Abstract
In the article the process of Fe, Co, Ni and Cu hydroxides modified nanoparticles of layered double hydroxides (LDH) based on Zn and Al (Zn-Al LDH) was successfully presented. The precipitation method allowed to obtain nanoparticles of high crystallinity with lateral dimensions below 100 nm and thickness below 20 nm. The photocatalytic activity of the modified LDH in the degradation process of quinoline yellow was over 99%, while for the unmodified LDH the efficiency was only 30%. The study confirmed that modification of LDH with divalent ions had a significant effect on both photocatalytic and sorption properties. Furthermore, the study also investigated the effect of the nature of the type of light on the photodegradation efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Layered double hydroxides (LDH) are a group of two-dimensional materials generally described by the formula \({\left[{\mathrm{M}}_{1-x}^{2+}{\mathrm{M}}_{x}^{3+}{\left(\mathrm{OH}\right)}_{2}\right]}^{x+}{\left[{\mathrm{A}}^{n-}\right]}_{x/n}\bullet m{\mathrm{H}}_{2}\mathrm{O}\), where M2+ refers to metal cations e.g. Mg2+, Ca2+, Co2+, Zn2+, Cu2+, Ni2+, and M3+ indicates the trivalent metal cations, e.g. Al3+, Co3+, Fe3+, Cr3+, Ni3+, Co3+ [1]. The M2+ and M3+ cations are distributed in the hydroxide layer and An− are anions located in the interlayer space, which can be inorganic (\({\mathrm{O}}_{3}^{2-}, {\mathrm{NO}}_{3}^{-}, {\mathrm{Cl}}^{-})\), organic (e.g. carboxylate, oxoanion) or complex (e.g. coordination compounds or polyoxometalates) [2]. The individual layers of cations in the LDH structure are held together by bonds formed between metal cations and anions in the interlayer space. An example of a naturally occurring LDH is hydrotalcite, which consists of magnesium or aluminium ions (Mg2+ and Al3+) with carbonate ions CO32− [3].
Due to the possibility of manoeuvring the composition of LDH nanoparticles and the selection of ions, these materials exhibit a wide range of properties and find applications as catalysts, sorbents, photocatalysts, drug carriers, flame retardant materials, additives with antimicrobial properties, etc. [4]. An unquestionable advantage of these materials is their developed specific surface area resulting from the layered structure [5]. Nuzhdin et al. have shown that copper- and clay-based LDH catalyses the N-methylation reactions of p-anisidine [2]. The developed surface enabled the initiation of surface reactions increasing the catalytic efficiency of the materials. Layered double hydroxides are also increasingly proposed for biomedical applications [6, 7]. Their biocompatibility, anion-exchange capacity, nanometric particle size, high chemical stability, and the ability to adjust the surface charge of the particles enable controlled release and permeation of active substances without the deleterious effects of LDHs themselves. Furthermore, LDHs presenting hydrophilic character are used as inorganic nanofillers to produce polymeric nanocomposite membranes for water treatment [8].
LDH materials can assist in the treatment of dye wastewater from the textile, leather, paper, cosmetics, and plastics industries and beyond [9,10,11]. There are several types of dyes used in industry, most of which are toxic, mutagenic, and carcinogenic. Among the known methods of wastewater treatment, there are physical (evaporation, distillation, sedimentation, adsorption) and chemical methods (chlorination, ozonation), but photocatalysis, being a physico-chemical method, is one of the most promising, as organic pollutants are converted into non-toxic simple compounds, mainly CO2 and H2O [10, 12, 13]. The challenge, however, is to develop materials that would increase the rate of dye removal. This effect would be achieved by combining materials with high photocatalytic activity and good sorption properties. In recent years, layered double hydroxides and their derivative structures have gained interest in photocatalytic applications due to their ability to efficiently sorb pollutants and their progressive photodegradation under both UV and visible light.
The aim of this study was to modify the structure of layered double hydroxides based on zinc and aluminium by adding divalent hydroxides (Me(OH)2). The modified materials differed in structure, surface area and particle size, which affected their sorption and photocatalytic properties. In this manner, active materials were obtained, which can be successfully used to remove a wide range of contaminants [14, 15].
2 Experimental section
2.1 Materials
The syntheses of layered double hydroxides were carried out with use of zinc nitrate (V) (Zn(NO3)2∙H2O, Sigma-Aldrich), aluminium nitrate (V) (Al(NO3)3∙H2O, Merck), sodium hydroxide (NaOH, Sigma-Aldrich) and sodium carbonate (Na2CO3, Sigma-Aldrich). The Zn-Al LDH were modified by introducing selected metal ions into hydroxide layers. For this reason, iron(II) chloride (FeCl2∙H2O, Acros), nickel chloride (NiCl2∙H2O, Sigma-Aldrich), cobalt(II) chloride (CoCl2∙H2O, Sigma-Aldrich), and copper(II) chloride (CuCl2, Sigma-Aldrich) were used.
2.2 Methods of LDH nanoparticles synthesis
Process of synthesis of layered double hydroxides (Zn-Al-LDH) was carried out in batch system. For this reason 10 g Zn(NO3)2 and 15 g Al(NO3)3 were dissolved in 200 cm3 deionized water. Simultaneously, 5 g NaOH with 4 g Na2CO3 were dissolved in 30 cm3 of water. The solution of metal ions was heated into 50 °C and 30 cm3 of alkalic solution was dropped. The solution was mixed in 200 rpm by 4 h, filtered, washed, and dried in 50 °C overnight. Syntheses of Me-LDH (Fe–Zn–Al LDH, Cu–Zn–Al LDH, Co-Zn-Al LDH and Ni–Zn–Al LDH) were carried out by adding selected metal salt into solution of Zn(NO3)2 and Al(NO3)3. The molar ratio of Me:Zn was 0.5:1, 0.75:1 and 1:1 (designation for example Fe0.5-Zn-Al LDH, Fe0.75-Zn-Al LDH and Fe1-Zn-Al LDH).
2.3 Instrumental analysis
The size of the resulting nanoparticles and LDH crystallites were determined by DLS and XRD analyses (Philips X’Pert camera with monochromator PW 1752/00 CuKα), respectively. The phase composition was described using the Rietveld method based on Match! program. The XRD analysis, combined with Scherrer equation for crystallite size, enabled the determination of actual LDH crystallite sizes. Differences in morphology of the obtained products were observed based on SEM microphotographs. The SEM–EDS analysis allowed determining the distribution of individual elements in LDH structures. Impact of addition of the metals on band gaps of the LDH material was established based on UV–Vis spectrophotometer Rayleigh 1800. The materials were also compared in terms of the surface character of the materials obtained. In this purpose, the point of zero charge was established in the Zeta Sizer Malvern. Based on the DLS method, the hydrodynamic diameter of LDH particles suspended in water was also determined. The presence of the special groups in the materials, including the occurrence of –CO3, –OH, –NO3, etc., was confirmed by FTIR. Active surface area of LDH nanoparticles and pore volume were measured on a Gemini VI apparatus from Micromeritics USA. The qualitative composition of the materials was determined by XPS X-ray Photoelectron Spectroscopy analysis. The analyses were carried out on a PHI Quantum 2000, Physical Electronics, Inc.: Photoelectron excitation source: a VG Scienta SAX 100 X-ray tube with an aluminium anode, equipped with a VG Scienta XM 780 monochromator, emitting radiation with an Al Kα characteristic line and an energy of 1486.7 eV.
2.4 Photocatalytic studies
2.4.1 Photodegradation properties of Zn-Al LDH
Photocatalytic properties of Zn-Al LDH nanoparticles were determined during photodegradation of quinoline yellow (Sigma-Aldrich). In each process, 50 mg of the material was mixed with 200 cm3 of 100 mg/dm3 solution of the dye. After 30 min in the darkness, samples were mixed in the presence of UV light (365 nm) for 60 min. The effectiveness of photodegradation was calculated based on the equation:
where C0 is an initial concentration of the dye, Ct is a concentration of the dye after t min of the photodegradation. The absorbance of the degraded dye was measured by UV–Vis spectroscopy (Rayleigh UV-1800 spectrophotometer).
2.4.2 Cycle efficiency of materials
The stability of the materials and their photocatalytic activity was investigated in cyclic processes. After photodegradation, each material was washed, filtered, and dried. In each catalytic step 200 mg of the material was mixed with new 200 cm3 of 100 mg/dm3 quinoline yellow solution.
2.4.3 Scavenging experiments for reactive species
To characterise the mechanism of photocatalysis on LDH nanoparticles, photodegradation processes were carried out in the presence of selected scavengers: triethanolamine (TEOA (h+), C0 = 1 mmol/dm3), benzoquinone (BQ (O2−), C0 = 0,5 mmol/dm3) and mannitol ((⋅OH), C0 = 1 mmol/dm3) and silver nitrate(V) (AgNO3 (e−), C0 = 1 mmol/dm3). For this purpose, 50 mg of material was added to 20 cm3 of solution in which the dye concentration was 100 mg/dm3 and the scavenger concentration was 1 or 0.5 mmol/dm3, respectively. After 30 min of running the process in the dark, the suspensions were stirred for 60 min in the presence of UV light (365 nm). The solutions were then filtered and the filtrate was analysed in terms of quinoline yellow concentration. The addition of selected compounds: triethanolamine, benzoquinone, mannitol, and silver nitrate(V) allowed the determination of which radicals are involved in the photodegradation process. Based on the results it was possible to investigate the mechanism of photodegradation on LDH photocatalysts.
3 Results
3.1 Selection of Me2+ modification for LDH of nanoparticles
3.1.1 Effect of Me 2+ modification on LDH structure
Zn-Al LDH nanoparticles with addition of Fe, Cu, Ni, and Co hydroxides with molar ratio of Me to Zn equal to 0.5, 0.75, and 1.0 were prepared in batch processes. The composition of materials is shown in Table 1. The obtained materials were examined to select the materials with the highest photocatalytic activity.
All diffractograms confirmed the presence of six diffraction peaks characteristic of LDH structures, at 11.6° (003), 23.3° (006), 34.5° (102), 39.12° (105), 46.6° (108), and 60.1° (110) [16]. No other phases indicative of impurities were observed. Characteristic peaks confirming LDH formation are those indexed as 003 and 006, appearing as strong/sharp, narrow, and symmetrical diffraction peaks (Fig. 1). These peaks determine the basal reflections of intercalated \({\mathrm{NO}}_{3}^{-}\), \({\mathrm{CO}}_{3}^{2-}\) anions located in the LDH interplane space, with the first basal reflection (003) appearing with greater intensity than the second basal reflection (006), as confirmed by literature sources [17, 18]. Materials without the addition of Me hydroxides showed higher crystallinity than materials with the addition of Fe, Cu, Co, and Ni hydroxides. These differences can be explained by an enhanced randomness in the formation of layers which included cations of different sizes [16].
XRD patterns of LDH nanoparticles (■—layered double hydroxide LDH, ●—ZnO): A: Fe–Zn–Al LDH, B: Cu–Zn–Al LDH, C: Co–Zn–Al LDH, D: Ni–Zn–Al LDH; designations for each diffractogram: a pattern of Zn-Al LDH, b Me0.5-Zn–Al LDH (the molar ratio of the added hydroxide to Zn was 0.5:1), c Me0.75-Zn–Al LDH (the molar ratio of the added hydroxide to Zn was 0.75:1), d Me1.0-Zn–Al LDH (the molar ratio of the added hydroxide to Zn was 1:1)
Based on crystallographic analysis, the nanoparticles were revealed to be hexagonal in nature, corresponding to an R-3 m structure (166) [19]. LDH nanoparticles were analysed to determine the basal distances and crystallite sizes of the nanoparticles. Basal distance is defined as the distance from the plane of the layer to the adjacent layer. The basal distance and crystal size data corresponding to the individual characterization peaks are given in Table 1. The lattice parameters of LDH with Me(OH)2 addition assume slightly larger basal space values (d(003)) compared to basal LDH, the exception being the addition of Fe(OH)2. This may be due to the smaller ionic radius of Fe2+ (63 pm) compared to the ionic radii of Zn2+ (74.5 pm), Ni2+ (70 pm), Cu2+ (73 pm) and Co2+ (73.5 pm) [20]. The cell parameter a, which represents the distance between cations within the LDH layer (a = 2. d (1 1 0)), remains approximately constant (Table 1). The parameter c, whose adhesion distance to the hydroxide layer is given by c = 3. d (0 0 3), where d = λ/2 sin θ, varies with the added Me(OH)2. This behaviour is related to the presence of oxygen-containing functional groups in the growth of LDH crystallites.
The preparation of LDH by changing the metal ion ratio can result in variation in nanostructure growth rates and network parameters of the materials (Table 1). An increase in the proportion of Me(OH)2 in the LDH structure resulted in a decrease in LDH crystallinity, with the greatest differences observed for materials with Ni2+ or Fe2+. Kulkarni et al. revealed variations in the platelet size of the obtained Ni–Co LDHs (Ni:Co ratio = 1:0, 0.75:0.25, 0.5:0.5, 0.4:0.6, 0.25:0.75, and 0:1), as well as the occurrence of differences in the pore size and number of layers of the materials depending on the proportion of each ion. The authors noted that the amount of nickel in the layer is a determining factor in LDH morphology. Changing the molar ratio of Ni–Co metal ions of 0, 0.3, 0.6,1, 3, and 1 revealed a reduction in the crystallinity of the materials, in which a reduction in the content of accessory metal ions leads to sharp and intense peaks of the hydrotalcite phase, showing good crystallinity of the LDHs [21]. It is suggested that increasing the molar ratio of additional hydroxide to Zn(OH)2 increases the amorphous character of LDH. Concurrently, the use of various metals with different ionic radii, affect the rate of hydroxide crystal formation and deform the layered structure of LDH.
3.2 Effect of Me(OH) 2 addition on photocatalytic properties of Zn–Al LDH
Table 2 shows the efficiency of LDH nanoparticles in quinoline yellow (QY) photodegradation process, depending on its concentration in the product. Based on the results, it was found that the highest photodegradation efficiency of QY is obtained for the materials with 0.5 addition in relation to the number of moles of Zn2+. In parallel, among the added metal ions, the highest efficiency was obtained for Ni0.5-Zn–Al LDH. As mentioned, the content of metal ions above 0.5 mol in relation to Zn2+ ions cause a decrease in crystallinity of materials and modification of their structure, which results in deterioration their photocatalytic properties. However, a positive correlation was observed between the presence of Me(OH)2 and the improvement of photocatalytic properties. Due to the developed LDH surface area, a significant contribution of dye sorption was observed, which influences at a further stage the surface processes allowing the degradation of the dye structure to simpler forms. It is suggested that the addition of Me hydroxide may have limited effect of reducing the energy gap width of LDH but enhancing the sorption efficiency of the materials by disrupting their structure. On this basis, LDH nanoparticles with addition of Ni(OH)2 with a molar ratio of Ni:Zn equals to 0.5:1:0 were selected for subsequent studies.
Similar results related to the change in intensity of diffraction peaks with increasing Ni(OH)2 content were confirmed by Paikaray et al. The authors suggested that nickel ions build into the structure of the forming LDH particles, as noted by the decreasing parameter a. An increase in Ni(OH)2 content decreases the distances between cations, which is due to the replacement of Zn2+ ions with Ni2+ ions in the crystal structure. Similarly, as described by the authors in their study, higher values of parameter c were obtained in Ni–Zn–Al LDH materials, indicating an expansion of the interlayer spacing. The reported results, combined with the decreasing size of the crystallites, confirm the growth of disorder in the LDH structure [22]. Compared with literature data, it was confirmed that LDH crystals become less amorphous with the increase in their size running in parallel with the nucleation process [23, 24].
3.3 Characterization of Zn–Al LDH and Ni–Zn–Al LDH nanoparticles
3.3.1 Analysis of SEM and SEM–EDS LDH nanoparticles
SEM microphotographs of the obtained LDH nanoparticles are shown in Figs. 2 and 3. Nanoplates with diameters of about 100–272 nm and thicknesses ranging from 13.5–36.0 nm were obtained, depending on the method used. SEM–EDS analysis confirmed a uniform distribution of Zn, Ni, Al, O, and C (Fig. 2.II). Ma et al. obtained γ-Fe2O3/Cd2+–Ni2+–Fe3+–LDHs in a batch process consisting of homogeneous hexagonal platelets with an average transverse size of 6–8 μm and an approximate thickness of 50–100 nm [25]. The addition of nickel (Fig. 2B) further influences the reduction in size of LDH nanoparticles regardless of the modification of the method.
3.3.2 Particle size analysis by DLS and the point of zero charge analysis
Table 3 shows the particle sizes of the obtained LDH materials and the point of zero charge (PZC) values. The addition of nickel hydroxide resulted in nanoparticles with smaller sizes for LDH materials. The surface charge of the materials was investigated by analysing the results with the photodegradation efficiency of QY and MB, which, with similar molecular weight, differ in ionic character. The determined PZC values were 7.99 for Zn–Al LDH and 7.05 for Ni0.5–Zn–Al LDH. The value of the point of zero charge for nickel-modified LDH materials resulted in a shift of the charge value towards neutral. The shift of the surface character from negative to neutral facilitates the sorption of negatively charged materials, which increases the applicability of the material, being more versatile for a wide range of compounds.
The energy band gap values equalled 3.29 eV for both Zn–Al LDH and Ni0.5–Zn–Al LDH. These values were comparable to those obtained for ZnO [26]. Despite the increased energy gap of nickel, there was no increase in the BG of modified LDH. This confirms that the presence of nickel hydroxide in the structure favourably increases the active surface area of the photocatalyst, without a decrease in the energy band gap value, which would require the use of high-energy radiation, preventing the catalyst from being active under visible light as well.
3.3.3 The BET analysis
The active surface area of Zn–Al LDH and nickel hydroxide-modified LDH nanoparticles (Ni0.5–Zn–Al LDH) was determined by BET analysis (Table 4.) The active surface area more than doubles after nickel addition to the LDH structure. Zn–Al LDH consists mainly of nanopores with diameters below 1 nm and a small proportion of micropores with sizes of 5–50 nm (Fig. 4). The P/Po plot takes the form of type II hysteresis, which corresponds to the formation of pores between the two planes associated with the plane structure of LDH nanoparticles [27]. The addition of nickel hydroxide changes the shape of the hysteresis, indicating an increase in the interplane space. This change correlates with an increase in the active surface area of the Ni0.5–Zn–Al LDH material, compared to Zn-Al LDH. In addition to micropores, the presence of mesopores with a size of 5–30 nm is also observed, so that the pore volume is increased by four times compared to Zn-Al LDH [28].
Hysteresis loops of adsorption isotherms of Zn–Al LDH (A1), pore distribution of Zn–Al LDH (A2), cumulative pore distribution of Zn-Al LDH (A3), hysteresis loops of adsorption isotherms of Ni0.5–Zn–Al LDH (B1), pore distribution of Ni0.5–Zn–Al LDH (B2), cumulative pore distribution of Ni0.5–Zn–Al LDH (B3)
3.4 FTIR analysis of LDH nanoparticles
The type and structure of ions forming the Zn–Al LDH and Ni0.5–Zn–Al LDH layers were investigated by FTIR spectroscopy (Fig. 5). The broad absorption band with a maximum at 3422 cm−1 comes from –OH stretching vibrations. Due to the absence of a band at about 1600 cm−1, it is suggested that the –OH bonds correspond to the hydroxide groups of LDH structures [27]. The high-intensity absorption peaks at 1360 and 779 cm−1 derive from symmetric stretching vibrations and out-of-plane deformation vibrations of CO32− ions, indicating the presence of interstitial carbonate ions [8]. The higher band intensity at 1360 cm−1 suggests an ordered arrangement of CO32− ions between layers of LDH nanoparticles. The weak absorptions in the low-frequency region of 550 and 460 cm−1 are attributed to the stretching bonds of –MeO and bending bonds of MeOH octahedral hydroxyl sheets [25]. Absorption peaks in the 800–650 cm−1 range are associated with vibrations of the Me–O–Me and O–Me–O bonds [29].
After the quinoline yellow photodegradation process, the materials were studied again by FTIR analysis. No significant differences were observed between the spectra, indicating the absence of structural changes in the materials. The lack of differences between the spectra also confirms the photocatalysis process, by which the dye molecules are degraded. If only the process of sorption of the dye had taken place, it would have been possible to observe additional bands corresponding to the C=C, C–C, C–H bonds, among others, forming the dye molecules [27].
3.4.1 The XPS analysis
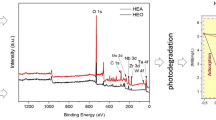
In order to better understand the composition of the LDH nanoparticles as well as the existing functional groups that build up the LDH layers, XPS analysis was performed. The broad spectrum (Fig. 6) confirmed the presence of Zn, Al, Ni, C, O, Cl. Figure 5 shows the deconvolution of the main peaks (Ni-2p, Al-2p, Zn-2p, C-1 s, and O-1 s) of the high-resolution XPS spectrum. Table 5 shows the quantitative composition of the LDH materials and in which functional groups the elements occur. Ni-2p, Zn-2p, and Al-2p indicate that the oxidation state of these elements is mainly based on ions [30].
The results show that the valence state of zinc on the Ni0.5-Zn-Al LDH surface is + 2 (Lu et al. 2017). The high-resolution Zn-2p spectrum showed three deconvoluted peaks located at 1021.91, 1022.62, and 1023.74 eV, which were assigned to Zn–OH, Zn–CO3, and in a small proportion of Zn-Cl, respectively [11]. The O1s spectrum displays signals from all oxygen species on the surface. The oxygen peak at 531.15 eV corresponds to a mixture of oxygen from the Ni0.5–Zn–Al LDH structure. XPS analysis shows that Ni2+ and Al3+ elements coexist in the product, which is in an agreement with predictions [31]. Analysis confirmed the formation of Ni(OH)2, Zn(OH)2, and Al(OH)3 [32]. The formation of additional forms of metal carbonates, mainly ZnCO3, was also confirmed.
3.5 The photocatalytic properties of LDH nanoparticles
3.5.1 Photodegradation of quinoline yellow QY under UV and visible light
To determine the photocatalytic activity of the obtained LDH, tests were carried out in the presence of various source of light. Based on previous tests, the influence of the type of light source was investigated on two materials: Zn–Al LDH and Ni0.5-Zn–Al LDH. The reactions were carried out at natural pH (6.8). In the first step, the samples were mixed with the dye solution for 30 min in the darkroom. In the processes, the proportion of sorption was between 40 and 60%, which can be explained by the developed layered structure of the materials, as well as by the good adhesion of neutral QY to the neutral character of the photocatalyst surfaces.
Figure 7 presents the decomposition process of the dye. Graphs presenting the concentration of QY as a function of exposure time showed that regardless of the addition of metal ions, all samples exhibited higher photocatalytic activity compared to the base LDH, but the addition of Ni improved the photodegradation efficiency of QY to the highest extent. The high photocatalytic activity of LDH with Ni addition may be due to the formation of smaller particles and the reduction in the anionic character of the catalyst surfaces [34]. The addition of ions also improves the adhesion of the inert dye to the surface of the materials. It was shown that for Ni0.5-Zn–Al LDH materials irrespective of the method of preparation, the percentage of decolorization was greater than 90%, which corresponds to the removal of 27 mg QY/g of catalyst and is higher than for base LDH (82% corresponds to 24.5 mg/dm3). The process rate constants after LDH modification with Ni are more than 5 times higher, indicating a significant improvement in reaction rates.
Photodegradation efficiency of quinoline yellow (QY): without presence of photocatalyst, in presence of Zn-Al LDH and Ni0.5-Zn-Al LDH nanoparticles in different source of light: incandescent light with a Kelvin colour temperature of 2700 K, halogen light with a Kelvin colour temperature of 4000 K, white light with a Kelvin colour temperature of 6500 K and UV light with a Kelvin colour temperature of more than 12000 K (time = 60 min, m = 50 mg, \({V}_{\mathrm{dye}}=\) 200 cm3, \({C}_{0,\mathrm{dye}}\)=100 mg/dm3)
3.5.2 Cyclic investigation of LDH nanoparticles
The stability of LDH photocatalysts was investigated in three cycles of QY photodegradation operation. The materials were stable and provided almost constant percentage of photodegradation after three cycles (Fig. 8). Liji confirmed the stable performance of LDH-based materials. The authors found that Au–Pd nanoparticles immobilized on Ni–Fe–CO3 LDH are good photocatalysts degrading about 95% of the dye (25 mg/dm3) after 60 min and the activity remains almost the same after recycling the catalyst [35].
In successive cycles of operation, a decrease in the sorption contribution to the removal of QY was observed, but the rate of photodegradation of the dye remained constant ensuring their effectiveness at longer time of use. In all materials, the slope curve of the dye concentration over time becomes steeper, indicating an increasing contribution of photocatalysis to dye removal and an improvement in the photocatalytic properties of the materials. The results of the high stability performance of LDH nanoparticles were confirmed by Abderrrazek et al. The Zn–Al based photocatalysts were stable and after three cycles provided an almost constant percentage of photodegradation [36].
3.5.3 Photodegradation mechanism using LDH nanoparticles
The presence of compounds acting as scavengers, i.e. benzoquinone, triethanolamine, isopropanol and silver nitrate(V), made it possible to determine which scavengers disturb the equilibrium of photocatalytic reactions and which radicals take part in the photocatalysis reaction, making it possible to predict the mechanism of this process. Different scavengers were used individually in the photocatalytic reaction to quench a specific reactive species. It was observed that the presence of TEA resulted in a reduction in photodegradation to the highest extent, as well as an inhibition of \(\cdot {\mathrm{O}}^{2-}\) radicals causing a decrease in the activity of the materials. This indicates that these radicals are involved in the photodegradation mechanism. The lack of a significant decrease in the removal efficiency of dye is associated with a significant proportion of sorption (Fig. 9).
The results indicate that the ·\({\mathrm{O}}^{2-}\) radical and the presence of h+ holes increase the photocatalytic activity and both the ·\({\mathrm{O}}^{2-}\) radical and the h+ holes are involved in the photodegradation mechanism of QY. In the study, it was confirmed that the Ni content increases the activity of the materials and the presence of Ni2+ ions effectively enhance the activity of the materials. It is suggested that nickel acts as a dopant to the LDH framework, which can capture photogenerated electrons and suppress their recombination, increasing the lifetime of the photocatalyst. However, nickel at higher concentrations does not act on the trapping sites but acts on the electron–hole recombination centres, hence the highest activity was obtained for materials with 0.5 mol Ni/mol Zn addition.
When Ni0.5–LDH is exposed to UV light, e−-h+ pairs are generated. The photogenerated holes move to the catalyst surface and react with water and OH− ions to give ∙OH radicals. Concurrently, to a greater extent, electrons can react with oxygen adsorbed on the catalyst surface to form the superoxide ∙\({\mathrm{O}}^{2-}\). The ∙\({\mathrm{O}}^{2-}\) radicals are highly reactive forms that, when acting on QY, cause its gradual degradation to simple molecules [37, 38]. On the other hand, photogenerated electrons reduce Ni3+ present on the catalyst surface (electron trap), promoting charge separation and producing h+ accumulation. To recover the oxidized form of Ni3+, the captured e- are transferred towards H+ adsorbed on the surface and lead to the formation of H2. Qi et al. analysed the effect of nickel addition in LDH structure, and suggested that photogenerated electrons are gradually reducing Ni3+ to Ni2+, promoting charge separation and producing accumulation of h + , which can move towards OH− adsorbed and produce ∙OH [39]. Sun et al. proposed a comparable mechanism for a material based on double layered hydroxides consisting of zinc, nickel, and cobalt deposited on NiMoO4 nanoplatelets. The O2 and H2O adsorbed on the surface of the nanoparticles, reacting with electron–hole pairs, are transformed into ∙O2− superoxide radicals and hydroxyl radicals, which directly react with organic dyes [40]. The proposed mechanism involving the reaction sequence is illustrated in Scheme 1.
4 Conclusion
This paper presents processes for the preparation of LDH nanoparticles modified with Fe, Ni, Cu and Co hydroxides using batch precipitation processes. The materials after modification with Ni(OH)2 exhibited high photocatalytic activity and high sorption capacity towards quinoline yellow. QY photocatalysis studies allowed the determination of QY removal efficiency over 99%. Cyclic analysis of the materials revealed a decrease in the sorption contribution to QY removal processes, increasing the photocatalytic activity. This demonstrates the applicability of Ni0.5–Zn–Al LDHs as photocatalysts in organic compound removal processes.
References
X. Feng, Z. Yu, R. Long, X. Li, L. Shao, H. Zeng, G. Zeng, Y. Zuo, Sep Purif Technol 253, 117525 (2020)
A.L. Nuzhdin, M.V. Bukhtiyarova, G.A. Bukhtiyarova, J. Chem. Technol. Biotechnol. 95, 3292 (2020)
I. Clark, R.L. Gomes, C. Crawshaw, L. Neve, R. Lodge, M. Fay, C. Winkler, M. Hull, E. Lester, React. Chem. Eng. 4, 663 (2019)
Z. Cao, B. Li, L. Sun, L. Li, Z.P. Xu, Z. Gu, Small Methods 4, 1900343 (2020)
R. Soltani, R. Pelalak, M. Pishnamazi, A. Marjani, A.B. Albadarin, S.M. Sarkar, S. Shirazian, Sci. Rep. 2021(11), 1 (2021)
R. Rojas, G. Mosconi, J.P. Zanin, G.A. Gil, Appl Clay Sci 224, 106514 (2022)
T. Hu, Z. Gu, G.R. Williams, M. Strimaite, J. Zha, Z. Zhou, X. Zhang, C. Tan, R. Liang, Chem Soc Rev 51, 6126 (2022)
E. Abdollahi, A. Heidari, T. Mohammadi, A.A. Asadi, M. Ahmadzadeh Tofighy, Sep Purif Technol 257, 117931 (2021)
Z. Yang, F. Wang, C. Zhang, G. Zeng, X. Tan, Z. Yu, Y. Zhong, H. Wang, F. Cui, RSC Adv. 6, 79415 (2016)
Q. Fang, S. Ye, H. Yang, K. Yang, J. Zhou, Y. Gao, Q. Lin, X. Tan, Z. Yang, J. Hazard Mater 420, 126569 (2021)
G. George, M.P. Saravanakumar, Environ. Sci. Pollut. Res. 2018(25), 30236 (2018)
B. Yuan, C. Zhang, Y. Liang, L. Yang, H. Yang, L. Bai, D. Wei, W. Wang, Q. Wang, H. Chen, Adv. Sustain. Syst. 5, 2000245 (2021)
Y. Zhang, J. Qin, X. Wang, Z. Chen, X. Zheng, Y. Chen, J. Environ. Manage 296, 113203 (2021)
A. A. Wani, A. M. Khan, Y. K. Manea, M. A. S. Salem, J. Rare Earths (2021).
T. Sadeghi Rad, A. Khataee, S. Sadeghi Rad, S. Arefi-Oskoui, E. Gengec, M. Kobya, Y. Yoon, Ultrason Sonochem 82, 105875 (2022).
A. F. da Silva, J. L. da S. Duarte, L. Meili, Sep Purif Technol 264, 118353 (2021).
A.F. Morais, I.G.N. Silva, B.C. Lima, F.A. Garcia, D. Mustafa, ACS Omega 5, 23778 (2020)
M. Serdechnova, A.N. Salak, F.S. Barbosa, D.E.L. Vieira, J. Tedim, M.L. Zheludkevich, M.G.S. Ferreira, J. Solid State Chem. 233, 158 (2016)
S. Chakraborty, I. Sarkar, A. Ashok, I. Sengupta, S.K. Pal, S. Chakraborty, Appl. Therm. Eng. 141, 339 (2018)
Y. Wang, S. Guo, X. Xin, Y. Zhang, B. Wang, S. Tang, X. Li, Appl. Surf. Sci. 549, 149108 (2021)
S.A. Kulkarni, S.-S. Feng, Pharm. Res. 2013(30), 2512 (2013)
S. Paikaray, M. A. Gomez, M. Jim Hendry, J. Essilfie-Dughan, Appl. Clay. Sci. 101, 579 (2014).
C. Tessier, L. Guerlou-Demourgues, C. Faure, A. Demourgues, C. Delmas, J. Mater. Chem. 10, 1185 (2000)
R. Qin, Y. Pan, Z. Duan, H. Su, K. Ren, W. Wang, Y. Li, N. Xi, Y. Wang, L. Zhang, S. Han, J. Electrochem. Soc. 168, 070539 (2021)
X.-R. Ma, X.-Y. Wei, R. Dang, W. Guo, Y.-H. Kang, X. Li, Y. Gao, J.-J. Bai, Y. Zhang, Z.-F. Zhang, Y.-J. Ma, Z.-M. Zong, Appl. Clay. Sci. 211, 106191 (2021)
M.M. Khan, N.H. Saadah, M.E. Khan, M.H. Harunsani, A.L. Tan, M.H. Cho, BioNanoScience 2019(9), 334 (2019)
V. Dubovoy, R. Subramanyam, M. Stranick, L. Du-Thumm, L. Pan, JoVE (J. Vis. Experim.) 2017, e55423 (2017)
L. Meili, P.V. Lins, C.L.P.S. Zanta, J.I. Soletti, L.M.O. Ribeiro, C.B. Dornelas, T.L. Silva, M.G.A. Vieira, Appl Clay Sci 168, 11 (2019)
R. Dang, X. Ma, J. Liu, L. Yan, W. Gao, J. Li, B. Chen, 24, 1 (2016). https://doi.org/10.1080/09276440.2016.1180733
A. Nait-Merzoug, O. Guellati, S. Djaber, N. Habib, A. Harat, J. El-Haskouri, D. Begin, M. Guerioune, Appl. Sci. 2021(11), 8899 (2021)
F. Zhang, L. Guo, S. Xu, R. Zhang, Langmuir 31, 6704 (2015)
X. Li, M. Fortunato, A.M. Cardinale, A. Sarapulova, C. Njel, S. Dsoke, J. Solid State Electrochem. 2021(1), 1 (2021)
M. Richetta, L. Digiamberardino, A. Mattoccia, P.G. Medaglia, R. Montanari, R. Pizzoferrato, D. Scarpellini, A. Varone, S. Kaciulis, A. Mezzi, P. Soltani, A. Orsini, Surf. Interface Anal. 48, 514 (2016)
A. Razzaq, S. Ali, M. Asif, S.-I. In, Catalysts 2020(10), 1185 (2020)
L.S. Liji, R. Mehedi, M. Malmivirta, P. Paturi, M. Lastusaari, M.M. Dîrtu, Y. Garcia, P. Fardim, Appl. Clay. Sci. 132–133, 641 (2016)
K. Abderrazek, F.S. Najoua, E. Srasra, Appl. Clay Sci. 119, 229 (2016)
L. Zhang, C. Hua Dai, X. Xiu Zhang, Y. Nian Liu, J. Hui Yan, Trans. Nonferrous Metals Soc. China (Engl Ed) 26, 2380 (2016).
X. Wang, P. Wu, Y. Lu, Z. Huang, N. Zhu, C. Lin, Z. Dang, Sep. Purif. Technol. 132, 195 (2014)
M. Qi, L. Fan, Y. Shen, H. Zou, X. Tian, D. Liu, S. Li, J. Nanosci. Nanotechnol. 18, 753 (2018)
Y. Sun, J. Li, L. Zhang, B. Jiang, X. Yang, N. Yang, F. Peng, M. Xu, X. Xiao, Sep. Purif. Technol. 259, 118116 (2021)
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Długosz, O., Banach, M. Synthesis of layered zinc-aluminium double hydroxides modified with metal ions as photocatalysts with enhanced sorption properties. Appl. Phys. A 128, 919 (2022). https://doi.org/10.1007/s00339-022-06045-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06045-3