Abstract
Nanocomposites containing different contributions of vanadium oxide (V2O5) and magnetite (Fe3O4) modified with manganese (Mn) ions were fabricated upon the formula of xMn0.4Fe2.6O4/(1-x)V2O5, whereas x = 0.0, 0.2, 0.4, 0.5, 0.6, 0.8, and 1.0. The obtained compositions were characterized upon their structure, morphology, besides their magnetic susceptibility. The TEM micrographs depicted that V2O5 was formed as rod shapes with diameters of 20–30 nm and length of 450 nm, while Mn–magnetite was configured in ellipsoidal shapes with dimensions 25–40 nm. Moreover, the Maximum height of the roughness(Rt) changed from 507.0 to 220.6 nm, while the skewness (Rsk) increased from 0.104 to 0.122 for (x = 0.2, and x = 1.0), respectively. Furthermore, Curie Weiss constant (θ) increased from 830 to 890 K and then decreased to 520 K, for x = 0.2, 0.5, and x = 1.0, respectively. The photo-activated antibacterial activity was examined and showed that the inhibition zone increased significantly by increasing the V2O5 content as well as it increased also from dark to light conditions. It was changed from 17.4 ± 1.1 and 16.9 ± 1.3 mm to 19.2 ± 1.4 and 18.9 ± 1.5 mm for pure V2O5 (x=0.0) against E. coli and S. aureus, respectively. The degradation of methylene blue (MB) was tested, and the efficiency of removal reached around 97.1% for the highest contribution of V2O5 after 35 min of visible light exposure. Hence, the fabricated nanocomposites can be suggested for a deep investigation to be highlighted for disinfection and water treatment applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last decades, the rapid development of printing and dyeing has caused a tremendous increase in environmental pollution [1, 2]. The dyes, organic molecules, and the pharmaceutical reagents that reach the rivers and seas lead to significant consequences on the drinking water [3, 4]. Furthermore, organic dyes such as methylene blue, congo-red, and malachite green are toxic compounds and can cause health problems against the kidney and the liver and even lead to cancer cell formation [5, 6]. Therefore, an economical treatment methodology is necessary to keep the pollution heath level [7].
Numerous techniques have been developed to degrade dyes from aqueous solutions, such as absorption, coagulation, and photocatalysis. Among those, the photocatalysis approach can be suggested as promising for water treatment and dye degradation owing to its feasibility and low cost [8, 9]. However, photocatalysts that can work only under ultraviolet (UV) consume intensive energy and attain low effectiveness. Thus, large bandgap and fast recombination of the formed charge carriers are considered the main drawbacks of the designed photocatalysts materials [10].
Therefore, composite materials could be suggested to gain high potency of dye degradation. Substances with low bandgaps can offer a high intensity of pumped electron–hole pairs [11]. However, a large number of electrons does not guarantee a high decolorization efficiency, because charge recombination might deteriorate the flow of electron current. Furthermore, the inability to collect the adsorbent agents after dye removal can cause secondary pollution. Therefore, the design of recoverable substances is crucial to deal with wastes. In addition, using an adsorbent material for one time could increase the cleaning costs [12, 13]. Hence, versatile requirements are vital for an appropriate approach to manage wastes through the water.
On the other hand, keeping a high level of care system requires remaining surfaces clear from bacterial organisms. Photocatalytic materials can generate reactive oxygen species (ROS) with a high affinity to interact with cellular walls. Thus, photoactivated antibacterial agents can play a crucial role in the disinfection of surfaces [14, 15].
In this regard, the development of nanomagnetic materials such as magnetite (Fe3O4) due to its high magnetic properties, surface area, recoverability, and reusability has attracted intensive interest. Many attempts have been made to improve the properties of modified magnetite [16, 17]. In general, the replacement of diamagnetic of paramagnetic ions into the magnetic structure induces lattice distortion, and thus, magnetic, electrical, and optical behaviors of the host composition can be changed significantly. Ionic substitution of magnetite doping can introduce a simple way to promote physical–chemical properties of ferrites. Among diverse metallic ions, Mn2+ is considered one of the best candidates for magnetite ionic replacement. Due to its magnetic moment value (5μB), which is larger than that of iron ions (4μB), Mn-doped magnetite has a cubic spinel structure, which is stable chemically and has a wide range of technological applications [18,19,20]. Many attempts have been done for improving the properties of doped magnetite. In general, adding diamagnetic or paramagnetic materials to magnetic sample (Mn doped magnetite) causes modification in structure, magnetic, electrical behavior of the host sample. As, the grain size formation depends on impurities concentration [21, 22].
Furthermore, V. A. R. Villegas et al. investigated the photocatalytic activity of nitrobenzene (NB) via magnetic nanoparticles under UV irradiation. The results depicted that the photocatalytic degradation reached around 73% after 2 h of irradiation at pH 2 [23]. Moreover, S. M. Sajadi et al. fabricated CuO@magnetite@hen bone using for the reduction of polycyclic aromatic hydrocarbons. The results illustrated that 7 mg of the nanocomposites were able to degrade methylene blue after 20 min of exposure [24]. On the other hand, M. Moztahida et al. used magnetite loaded into reduced graphene oxide for photocatalytic degradation of 2-methylisoborneol (MIB). The results showed that pure magnetite degraded only 22.5% of MIB, while the nanocomposite was able to remove around 99% after 180 min of continuous irradiation [25].
Vanadium pentaoxide (V2O5) is one of the unique transition metal oxides because of its physical and chemical properties, which enable it to be used widely in various applications as gas sensors and photo-catalysis. Thus, nanocomposites based on vanadium oxide (V2O5) have been investigated for dye removal for decades. For instance, H. EL-Sheshtawy et al. examined the removal of MB using composites of g-C3N4/V2O5 modified with Ag nanoparticles. The results indicated that the composition was able to degenerate MB completely after 60 min of irradiation [26]. The bandgap of V2O5 is around 2.6 eV, while its calculated conduction and valence edges are around 0.3 eV and 2.9 eV [27]. Concerning their biological utilization, W. Ma et al. used vanadium oxide nanorods for antibacterial applications. The results showed that the released ROS were able to degenerate E. coli and S. aureus bacterial cells and thus accelerated wound healing [28].
In this regard, this work aims to investigate the effect of compositional variation of Mn–magnetite/V2O5 on their ability to degrade dyes, besides their antibacterial activity based on their magnetic.
2 Experimental techniques
2.1 Materials
Analytical grade of oxalic acid dihydrate, ammonium metavanadate, ferrous chloride tetrahydrate (FeCl2·4H2O), nitric acid, ferric chloride hexahydrate (FeCl3·6H2O), manganese chloride tetrahydrate (MnCl2·4H2O), sodium metavanadate and ammonium chloride were used without further purification and were obtained from Sigma-Aldrich Company.
2.2 Synthesis of Mn0.4 Fe2.6O4/V2O5 nanocomposites
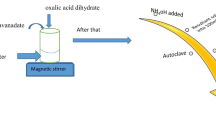
Figure 1A reveals formation of Mn-doped magnetite by co-precipitation method. First, suitable amounts of (FeCl2·4H2O), (MnCl2·4H2O), and (MnCl2·4H2O) have been dissolved in deionized water at room temperature. Then, the pH value was adjusted to be 11 by adding droplets of ammonia solution under vigorous stirring. Finally, the resultant precipitate was washed by ethanol and distilled water several times and then dried at 90 °C
On the other hand, Fig. 1b shows that V2O5 was synthesized upon the dissolving of sodium metavanadate in distilled water. Then, the ammonium chloride (1.2 g) was droped into the solution, followed by an increase of the temperature to 80 oC. Then, pH value was increased to 8. The obtained produced was then annealed at 600 oC for 4 h.
Different weights of both V2O5 and Mn0.4Fe2.6O4 were added into a Falcone tube containing 50 ml of distilled water. The solution was then sonicated under a high-frequency probe sonicator for 30 min. The obtained gel was then precipitated and dried at 50–70 °C.
3 Characterization of prepared samples
Obtained samples were characterized by XRD (analyticalx, Pertpro, Cu kα1 target, λ = 1.5404 Å, 45 kV, 40 mA, Netherlands). The XRD was used in the range 2θ (5° ≤ 2θ ≤ 70°). Samples morphology was observed by Field emission scanning electron microscopy (FESEM) (FE-SEM, QUANTA-FEG250, Netherlands, the operating volt is about 20–30 kV). Magnetic properties were studied through a homemade setup of Faraday's method.
The optical properties, absorbance, and reflection spectra were acquired via the UV–Vis spectrophotometer (Bio Aquarius CE 7250, UK) and information about the bandgaps.
The degradation of methylene blue (MB) was done under a visible light irradiation source of (500 W). First, 0.1 mg of each composite was added into a 10 mL solution of the MB that contains ~ 10 ppm (20 ml). The total time of exposure is 35 min; every 5 min, around 3 mL aliquot of the dye was taken for testing using the double beam spectrophotometer (Bio Aquarius CE 7250, UK). The degradation effectiveness (DR) was calculated [29, 30]:
where Co is the initial at t = 0.0 min; Ct is the absorbance at any time of the interval.
The antibacterial effectiveness was investigated against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Standard agar plate assays were used, whereas the initial concentration of V2O5/Mn–magnetite powders was 0.5 mg/mL. The test was repeated in the dark and in the light conditions under visible light at 37 °C [60]. Each test was repeated three times to calculate the standard deviation. The results were compared with the standard sample, which was selected as amoxicillin with a concentration of 30 µg/mL. The control sample was considered as a bacterial disk without additional nanocomposites.
4 Results and discussion
4.1 Crystal structure study
The crystal configuration of the fabricated nanocomposites was investigated by XRD, as obvious in Fig. 2. It can be observed that the main characteristic peaks related to the cubic structure of pure Mn0.4Fe2.4O4 appeared at 2θ = 35.33°, 62.37° and corresponded to miller indices of (311) and (440), respectively. While the major peaks of the orthorhombic crystal structure of pure V2O5 existed at 2θ = 15.380°, 21.720°, 26.150°, and 31.046°, which are accompanied to (200), (101), (110), and (400) respectively. It is noticed that increasing V2O5 content leading to the intensities of the peaks of Mn0.4Fe2.6O4 decreased. The average crystallite size of obtained compositions was calculated by the Scherer's equation [31]:
where D is the crystallite size, 0.9 represented the shape factor, λ is the wavelength of X-ray, \(\upbeta\) donates the full width at half maximum, and θ reveals to the Bragg angle.
The crystallite size of xMn0.4Fe2.6O4/(1-x) V2O5; x = 0, 0.2, 0.4, 0.5, 0.6, 0.8 and 1. was calculated and listed in Table 1. The data in Table 1 show that by increasing V2O5 content, the crystallite size decreased as the lattice distortion increased. The calculated crystallite size depicts a decreasing behavior upon the increasing of V2O5 contribution starting from 79 to 50 nm. Thus, the crystallinity of V2O5 seems to be lower than that of the Mn–magnetite nanoparticles. This behavior is assigned to the difference in preparation methods.
4.2 Microstructural features
In Fig. 3a, b, TEM micrographs exhibit the formation of dual phases of Mn–magnetite and V2O5. It could be distinguished that V2O5 was formed as rod shapes with diameters in the range of 20–30 nm and length exceed 450 nm. On the other hand, Mn doped magnetite seems to be configured with ellipsoidal shapes with dimensions around 25–40 nm. Good distribution of the particles can be detected, while the smaller size of V2O5 than magnetite matches well with the calculated crystallite size from XRD results.
4.3 Surface morphology
The surface morphology of the composition of xMn0.4Fe2.6O4/(1-x)V2O5, whereas x = 0.2, 0.4, 0.5, 0.6, 0.8 and 1.0, was done via SEM, as shown in Fig. 4a–f. At x = 0.2, Fig. 4a shows that the pristine vanadium oxide was formed as fine agglomerated grains with narrow dimensions distribution in a range of 65–95 nm. Porosity and surface roughness seems to be high. The composition of x = 0.4 is illustrated in Fig. 4b. The grains tend to be agglomerated with a fluffy surface, with dimensions of 62–70 nm. Figure 4c shows the composition of x = 0.5with crumble grains in the range of 54–95 nm. Furthermore, the surface porosity is shown to be lower than the former compositions. Figure 4d exhibits the composition of x = 0.6 with randomly oriented grains with dimensions of 50–78 nm. The compositions of x = 0.8 and x = 1.0 were formed with a wide range of size distribution around 40–85 and 47–102 nm, respectively. The surface defects and crystallographic misalignment between vanadium oxide and Mn–magnetite are the main responsible for the change of topography.
Figure 5 shows the surface roughness of the xMn0.4Fe2.6O4/(1-x)V2O5 at different contributions, while Table 2 reports the roughness parameters. It can be noticed that the roughness average (Ra) decreases from 40.3 significantly to 22.8, while the root mean square roughness (Rq) decreases from 57.8 to 29.2 nm from the lowest additional Mn–magnetite to the highest one. Furthermore, the maximum height of the roughness (Rt) starts from 507.0 and plunges to 220.6 nm, while the maximum roughness valley depth (Rv) begins with 281.3–117.6 nm. The high divergence between values of Rt and Rv indicates the inhomogeneity of roughness components. In other words, the surface topography can denote two components, heights, and notches. Herein, the expression of skewness might help to differentiate between heights and notches. The positive values indicate that heights are predominant than notches. The interaction between the surrounding substances depends strongly on the contribution ratios of heights and notches on the surface topography. While the physical interaction is encouraged to be done via heights, notches are vital for chemical bonding. This approach comes from the change in nature of both heights and notches. The incorporation of low crystalline V2O5 through the grains Mn–magnetite causes increasing of notches. Moreover, the presence of crystallographic defects on the grain surface acts as ionic traps for surrounding ions [32, 33]. This ability to establish chemical bonds with the ambient environment is vital for degrading dyes or interact with cellular walls. Furthermore, the physical adhesion is promoted as a result of the heights, and it can be crucial to initiate the chemical one [34]. Controlling the compositional contribution is a good tool to manipulate the surface topography and thus promoting the dye removal effectiveness.
4.4 Magnetic study
Relation between molar magnetic susceptibility as a function of temperature of xMn0.4Fe2.6O4/(1-x)V2O5; x = 0.0, 0.2, 0.4, 0.5, 0.6, 0.8, 1.0 is shown in Fig. 6. The magnetic susceptibility was measured upon the variation of temperature under different applied fields. It is obvious that curves start from different points on the axis of magnetic susceptibility. These values are inversely proportional to the applied field. The behavior of magnetic susceptibility seems to gradually deteriorate upon the increase of temperature reaching the zero value (Curie temperature). The raising of thermal energy due to the increase of temperature encourages thermal agitation and induces entropy through the system, which enlarges the dipoles disordered, and thus, susceptibility deteriorates.
Furthermore, the inversely proportional between the magnetic susceptibility and magnetic field intensity is assigned to the disability of dipoles to be aligned with the field direction. The temperature point in which the dipoles cannot follow the direction of the applied field. By increasing V2O5 content from 1 up to x = 0.6, the curie temperature decreased due to diamagnetic nature of V2O5. From x = 0.5 to x = 0.2, the curie temperature has higher value due to enhancement of grain growth which is attributed to existence of large number of cation vacancies presented by presence of V.+5
From Fig. 7, the straight part of all prepared samples fitted with Curie–Weiss law, from the paramagnetic region, some magnetic parameters as Curie constant, Curie Weiss constant and effective magnetic moment can be calculated, from the linear fitting of the obtained curves, the paramagnetic region obey to Curie–Weiss law, and thus, Curie constant (C), Curie Weiss constant (θ), and effective magnetic moment (μeff) can be calculated by the following equations:
The obtained magnetic constants were scheduled in Table 3. It could be noticed that the critical concentration of V2O5 is x = 0.5, at this ratio, there is a fetal change in magnetic parameters, which is suitable for various applications.
Finally, the magnetic behavior of the nanocomposite with varied x values can be explained by the presence of diamagnetic behavior related to V2O5 at all the indicated temperatures [10] but in another hand we found that V2O5 has small ferromagnetic component due to the presence of small amount of V+4 in the sample in addition to enhancement of grain growth which is attributed to existence of large number of cation vacancies presented by presence of V+5. Consequently, the curie temperature, Curie constant, curie Weiss constant and effective magnetic constant have their lowest values for x = 0.6 and 0.8.
4.5 Antibacterial activity
The improvement of the ability of materials to interact with the bacterial cell under light irradiation denotes its photoactivated antibacterial effectiveness. As obvious from Fig. 8, the antibacterial offense has been investigated in dark and light conditions. The inhibition zone increased upon the increasing of V2O5 content through the nanocomposite starting from 17.4 ± 1.1 and 16.9 ± 1.3 mm to 10.6 ± 0.8 and 11.3 ± 1.4 mm in the dark, while it plunged from 19.2 ± 1.4 and 18.9 ± 1.5 mm to 10.5 ± 0.7 and 11.9 ± 0.9 mm under visible light, against E. coli and S. aureus, respectively. It can be noticed that there is a significant change in inhibition zones from dark to light conditions, whereas the inhibition zone increased from 17.4 ± 1.1 to be 19.2 ± 1.4 mm against E. coli from dark to light conditions. The release of ionic species [5, 11, 16, 28, 35] from the nanocomposite, including V5+, Mn2+, Fe2+, besides the oxyanions of OH−, O2− possess high reactivity against the cellular tissue of bacteria. It can be noticed that the incidents of photons due to light irradiation can generate charge carriers to be pumped through the conduction band (C.B.). These charges represent an additional source of ionic species which are able to degenerate bacterial cells.
4.6 Dye degradation
The degradation of methylene blue (MB) under visible light irradiation was examined using adsorbents of nanocomposites containing different contents of V2O5 and Mn–magnetite. As shown in Fig. 9a, b, the degradation of MB is shown as a lowering of the absorbance upon the increasing of exposure time. The effectiveness of degradation increased reaching to be 97.1%, 91.0%, 88.5%, 79.3%, 74.7%, 60.9% and 58.8% for the compositions of x = 0.0, 0.2, 0.4, 0.5, 0.6, 0.8, and 1.0 after 35 min of exposure to visible light, respectively. The ability to degrade MB was improved upon the rising of V2O5 contributions and vice versa.
The photocatalytic degradation is based on the ability of an adsorbent substance to pump electrons due to the bombarding of photons with moderate energy [35]. The generated photoelectrons jump to the C.B. to participate in the current. The increasing of V2O5 with its low bandgap (2.28 eV) can facilitate the motivation of electrons. Furthermore, the high surface area of the fabricated compositions might decrease the charge collisions, and thus, the recombination ratio. The produced oxyanions, including OH−, O2−, and other cations, are responsible for the high degradability of the MB. The degradation behavior was studied using the pseudo-first and pseudo-second-order kinetics as follows [36]:
Pseudo first-order model:
Pseudo-second-order model:
where k1 and k2 are the pseudo first-, second-order and inter-particle diffusion rate constants in (min− 1) and (g mg− 1 min− 1), respectively.
The kinetics constant (k) was calculated from the fitting of the equation and summarized in Table 4. The correlation coefficients related to the pseudo-second-order model (0.94–0.99) were higher than those for the pseudo-first-order model (0.74–0.94), which recommends that the adsorption process follows a pseudo-second-order model, and it was noticed that values of the kinetics constant correlated with the contribution of V2O5. The highest value was 8.3 × 10–2 min− 1 and 0.191 g/mg.min.− 1, while its lowest value was 2.8 × 10–2 min− 1 and 0.137 g/mg.min− 1 for the pseudo-first- and pseudo-second-order constant respectively, which accompanied the highest and the lowest additional V2O5.
Table 5 compares the antibacterial activity and Dye removal efficiency between xMn0.4Fe2.6O4/(1-x) V2O5; (x = 0. 0.2, 0.4) nanocomposites and other materials from the literature. It can be noticed from the table that the antibacterial activity as well as the dye removal efficiency of the considered samples is superior to most of the other materials which make the xMn0.4Fe2.6O4/(1-x) V2O5 nanocomposite a useful material for the dye degradation and antibacterial activity.
5 Conclusion
Nanocomposites based on different contents of V2O5/Mn–magnetite were fabricated. The TEM graphs exhibited that the V2O5 were formed in a rod shape with diameters of 20–30 nm and length exceed 450 nm, while the Mn-were looked like ellipsoidal shapes with dimensions of 25–40 nm. The surface morphology investigation showed that the topography was significantly changed upon the compositional variation. The grain size was changed from 65–95 to 47–102 nm for the highest and the lowest additional V2O5 (x = 0.2 and x = 1.0), respectively. Besides, it was shown that the roughness average changed from 40.3 to 22.8 nm for (x = 0.2 and x = 1.0), respectively. The Curie temperature increased from 910 to 960 K and plunged to 870 K for x = 0.2, 0.5, and x = 1.0, respectively. Moreover, the photo-activated antibacterial effectiveness was examined and showed that the inhibition zone increased from 17.4 ± 1.1 and 16.9 ± 1.3 mm to 19.2 ± 1.4 and 18.9 ± 1.5 mm for pure V2O5 against E. coli and S. aureus, in the dark and under visible light conditions, respectively. Furthermore, the efficiency to degrade MB was tested and reached around 97.1%, 79.3%, and 58.8% for the compositions of x = 0.0, 0.5, and 1.0 after 35 min of exposure to visible light, respectively.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Change history
22 May 2024
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s00339-024-07504-9
References
R. Dou, H. Cheng, J. Ma, S. Komarneni, Manganese doped magnetic cobalt ferrite nanoparticles for dye degradation via a novel heterogeneous chemical catalysis. Mater. Chem. Phys. 240, 122181 (2020)
S.A. Al Kiey, R. Ramadan, M.M. El-Masry, Synthesis and characterization of mixed ternary transition metal ferrite nanoparticles comprising cobalt, copper and binary cobalt–copper for high-performance supercapacitor applications. Appl. Phys. A 128, 473 (2022). https://doi.org/10.1007/s00339-022-05590-1
L. Zou, H. Wang, X. Jiang, G. Yuan, X. Wang, Enhanced photocatalytic efficiency in degrading organic dyes by coupling CdS nanowires with ZnFe2O4 nanoparticles. Sol. Energy 195, 271–277 (2020)
R. Ramadan, A.M. Ismail, Tunning the physical properties of PVDF/PVC/ Zinc ferrite nanocomposites films for more efficient adsorption of Cd (II). J. Inorg. Organomet. Polym. Mater. (2022). https://doi.org/10.1007/s10904-021-02176-x
C. Zhu, Y. Li, Y. Yang, Y. Chen, Z. Yang, P. Wang, W. Feng, Influence of operational parameters on photocatalytic decolorization of a cationic azo dye under visible-light in aqueous Ag3PO4. Inorg. Chem. Commun. 115, 107850 (2020)
B. Zhu, H. Cheng, Y. Qin, J. Ma, Y. Kong, S. Komarneni, Copper sulfide as an excellent co-catalyst with K2S2O8 for dye decomposition in advanced oxidation process. Sep. Purif. Technol. 233, 116057 (2020)
K. Shen, Y. Cui, D. Zhang, M. Liu, H. Huang, X. Sha, F. Deng, N. Zhou, X. Zhang, Y. Wei, Biomimetic preparation of MoS2-Fe3O4 MNPs as heterogeneous catalysts for the degradation of methylene blue. J. Environ. Chem. Eng. 8, 104125 (2020)
Z. Wu, W. Huang, X. Shan, Z. Li, Preparation of a porous graphene oxide/alkali lignin aerogel composite and its adsorption properties for methylene blue. Int. J. Biol. Macromol. 143, 325–333 (2020)
W. Wei, X. Han, M. Zhang, Y. Zhang, Y. Zhang, C. Zheng, Macromolecular humic acid modified nano-hydroxyapatite for simultaneous removal of Cu(II) and methylene blue from aqueous solution: experimental design and adsorption study. Int. J. Biol. Macromol. 150, 849–860 (2020)
Z.R. Xiao, G.Y. Guo, Structural, electronic and magnetic properties of V2O5−x: An ab initio study. J. Chem. Phys. 130, 214704 (2009). https://doi.org/10.1063/1.3146790
H. Dhoundiyal, P. Das, M.C. Bhatnagar, Electrical and magnetic properties of V2O5 microstructure formed by self-assembled nanorods. Phys. B 603, 41262 (2021)
R. Saleh, A. Taufik, Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II, III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 210, 563–573 (2019)
M. Mai, El-Masry, Rania Ramadan, Enhancing the properties of PVDF/MFe2O4; (M: Co–Zn and Cu–Zn) nanocomposite for the piezoelectric optronic applications. J Mater Sci Mater Electron (2022). https://doi.org/10.1007/s10854-022-08493-2
F. Soltani-Nezhad, A. Saljooqi, A. Mostafavi, T. Shamspur, Synthesis of Fe3O4/CdS-ZnS nanostructure and its application for photocatalytic degradation of chlorpyrifos pesticide and brilliant green dye from aqueous solutions. Ecotoxicol Environ Saf 189, 109886 (2020)
L. Dong, M. Li, S. Zhang, X. Si, Y. Bai, C. Zhang, NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions. Desalination 476, 114227 (2020)
M. Mousavi, A. Habibi-Yangjeh, Ternary g-C3N4/Fe3O4/Ag3VO4 nanocomposites: Novel magnetically separable visible-light-driven photocatalysts for efficiently degradation of dye pollutants. Mater. Chem. Phys. 163, 421–430 (2015)
M. Ghaedi, S. Hajjati, Z. Mahmudi, I. Tyagi, S. Agarwal, A. Maity, V.K. Gupta, Modeling of competitive ultrasonic assisted removal of the dyes–Methylene blue and Safranin-O using Fe3O4 nanoparticles. Chem. Eng. J. 268, 28–37 (2015)
M.M. El-Masry, R. Ramadan, The Effect of CoFe2O4, CuFe2O4 and Cu/CoFe2O4 nanoparticles on the optical properties and piezoelectric response of the PVDF polymer. Appl. Phys. A 128, 110 (2022). https://doi.org/10.1007/s00339-021-05238-6
S.F. Mansour, R. Al-Wafi, M.K. Ahmed, S. Wageh, Microstructural, morphological behavior and removal of Cr(VI) and Se(IV) from aqueous solutions by magnetite nanoparticles/PVA and cellulose acetate nanofibers. Appl. Phys. A (2020). https://doi.org/10.1007/s00339-020-3377-z
R. Ramadan, Preparation, characterization and application of Ni-doped magnetite. Appl. Phys. A 125, 586 (2019). https://doi.org/10.1007/s00339-019-2887-z
R. Ramadan, S.I. El-Dek, M.M. Arman, Enhancement of Mn-doped magnetite by mesoporous silica for technological application. Appl. Phys. A 126, 900 (2020). https://doi.org/10.1007/s00339-020-04059-3
R. Ramadan, A.M. Ismail, Tunning the physical properties of PVDF/PVC/Zinc ferrite nanocomposites films for more efficient adsorption of Cd (II). J. Inorg. Organometall. Polym. Mater. (2022). https://doi.org/10.1007/s10904-021-02176-x
V. Alfredo Reyesillegas, J. de IsaíasLeónamírez, E. Hernandez Guevara, S. Perez Sicairos, L. Angelica Hurtado Ayala, B. Landeros Sanchez, Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. J. Saudi Chem. Soc. 24, 223–235 (2020)
S.M. Sajadi, K. Kolo, S.M. Hamad, S.A. Mahmud, M. Pirouei, K. Amjadian, K.M. Khalid, Biosynthesis of reusable and recyclable CuO@Magnetite@Hen Bone NCs and its antioxidant and antibacterial activities: a highly stable magnetically nanocatalyst for excellent reduction of organic dyes and adsorption of polycyclic aromatic hydrocarbons. IET Nanobiotechnol 13, 124–133 (2019)
M. Moztahida, M. Nawaz, J. Kim, A. Shahzad, S. Kim, J. Jang, D.S. Lee, Reduced graphene oxide-loaded-magnetite: a Fenton-like heterogeneous catalyst for photocatalytic degradation of 2-methylisoborneol. Chem. Eng. J. 370, 855–865 (2019)
H.S. El-Sheshtawy, H.M. El-Hosainy, K.R. Shoueir, I.M. El-Mehasseb, M. El-Kemary, Facile immobilization of Ag nanoparticles on g-C3N4/V2O5 surface for enhancement of post-illumination, catalytic, and photocatalytic activity removal of organic and inorganic pollutants. Appl. Surf. Sci. 467–468, 268–276 (2019)
R. Saravanan, V.K. Gupta, E. Mosquera, F. Gracia, Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J. Mol. Liq. 198, 409–412 (2014)
M. Mai, M. El-Masry, R.R. Elshahat, R.M. Abdelhameed, selective photocatalytic reduction of nitroarenes into amines based on cobalt/copper ferrite and cobalt-doped copper ferrite nanophotocatalyst. J. Mater. Sci. Mater. Electron. 32, 18408–18424 (2021)
H.B. Sales, R.R. Menezes, G.A. Neves, J.JNd. Souza, J.M. Ferreira, L. Chantelle, A.L. Menezes, Hd.L. de OliveiraLira, Development of sustainable heterogeneous catalysts for the photocatalytic treatment of effluents. Sustainability 12, 7393 (2020)
M.M.G. Fouda, J.S. Ajarem, S.N. Maodaa, A.A. Allam, M.M. Taher, M.K. Ahmed, Carboxymethyl cellulose supported green synthetic features of gold nanoparticles: antioxidant, cell viability, and antibacterial effectiveness. Synth. Met. 269, 116553 (2020)
K. Xinga, S. Zhangb, A. Tsaic, H. Xiaob, D.L. Creedonc, S.A. Yiannia, J.C. McCallumc, C.I. Pakesa, D.-C. Qi, High-electron-affinity oxide V2O5 enhances surface transfer doping on hydrogen-terminated diamond. Diam. Relat. Mater. 108, 107865 (2020)
M.T. Elsayed, A.A. Hassan, S.A. Abdelaal, M.M. Taher, M.k. Ahmed, K.R. Shoueir, Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J. Mater. Res. Technol. 9, 13927–13936 (2020)
R. Al-Wafi, S.F. Mansour, M.K. Ahmed, Mechanical, microstructural properties and cell adhesion of Sr/Se-hydroxyapatite/graphene/polycaprolactone nanofibers. J. Thermoplas. Compos. Mater. 34, 536–556 (2020)
R. Ramadan, M.K. Ahmed, V. Uskoković, Magnetic, microstructural and photoactivated antibacterial features of nanostructured Co–Zn ferrites of different chemical and phase compositions. J. Alloy. Compd. 856, 157013 (2020)
M.K. Ahmed, M.E. El-Naggar, A. Aldalbahi, M.H. El-Newehy, A.A. Menazea, Methylene blue degradation under visible light of metallic nanoparticles scattered into graphene oxide using laser ablation technique in aqueous solutions. J. Mol. Liq. 315, 113794 (2020)
Z.A. Al-Ahmed, N.S. Al-Radadi, M.K. Ahmed, K. Shoueir, M. El-Kemary, Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 13, 8626–8637 (2020)
D.G. Nkosinathi, B.K. Albertus, S.S.E. Jabulani, S.S. Mavuso, R.V.S.R. Pullabhotla, Biosynthesis, characterization, and application of iron nanoparticles: in dye removal and as antimicrobial agent. Water Air Soil Pollut 231, 130 (2020). https://doi.org/10.1007/s11270-020-04498-x
K.N. Pham, K.M. Trung, T.N. Huu, T.V. Do, D.T. Tran, V. Van Pham, T.P. Bach, T.D. Anh, D. Raja, Superior organic dye removal by CoCr2O4 nanoparticles: adsorption kinetics and isotherm. J. Sci. Adv. Mater. Devices 7, 100438 (2022)
U. Enerelt, E.B. Bum, G. Aminaa, S. Nominchimeg, M.O. Tsogbadrakh, Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Scientifc Reports 11, 21047 (2021). https://doi.org/10.1038/s41598-021-00520-2
Acknowledgements
The authors gratefully acknowledge the support provided by the Egypt and Suez University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R R: methodology, formal analysis, investigation, data curation, visualization, writing, and review. M.K. A: conceptualization, methodology, formal analysis, data curation, investigation, writing, and review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Hereby, we consciously assure that for the manuscript optimizing microstructure and magnetic properties of xMn0.4Fe2.6O4/(1-x) V2O5 nanocomposite for dye removal and antibacterial applications. the following is fulfilled: this material is the authors' own original work, which has not been previously published elsewhere. This paper is not currently being considered for publication elsewhere. This paper reflects the authors' own research and analysis in a truthful and complete manner. This paper properly credits the meaningful contributions of co-authors and co-researchers. The results are appropriately placed in the context of prior and existing research. All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such using quotation marks and giving proper reference. we have been personally and actively involved in substantial work leading to this paper, and will take public responsibility for its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, R., Ahmed, M.K. Impact of adding vanadium pentoxide to Mn-doped magnetite for technological uses. Appl. Phys. A 128, 1056 (2022). https://doi.org/10.1007/s00339-022-06197-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06197-2