soc. Lond. ALond. A 240(826), 599–642 (1948)
E.E. Ateia, A.T. Mohamed, J. Magn. Magn. Mater. 426, 217–224 (2017)
A. Amirabadizadeh, T. Amirabadi, WJCMP 3, 131–135 (2013)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dalia N. Ghaffar: Methodology, and written the manuscript. M. M. Arman: Methodology and write the manuscript. S.I. El-Dek Preparation of the samples, and write the manuscript. Rania Ramadan: Preparation of the samples, and write the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghaffar, D.N., Arman, M.M., El-Dek, S.I. et al. Studying the preparation, characterization, and physical properties of NiFe2O4, TiO2, and NiFe2O4/TiO2 nanocomposite. Appl. Phys. A 130, 254 (2024). https://doi.org/10.1007/s00339-024-07397-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07397-8