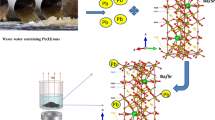
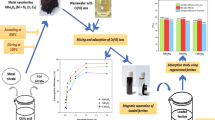
Abstract
Rare-earth doped calcium nano ferrites (CaFe2−x−yGdxSmyO4; x = y = 0.0; x = 0.025, y = 0.05) are synthesized by citrate nitrate auto combustion method. The prepared samples are characterized using X-ray diffraction (XRD), Fourier transform infra-red spectroscopy (FTIR), high-resolution transmission electron microscope (HRTEM), and vibrating sample magnetometer (VSM) analyses. After that, the samples were examined for Pb (II) ions removal from the water solution. The orthorhombic structure with space group Pnma is ratified from XRD. The average crystallite size of the synthesized powders is estimated from the broadening of the XRD lines in the range of 18–21 nm. FTIR confirms the molecular signature of the samples. The magnetic property is discussed based on super exchange interactions. The hysteresis loop shows two different magnetic behaviors of the investigated sample as well as increasing the coercivity from 14 to 240 G due to the doping. The electrical parameters are discussed in the basic hopping charge model, while the conduction mechanism of the samples will be discussed based on de-Boer and Verwey. The experimental results are subjected to kinetic and isotherm analyses. The Langmuir and Freundlich isotherms describe the adsorption of heavy metal ions as designated by the high correlation coefficient (R2). The use only 4 mg of the doped sample with low price ferrite nanoparticles has been utilized for 99.31% removal of Pb (II) from wastewater at a contact time of 10 min at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Innovative designs of nanocrystalline spinel ferrites have received much attention because of their exclusive properties related to their remarkable structure and outstanding electrical and magnetic properties. Furthermore, magnetic ferrites are of great interest due to their wide range of applications, such as the magnetically controlled transport of anti-cancer drugs [1, 2] and as contrast agents in magnetic resonant imaging (MRI) to detect cancer or other diseases [3]. Generally, magnetic nanostructures research has tremendously increased due to their specific applications in high-density magnetic storage, hyperthermia treatment and protein separation [4,5,6,7,8,9]. Magnetic nano ferrites are a group of ferrites with unique physical characteristics that make them competitive candidates for many biomedical applications such as detecting and diagnosing precancerous lesions; In addition, they are presently used for heavy metal removal from waste water and soil [7,8,9,10,11,12,13].
It was thought that magnetic ferrite composites can be frequently utilized to form cheap and highly controllable materials used in various applications of communications, radiofrequency devices, electronic appliances, solar cells, biosensors and microwave devices [14,15,16]. The development of such magnetic ferrite composites could conduce to the creation of a multi-functional devices promising for nano Opto-electromechanical systems, catalysts, memory storage, logic operations, and especially for multiple functional electronic devices that can be actively exchanged among transistor, inductor, capacitor and resistor to achieve multi-functions inside the same system [17, 18].
Nanocrystalline calcium ferrite (CaFe2O4) has a wide range of applicability, such as an anode material for the fabrication of electrodes in Lithium-ion batteries, p-type photocathode material, photo catalyst, drug delivery agent and gas sensors [19,20,21]. CaFe2O4 is an environmentally safe compound that is both thermally and chemically stable [22,23,24]. Another significant advantage is that it can be easily synthesized in the orthorhombic structure [25,26,27]. Most importantly, It is also an appropriate candidate for bone-like scaffolds manufacturing and hyperthermia treatment of cancer [28].
At low calcination temperature, calcium ferrite formed in the spinel structure which generally has the form AB2O4, described as a cubic closed-pack of oxygen anions [29]. These assemblies can be represented by the formula (Ax−1Bx) [AxB2−x] O4, where x denotes the inversion degree and equals zero and unity for the normal and inverse arrangements, respectively. At high calcination temperature, calcium ferrite formed in the orthorhombic structure where the large A-site cation (Ca) sits in a 12-fold site at the corners of the unit cell and the small B-site cation (Fe) is at the center of six octahedrally coordinated O atoms. Distortions from cubic symmetry, such as cation displacements within the BO6 octahedra and tilting of the octahedra, are common and result from electronic and structural considerations of the A and B-site elements. These distortions can be quantified by the Goldschmidt tolerance factor [30, 31].
Moreover, ferromagnetic and anti-ferromagnetic interactions are competing in-between Fe2+, Fe3+ and substituted cations. Therefore, electrical and magnetic properties originated from cation’s distribution at the two metallic sites as well as their charge, orbital and spin interactions. Additionally, the physical properties are greatly influenced by the synthesis route, which considerably controls the stoichiometry and morphology of the materials [32, 33].
The properties of the nano spinel ferrites are affected by the preparation conditions, calcination temperature and preparation methods. Several physical and chemical techniques have been developed for the preparation of stoichiometric and single phase spinel ferrite materials, e.g., spray pyrolysis, ball milling, sol–gel technique, hydrothermal method, co-precipitation method and citrate auto combustion technique [34,35,36,37,38,39]. The citrate auto-combustion technique among all available synthesis methods is intensively used for the preparation of homogeneous nano powders due to its advantages, i.e., high purity, crystallinity, great control of stoichiometry, low-cost, high yield, and effectiveness [40, 41].
It is well identified that RE ions can enter the spinel matrix up to a certain ratio, and its solubility limit is established by the following issues: (1) Relatively larger ionic radii of ions compared to the dimensions of A or B sites. and (2) the rate of cooling of the samples. The replacement of various cations of RE ions in spinel ferrites can also enhance the physical properties of the host spinel lattice [42, 43]. Among the RE elements, samarium (Sm) and gadolinium (Gd) have been widely studied due to their excellent mechanical hardness, chemical stability, and high magnetocrystalline anisotropy [44]. The substitution of Sm3+ and Gd3+ ions into the spinel ferrite would eventually bring structural distortion. Larger ionic radii of RE ions compared with Fe ions implies larger strain and as a result, the magnetic and electrical properties will be modified. Additionally, the interaction between the unpaired electrons in the 4f subshell of RE ions and that of the 3d subshell of Fe ions turns out the structural, magnetic and electrical properties [34]. Spinel ferrites substituted with rare-earth ions have been reported by numerous research groups as Almessiere et al. [45] and Slimani et al. [46].
In this work, we are doping CaFe2O4 with tiny amounts of rare earth RE elements, namely Sm and Gd, to enhance the physical properties of calcium ferrite nanoparticles. Boosting these structures properties will improve their qualifications and may open new areas of application. The chosen concentration was selected according to the previous work [47]. Moreover, if the concentration percentage of the doped RE ions increases over 3.75% in the B site of spinel nano ferrite, it will be indexed as a secondary phase. Additionally, the ad-sorption characteristics of Pb (II) ions on the CaFe2−x−yGdxSmyO4 were testified in a batch experiment at different ad-sorption parameters, the ad-sorption kinetics and ad-sorption isotherm for lead ions were determined. The obtained information will be useful for application in the treatment of wastewater containing lead ions.
2 Experimental
2.1 Sample preparation
Using the citrate auto-combustion method to achieve a relatively small particle size distribution of nanocrystalline calcium ferrite [47]. A schematic diagram of the citrate auto-combustion preparation method is shown in (Fig. 1), in this method, the stoichiometric quantities of calcium nitrate Ca(NO3)2.4H2O, ferric nitrate Fe(NO3)3.9H2O, gadolinium nitrate Gd(NO3)3.6H2O, and samarium nitrate Sm(NO3)3.6H2O were well mixed and dissolved in 20 ml of double distilled water with citric acid as a chelating agent. The solution was continuously stirred well with a magnetic stirrer until a homogenous solution is obtained. Then, the solution was stirred vigorously and heated for one hour till the solution turned into gel. Subsequently, the sample is heated until the gel is burned out and the sample is transformed into gray ash. Then, the gray ash is collected and grounded into fine powder. The powder is then calcined at 950 °C for 4 h in a furnace.
2.2 Characterization techniques
The structure and crystallite size were tested by X-rays diffractometer (XRD), Diano corporation with target Cu-Kα (λ = 1.5424 Å). The average nanoparticle size was estimated using Scherer's relationship. The surface composition and the chemical states of the elements in the prepared nano powders were studied by K-Alpha X-ray Photoelectron Spectrometer (XPS) System (Thermo Fisher Scientific, USA) using CASAXPS software.
The surface morphology or the microstructure was identified by a high-resolution tunneling electron microscope (model JEM-100S). The molecular signature of the samples was confirmed by Fourier transform infra-red spectrometer FTIR (IR-Affinity-1 FT-IR spectrometer) studies.
The saturation magnetization, the remanence and the coercive fields were measured by tracing M-H hysteresis loops for the powder samples at room temperature and in a varying magnetic field up to 20 kOe, using the LDJ model 9600, vibrating sample magnetometer (VSM). The surface area and pore volume were carried out using N2 adsorption/desorption isotherms at 77 K acquired with NOVA 2200 BET surface area analyzer.
2.3 Adsorption of heavy metals
The addition of 0.01 g of an adsorbent for 10 ml of a typical solution containing on lead ion with contraction 25 ppm and stirred at 200 rpm on electric shaker after adjusting pH at certain values for 1 h at room temperature. The concentration of heavy metal (HM) ions was measured by ICP for lead ions at pH extended from 2 to 8 [49].
3 Results and discussion
3.1 Structural analyses
3.1.1 X-ray analysis (XRD)
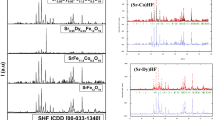
The nano ferrites CaFe(2-x–y)GdxSmyO4, x = y = 0; x = 0.025, y = 0.05, symbolized CaFeO and CaFeREO, respectively, are successfully obtained without any secondary phase even after the substitution of small amounts of soluble rare-earth (Gd-Sm) ions. The X-ray diffraction pattern of CaFeO and CaFeREO samples are recorded in the 2θ range 20–80º with a step size of 0.04º, as illustrated in (Fig. 2). The broadness of the peaks is a characteristic of nanoparticles and is indexed to the orthorhombic structure diffraction orthorhombic peaks with space group Pnma (No.62).
Noticeably, all peaks are well-matched with the standard CaFe2O4 spectrum (ICDD 00-008-0100), which has been allocated to the typical crystallographic planes of the high-purity calcium ferrite [50].
Generally, the calcium ferrite with the general formula CaFeO is described as a cubic spinel structure at low calcination temperature. The transformation from spinel structure to orthorhombic phase is due to the high calcination temperature (950 ºC), and no spherical nanoparticles are formed at this temperature.
The Williamson–Hall (W–H) plot method is utilized to define both the average crystallite size (D) and the micro strain (ε) for the synthesized samples [9, 51]. The average crystallite size and the strain can be calculated from XRD data using the following equation:
where β is the full-width half maximum in radian, θ is the Bragg’s angle in radian, λ is the wavelength of X-ray source, K is the shape factor approximately equals to 0.94. Consequently, the gradient (slope) of the fitted straight line and the y-intercept (\(\frac{\mathrm{K\lambda }}{D}\)) provide ε, and D respectively. The obtained data is shown in (Fig. 3) and tabulated in Table (1). The average crystallite size can be also estimated from the broadening of the XRD lines using the well-known Scherer as mentioned in the previous work [52, 53]. It is found as 21 and 18 nm for CaFeO and CaFeREO nanoparticles, respectively.
The experimental lattice parameters (aexp.) are computed based on orthorhombic unit cells, and tabulated in (Table 1). The large difference of ionic radius of Gd3+ (0.938 Å), Sm3+ (0.958 Å) and Fe3+ (0.645 Å) [54] lead to the increase in the lattice parameters.
The theoretical density Dx is determined and reported in (Table 1) using the following equation Dx = ZM/NAV [31, 55], where Z is the number of molecules per orthorhombic unit cell, M is the molecular weight, NA is the Avogadro’s number, and V is the unit cell volume. The data shows that the density Dx increases as a result of RE ions doping, which is directly explained by the dissimilarity of the average atomic mass numbers of Fe (55.847), with that of Gd (157.25), and Sm (150.36) [56].
The tolerance factor (T), for the orthorhombic structured materials, is calculated as mentioned in the previous work [31] and the results are displayed in (Table 1). Generally, the BO6 octahedra tilt cooperatively causes orthorhombic structure in such case the “T” can be written as 0.75 < T < 0.90. Whilst for 0.90 < T < 1.0, instead of buckling of BO6 a small distortion leads to a lower symmetry structure may be expected.
However, the perovskite oxides can be crystallized not only in orthorhombic but in hexagonal, rhombohedral, and monoclinic structures [57]. In the present case T > 1 indicates a hexagonal perovskite structure forming by neighboring hexagonal close-packed in the layers of Fe cations and O anions. The hexagonal structure is a variable of the oxygen packing in perovskite oxides leading to a structure with chains of face sharing BO6 octahedra. This can may be due to the increase of the A cation (Ca) size favors sharing its edges octahedrally, in addition to the B-site (Fe) distortion [58]
3.1.2 XPS spectroscopy
XPS spectroscopy is used to identify the valence states of the elements in the CaFeO and CaFeREO nano powders. The broad scanning of the XPS spectrum in (Fig. 4a) indicates the existence of Ca, Fe, Gd, Sm and O elements, which confirm the elemental surface composition for each of the two samples. The observed peak of C 1 s is attributed to the attaching of nano powder to an adhesive tape of carbon during measurement [59, 60].
XPS spectra of Ca 2p for CaFeO and CaFeREO samples are shown in (Fig. 4b). Calcium 2p peak is split into two components of 2p1/2 and 3p3/2 in addition to variable weak satellite loss peak. The split of Ca 2p peak is caused by the spin-orbital coupling while the shift in satellite peak of CaFeREO to lower binding energy is attributed to the change in the Plasmon energy loss [61, 62]. The peak of Ca 2p3/2 at 345.48 eV is attributed to Ca lattice which is indicated by the presence of oxygen vacancies in orthorhombic structure, whereas the peak of Ca 3p1/2 at 349.28 eV is attributed to surface bound Ca non lattice [63].
The broad peak of Fe ion in (Fig. 4c) may be raised from the spin–orbit interaction between the unpaired electrons in the 3d orbital shell and the core electrons in 2p of Fe ions. The two doublets spectrum of Fe atom are corresponding to the spin orbit of Fe 2p3/2 and Fe 2p1/2 valence states in both samples at 710.58 and 724.18 eV, respectively [62]. The Fe 2p3/2 peak is divided into two peaks revealing the existence of Fe2+ and Fe3+ ions of binding energies 710.08 and 711.78 eV, respectively [64]. Similarly, the Fe 2p1/2 peak is also divided into two peaks of Fe2+ at 727.08 eV and Fe3+ at 724.08 eV. The other two peaks located at 719.48 eV and 716.48 eV, are named the satellite peaks of Fe 2p1/2 for Fe2+ and Fe3+ ions, respectively. The absence of the corresponding satellite peak of the Fe 2p3/2 chemical state because it does not have satellite peaks [65]. Therefore, the existence of multivalent Fe ions together with the oxygen vacancies can be one of the primary factors to enhance the conduction process. On the other hand, as the ionic radius of Fe2+ is greater than that of Fe3+, then the partial substitution of Fe2+ destabilizes the orthorhombic structure of the samples through the oxygen deficiency and hence, the existence of Fe2+ instead of Fe3+ leading to the formation of hexagonal (metastable) phases under ambient conditions as will be clearly shown in HRTEM micrographs [64, 66].
XPS spectra of Sm 3d and, 4d Gd for CaFeREO sample are shown in (Fig. 4d). Gadolinium 4d spectrum provides two sets of peaks centered at 140.88 and 152.48 eV, that are associated with 4d5/2 and 4d3/2 peaks, respectively. Samarium 3d spectra also consist of two peaks that existed at 1082.5 and 1109 eV, which referred respectively to 3d5/2 and 3d3/2 oxidation states indicating the existence of Sm+3 ion in CaFeREO [67, 68].
The oxygen 1 s spectrum as displayed in (Fig. 4e) is deconvoluted into two main peaks at 529.08 eV and 531.08 eV. The O 1 s peak at lower binding energy is due to the trapped ionized atoms of oxygen, that are being individually in the grain boundary regions. While the O 1 s peak at higher binding energy is due to the interaction of oxygen atoms within the crystal lattice [64]. The conduction electrons created by that oxygen vacancies can be released and bind with Fe3+ ion to form Fe2+ states according to the Eq. (Fe3+ + eــ → Fe2+). Sup-peaks are observed in the XPS spectrum of the Oxygen 1S. The main peaks are split into two sub-peaks in CaFeO at 528.98 eV and 531.38 eV, indicating the presence of two types of oxygen species which assign to Fe–O and Ca–O bonds, respectively [69]. The O 1S sub-peak corresponding to the Fe–O bond in CaFeO exhibited a higher intensity compared with CaFeREO, which may be due to the filling of oxygen vacancies by RE ions in CaFeREO. As a result, the O 1S main peaks are splitting into four sub peaks and the appearance of two additional peaks at 533.08 eV (Gd–O bond) and 528.98 eV (Sm–O bond) in CaFeREO, indicating the presence of four types of oxygen species [70].
3.1.3 High resolution transmission electron microscope (HRTEM)
The HRTEM micrographs of the solution of the samples are shown in (Fig. 5). The micrographs reveal a uniform distribution of the particles of varying shapes, and well-defined boundaries. The corresponding particle size histograms of the samples are depicted in (Fig. 5c, f), which are obtained from the frequency sizes from the measurement of different particle sizes using ImageJ software [60]. The histogram depicts the size distribution of the nanoparticles in the range of 33–65 nm. The grains of the two samples are hexagonal, with a relatively lower agglomeration for the CaFeREO sample. Generally, the grains of the samples have a homogeneous distribution indicating a good physical mixing. There are three factors that led to the appearance of the hexagonal structure of the samples, the larger ionic radius of calcium ion in the B-site, the existence of Fe2+ instead of Fe3+ under ambient conditions as discussed in XPS analyses of the Fe 2p spectrum and the view plane of the crystal structure.
The HRTEM micrographs depend on the view plane of crystallography. For most cases, the features of orthorhombic structure can be identified by considering only hkl planes with small values of indices such as zero and one. Scarcely, the equilibrium structure shaping is contained faces with higher values of indices [71]. Figure 5g shows three prospective shapes of the crystal structure in different plane views which drew online using the SMORF crystal model depending on the calculated values of h, k and l from XRD [72]. The HRTEM micrographs of CaFeO and CaFeREO matched well with the view of the structure in the “bc” plane.
The high crystallinity of the samples is ratified from the typical lattice fringes as detected in (Fig. 5a). Moreover, the polycrystalline nature of the samples is identified through the selected area electron diffraction (SAED) pattern (Fig. 5e). The bright rings signify various diffraction planes in the unit cell.
3.1.4 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the parent and doped samples are testified in the 400–4000 cm−1 range as revealed in (Fig. 6). The bands in the wavenumber regions 580–440 cm–1 and 580–650 cm–1 are due to the deformation of FeO6 octahedral and the asymmetric stretching vibrations of Ca–O and Fe–O bonds respectively. The higher frequency band in the wavenumber region 650–745 cm–1 is assigned to a strong Fe–O stretching vibration mode, which involves internal motion of a change in Fe–O bond length. While, the lower band around 440 cm–1 corresponds to the bending mode, which is indicative of any changes in the Fe–O–Fe bond angle [73]. These findings confirm the formation of the calcium ferrite orthorhombic structure in accordance with the XRD data.
In the parent and doped samples, as shown in (Fig. 6), small bands at 3430 cm−1, matched to O–H stretching vibration. In addition, the bands at ≈ 1635 cm−1 correspond to the asymmetrical stretching of C–O [27]. These may be initiated from surface-adsorbed carbonate group species [74]. Since all the measurements are performed in ambient conditions, the existence of OH and CO functional groups in the spectra is predicted.
3.1.5 Energy dispersive X-ray analysis (EDAX)
EDAX analysis is employed to examine the configuration and stoichiometry of the CaFeO and CaFeREO nanoparticles (Fig. 7). Intense O, Ca,Fe, Sm and Gd peaks are observed, and the atomic ratio between Ca to Fe equals 2.01 (see inset in Fig. 7c. The successful synthesizing of the CaFeO and CaFeREO is confirmed.
3.1.6 Brunauer–Emmett–Teller (BET) surface area analysis
BET analysis includes the specific surface area, the pore size and the pore volume of the studied samples. The nature of the porous affects the crystallinity and the magnetic properties of the nanoparticles. (Fig. 8a, b) shows the variation of relative pressure (P/Po) with the volume for CaFeO and CaFeREO samples. The inset figures show the distribution of pore size versus pore volume of the synthesized samples. The investigated samples have a hysteresis loop characteristic, which is related to the capillary condensation/evaporation process in the mesopores [75].
Generally, the characteristic shape and size of the hysteresis loop are utilized to extrapolate the kind of pores encountered by the gas during a sorption technique. Therefore, the isotherm of ad-sorption/de-sorption of the samples in (Fig. 8) exhibits type IV behavior with H3-type hysteresis loop according to the IUPAC classifications [76]. The IV hysteresis is the characteristic of mesopores structures that have pore width intermediate between 2 and 50 nm [77].
The specific surface area, the pore volume and the pore width are calculated and tabulated in (Table 2). The surface area of CaFeREO are found to be significantly higher than CaFeO, which means that reactive sites increase inside the pores to adsorb organic pollutants of the waste water and hence increasing the photo/Fenton activity [9].
3.2 Magnetic properties
The magnetic hysteresis loop (M-H) allows us to accurately determine the magnetization behavior as well as the values of magnetic parameters like the saturation magnetization (Ms), the remnant magnetization (Mr) and the coercive field (Hc) [31]. The M˗H curves for the prepared nanoparticles CaFeO and CaFeREO are recorded and plotted in (Fig. 9) at room temperature.
It is clear from the figure that the CaFeO sample shows a paramagnetic trend due to the weak super exchange interaction between Fe ions inside the orthorhombic structure. It should be noted that the magnetic behavior of nano ferrite mainly depends on the microstructure so the transformation of the structure that occurs in the sample leads to a lack of the magnetization indicated by the paramagnetic phase [78].
The CaFeREO sample exhibits antiferromagnetic behavior, which is characterized by a lack of saturation S-shaped hysteresis loop. This shape can be attributed to the small loop area and the small values of magnetization. The substitution of various rare-earth ions (Gd3+, and Sm3+) at A and/or B sites in place of Fe3+ ions interrupts the A–O–B super exchange interactions and causes an increase of the coercive field as well as reduction of saturation magnetization.
The antiferromagnetic character of CaFeREO originates from the single-ion anisotropy and the mutual interactions between two magnetic sub lattices (electrons in a 4f subshell of rare earth ions and electrons in a 3d subshell of Fe ions) [79]. Furthermore, the antiferromagnetic alignment results from the coupling of iron and rare-earth cations via the π* orbitals of the oxygen ion. The magnetic moments of Gd and Sm are arranged not completely antiparallel but with a small angle to that of the nearest Fe as a result of the Dzyaloshinskii and Moriya antisymmetric exchange interaction [31]. However, this study shows that magnetization of RE doped calcium nanoparticles cannot be clarified on the basis of the magnetic moment of the individual ions. Additionally, the 3d–4f interaction along with the 3d–3d interaction in the ferrite will play an important to manipulating the magnetic properties of investigated samples.
The mixed-valence (Fe3+ and Fe2+) and the existence of Gd3+, Sm3+ can exhibit magnetic ordering, charge ordering, and orbital ordering. Some of the simplest magnetic orderings for B-site cation are shown in (Fig. 10) which represents one FM and four AFM (A-, C-, G-, and CE-type) ordering. The Sm3+ and Gd3+ RE ions also exhibit the Van Vleck effect due to the presence of multiplicity in both its first excited and ground spin states [13]. For the Gd3+(8.9 μB) ion, although the magnetic moment is high compared to the Fe3+ (5.9 μB) ion, numerous surface effects as spin pinning, spin canting, and disordered surface spins may be the main reason for the reduction of Ms as shown in (Table 3). The squareness ratio (MR/MS) is considered as a very significant parameter for determining the dimensional homogeneity of the nanoparticles. Its values for the loop of CaFeO is equal to 0.0007. This value is smaller than the super paramagnetic limit of 0.001 indicating the presence of multidomain and single-domain magnetic nanoparticles.
The variation of the Hc with particle size can be analyzed based on the anisotropy (K) of the crystal and domain structure as mentioned in the previous work [28]. From (Table 3), Hc increases from 14.323 G to 239.98 G for CaFeO and CaFeREO respectively which can be explained by the particle size and increasing magnetic anisotropy of CaFeREO. The increase in magnetocrystalline anisotropy resulted from the coupling of the spins of the Gd+3, Sm+3 and Fe+3 ions in the multi-domain regions of CaFeREO [80]. The coercivity Hc is seen to increase with the addition of Gd-Sm ions which specifies a stronger alignment of magnetic dipoles in define direction [81]. In other words, the reason for the highest coercivity has been explained in terms of the transition from paramagnetic to antiferromagnetic with magnetic multidomain as elucidated by Morrish and Yu [82, 83]. A recommended application is to achieve the high core loss which is desirable in some electrical appliances [84].
3.3 Electrical properties
Figure 11a, b shows the relation between the AC conductivity (lnσ) and the reciprocal of the temperature (1000/T) as a function of the applied frequencies. The samples exhibit a semiconducting nature, and the data obey the well-known Arrhenius equation [85]. The conduction mechanism of the samples will be elucidated by the de-Boer and Verwey models [86] mechanism, which includes electron exchange between the cations of the same element on the B site that exists randomly distributed in more than one valence state through the crystalline lattice. As cations are densely packed in the orthorhombic structure compared to cubic spinel structure, the distance between sequential Fe3+ ions is extremely small, leading to efficient electrons exchange [27]. Thus, the conduction in the samples is ascribed to electron hopping from Fe2+ to Fe3+ ions.
The activation energies (EI EII) are calculated using the Arrhenius equation and tabulated in (Table 4) [85]. Obviously, for the prepared samples, the EII at a high temperature is greater than the EI at a low temperature. That means more energy is needed to liberate the confined charges in the paramagnetic region to contribute to the conduction process.
The total conductivity σtotal at a given frequency can be written as the sum of DC conductivity σ0 due to band to band conduction and AC conductivity σac (ω) due to hopping conduction. Moreover, the σAC(ω) obeys the universal power-law
where ω is the angular frequency, A is a temperature-dependent constant, and s is an exponent whose value is in the range 0.0 ≤ s ≤ 1.0.
Figure 12a, b illustrates the dependence of ln σac versus frequency ln ω at numerous temperatures for the examined samples. The general characteristic of semiconductors is frequency-dependent. Their conductivity increases linearly with increasing frequency due to the increase in the hopping rate of the cation’s charge [87].
As the applied frequency increases, the net pumping force pushes the charge carrier between Fe2+ and Fe3+ ions to hop between the two different conduction states leading to improve the ion conduction process [88, 89].
Figure 13 shows the dependence of “s” exponent on absolute temperature (T) for the considered samples. For the CaFeO sample, the value of “s” initially increases slowly and then fluctuates at higher temperatures, whereas the CaFeREO sample shows an initial monotonic increase followed by a decrease of s value. The degree of correlation between AC conductivity and frequency is given by the exponents. The value of s defines the type of conduction mechanism that might change over the temperature range, as is evident for the investigated samples. Random hopping occurs when “s” equals zero; this means that AC conductivity is frequency independent. Small polaron (SP) and correlated barrier hopping (CBH) (Fe2+ ↔ Fe3+ + e) is usually related to an increase and decrease in “s” with temperature, respectively.
3.4 The removal of heavy metals
The removal of heavy metal (Pb2+) from an aqueous solution is detected using CaFeO and CaFeREO as ad-sorbent through a batch ad-sorption experiment. Various analytical parameters, including pH, the dosage of ad-sorbate and ad-sorption capacity are optimized. The removal efficiency [90] and the sorption capacity are calculated according to the following equations:
where Co and Cf are the initial and final concentration (mg/L) of lead ion solution, respectively. V is the volume of the aqueous phase (L) and m (g) is ad-sorbent’s mass.
3.4.1 Effect of pH for removal of Pb II ions
The removal efficiency of Pb II ions for CaFeO as a function of pH is illustrated in (Fig. 14). The investigational data designates that the ad-sorption of Pb (II) increases with changing pH from 2 to 8 at 30 ºC as illustrated in the graph. The maximum removal efficiency % η of Pb (II) at pH ≅ 7 is nearly 99.59%. The obtained results reveal that CaFeO can be considered an active adsorbent for Pb (II) removal in practical applications, where the Pb (II) concentration in wastewater is usually lower than 30 mg/L.
The minimum ad-sorption of Pb ions is detected at pH 2. This can be ascribed to the mobility, as well as the high content of hydrogen ions existent at lower pH, prefer the ad-sorption of H+ rather than Pb II ions. Consequently, with increasing pH, the available proton number decreases. This is due to the active sites turning more negatively and/or neutral charged. Though, the sorption of the metal ions that have a positive charge is increased through the electrostatic attraction force [91].
At pH \(>\) 7, the formation of hydroxyl complexes increases the ad-sorption activity as testified by Ali et al. [92]. Subsequently, the increase of participating metal ions in the ad-sorption process is detected which is not favorable for the efficiency of the process.
3.4.2 Effect of adsorbent dosage for removal efficiency η of heavy metals
The effect of magnetic CaFeO and CaFeREO dose on the η of pb (II) is determined using concentrations of 0.01 g to 0.06 g. The experiment is carried out under definite parameters (contact time ≅ 10 min, shaking speed ≅ 200 rpm, T ≅ 30 Co and pH ≅ 7). The heavy metal removal efficiency η as a function of ad-sorbent dosage is illustrated in (Fig. 15a).
The η at optimum ad-sorbent dosage (40 mg) of CaFeO, and CaFeREO are 96.32, and 99.31% respectively. The increase of the ad-sorbent dosage also increases the η of metal ions until an optimal dosage of 40 mg, after which the removal is more or less the same as detected from the obtained results. Increasing the percentage of ad-sorption with ad-sorbent dose is due to the increase in ad-sorbent surface area and the availability of more adsorption sites. The decrease of η for CaFeO after the optimal ad-sorbent dosage is due to overlapping of ad-sorption sites as a result of overcrowding of ad-sorbent particles [93].
On other side, the ad-sorbent dose studies after the addition of the rare-earth ions display an increase in η increasing the ad-sorbent dose compared to CaFeO. This is due to the presence of a tiny amount of rare-earth ions in the sample significantly reducing the agglomeration of the nano particles and providing greater surface area for ad-sorption. The accessibility of more ad-sorption sites with an increase in dosage results in more interactions occurring between CaFeREO with the negatively charged surface, and Pb (II) ions with + Ve charged [94].
3.4.3 Adsorption isotherms
Several isotherm models can be utilized to designate the distribution of Pb2+ ions between the solid phase and liquid phase as well as the efficiency of the ad-sorbent. The Langmuir and Freundlich models are the most testified models to investigate the sorption isotherm of Pb (II) ions as presented in (Fig. 16a, b) respectively. The adsorption process of Pb (II) on CaFeO and CaFeREO is clarified by the previous models [22]. The Freundlich and Langmuir equations can be expressed as [95]:
Langmuir isotherm
Freundlich isotherm:
where qe represents the amount of heavy metal ion (Pb2+) ad-sorbed by the ad-sorbent at equilibrium (mg/g); Ce is the equilibrium concentration of heavy metal ion (Pb2+) in the solution (mg/L); qmax denotes the maximum ad-sorption capacity corresponding to complete monolayer coverage on the whole ad-sorbent surface (mg/g); KL is the Langmuir ad-sorption equilibrium constant for the relative energy of ad-sorption (L/mg) and Kf and n are Freundlich constants associated with ad-sorption capacity and intensity of ad-sorption, respectively.
The Langmuir model is applied at particular binding sites on the homogenous ad-sorbent surface where a mono layer of ad-sorbed Pb2+ ions is wrapped completely on the surface of the ad-sorbent. Additionally, no interaction can occur between the ad-sorbed Pb2+ molecules on the ad-sorbent surface [96, 97]. This model describes the ad-sorption of heavy metal ions as designated by the high correlation coefficient (R2) of 0.981 and 0.999 for CaFeO and CaFeREO respectively.
The Langmuir constants, qmax and KL values are attained from the slope and the intercept of the linear scheme of Ce/qe against Ce as shown in (Fig. 16a). The obtained values are 22.49 mg/g, 0.148 L/mg for CaFeO, and 27.47 mg/g, 0.079 L/mg for CaFeREO respectively. The higher value of qmax signifies the higher Langmuir monolayer ad-sorption capacity of CaFeREO. While a lower KL value for CaFeREO implies the lower surface energy in the process.
Freundlich model is designated the process of chemical ad-sorption on multilayer physisorption in the heterogeneous surfaces [98, 99] by the high correlation coefficient (R2) of 0.998 and 0.999 for CaFeO and CaFeREO respectively.
Freundlich constants, Kf and n values are obtained from the slope and the intercept of the linear plot of log qe versus log Ce as illustrated in (Fig. 16b) and found to be 35.69 mg/g, 1.059 for CaFeO and 36.06 mg/g, 0.996 for CaFeREO, respectively. The great ad-sorption capacity of the ad-sorbent towards Pb2+ is ratified from the obtained results.
The High value of Kf and the low value of n for CaFeREO indicate the higher Freundlich multilayer ad-sorption capacity and the higher intensity of ad-sorption respectively. The preference of ad-sorption and heterogeneity degree on the surface of the ad-sorbent can be identified by the magnitude of 1/n. The increase of 1/n for CaFeREO ratifies the high sorption capacity and the formation of new ad-sorption sites [100].
The obtained results show that the CaFeO and CaFeREO have a good fit with the Freundlich and Langmuir models. The high correlation coefficients of the samples indicate that the entire isotherm models agree well with the results and hence a combination of homogenous monolayer and multilayer is convenient for the two samples. The obtained data indicate that the CaFeREO is a promising and better ad-sorbent for Pb (II) ions. The use of only 4 mg of CaFeREO with low price ferrite nanoparticles has been utilized for 99.31% removal of Pb (II) from wastewater at a contact time of 10 min at room temperature. This is the prime novelty of the investigation.
4 Conclusion
CaFe2−x−yGdxSmyO4 (with x = y = 0.0; x = 0.025, y = 0.05) nanoparticles were successfully synthesized in orthorhombic structure using citrate auto combustion method. The preparation of the samples in a single phase is ratified from XRD data. HRTEM micrographs show that the particle size is in the range of 33–65 nm. The parent nanoparticles exhibit paramagnetic nature at room temperature with low remanence while antiferromagnetic nature is manifested after doping by the rare-earth ions. The obtained data indicate that the CaFeREO is a promising and better ad-sorbent for Pb (II) ions. The use of only 4 mg of CaFeREO with low price ferrite nanoparticles has been utilized for 99.31% removal of Pb (II) from wastewater at a contact time of 10 min.
References
M. Vallet-Regí, J. Chem. Soc. Dalton Trans. (2001). https://doi.org/10.1039/b007852m
M. Bañobre-López, A. Teijeiro, J. Rivas, Rep. Pract. Oncol Radiother: J. Greatpoland. Cancer. Center. Poznan. Polish. Soc. Radiat. Oncol. 18, 397–400 (2013)
S.P. Mornet, S.B. Vasseur, F. Grasset, E. Duguet, J. Mater. Chem. 14, 2161–2161 (2004)
C. Cannas, A. Ardu, D. Peddis, C. Sangregorio, G. Piccaluga, A. Musinu, J. Colloid. Interface. Sci. 343, 415–422 (2010)
S. Laureti, G. Varvaro, A.M. Testa, D. Fiorani, E. Agostinelli, G. Piccaluga, A. Musinu, A. Ardu, D. Peddis, Nanotechnology 21, 31,5701 (2010). https://doi.org/10.1088/0957-4484/21/31/315701
T.E. Torres, A.G. Roca, M.P. Morales, A. Ibarra, C. Marquina, M.R. Ibarra, G.F. Goya, J. Phys: Conf. Ser. 200, 072101–072101 (2010)
A.R. Chavan, P.P. Khirade, S.B. Somvanshi, S.V. Mukhamale, K.M. Jadhav, J. Nanostructure. Chem. 11, 469–481 (2021)
P.P. Khirade, A.R. Chavan, S.B. Somvanshi, J.S. Kounsalye, K.M. Jadhav, Mater. Res. Express 7, 116102 (2020)
M. George, T.L. Ajeesha, A. Manikandan, A. Anantharaman, R.S. Jansi, E.R. Kumar, Y. Slimani, M.A. Almessiere, A. Baykal, J. Phys. Chem. Solids 153, 110010 (2021)
H. Tombuloglu, G. Tombuloglu, Y. Slimani, I. Ercan, H. Sozeri, A. Baykal, Environ. Pollut. 243, 872–881 (2018)
A.D. Korkmaz, S. Güner, Y. Slimani, H. Gungunes, M. Amir, A. Manikandan, A. Baykal, J. Supercond. Novel Magn. 32, 1057–1065 (2018)
E.E. Ateia, A.T. Mohamed, J. Inorg. Organomet. Polym. Mater. 32, 1389–1399 (2022)
T. Ajeesha, A. A, A. Mary George, J.A. Manikandan, Y. Mary, M.A. Slimani, A.B. Almessiere, Physica. B: Condensed. Matter. 606, 412660 (2021)
Y. Slimani, M.A. Almessiere, S. Güner, N.A. Tashkandi, A. Baykal, M.F. Sarac, M. Nawaz, I. Ercan, J. Mater. Sci: Mater. Electron. 30, 9143–9154 (2019)
I. Bibi, M. Muneer, M. Iqbal, N. Alwadai, A.H. Almuqrin, A.S. Altowyan, F.H. Alshammari, A.S. Almuslem, Y. Slimani, Ceram. Int. 47, 31518–31526 (2021)
M. Aamir, I. Bibi, S. Ata, F. Majid, N. Alwadai, A.H. Almuqrin, H. Albalawi, Y. Slimani, M. Bashir, M. Iqbal, Results. Phys. 23, 104023 (2021)
Y. Slimani, M.A. Almessiere, S.E. Shirsath, E. Hannachi, G. Yasin, A. Baykal, B. Ozçelik, I. Ercan, J. Magn. Magn. Mater. 510, 166933 (2020)
S. Noreen, K. Khalid, M. Iqbal, H.B. Baghdadi, N. Nisar, U.H. Siddiqua, J. Nisar, Y. Slimani, M.I. Khan, A. Nazir, Environ. Technol. Innov. 22, 101430 (2021)
N.H. Sulaiman, M.J. Ghazali, B.Y. Majlis, J. Yunas, M. Razali, Bio-Med. Mater. Eng. 26, S103–S110 (2015)
L. Khanna, N.K. Verma, S.K. Tripathi, J. Alloy. Compd. 752, 332–353 (2018)
N.H. Sulaiman, M.J. Ghazali, J. Yunas, A. Rajabi, B.Y. Majlis, M. Razali, Ceram. Int. 44, 46–50 (2018)
M.J. Pirouz, M.H. Beyki, F. Shemirani, Food Chem. 170, 131–137 (2015)
X. Liu, J. Jiang, Y. Jia, A. Jin, X. Chen, F. Zhang, H. Han, J. Energy Chem. 25, 381–386 (2016)
X. Liu, Y. Zhang, Y. Jia, J. Jiang, Y. Wang, X. Chen, T. Gui, Chin. J. Catal. 38, 1770–1779 (2017)
A. Sutka, M. Kodu, R. Pärna, R. Saar 224, 260–265 (2016)
A.C. Gandhi, R. Das, F.-C. Chou, J.G. Lin, J. Phys.: Condens. Matter 29, 175802–175802 (2017)
A. Manohar, C. Krishnamoorthi, J. Alloy. Compd. 722, 818–827 (2017)
E.E. Ateia, G. Abdelatif, F.S. Soliman, J. Mater. Sci.: Mater. Electron. 28, 5846–5851 (2017)
B. Issa, I.M. Obaidat, B.A. Albiss, Y. Haik, Int. J. Mol. Sci. 14, 21266–21305 (2013)
R. Mitchell, M. Welch, A. Chakhmouradian, Mineral. Mag. 81, 411–461 (2017)
M.A. Ahmed, N.G. Imam, M.K. Abdelmaksoud, Y.A. Saeid, J. Rare. Earths. 33, 965–971 (2015)
D. Jiles, Recherche. 67, 2–2 (1998)
A. Goldman, Handbook of modern ferromagnetic materials, 1st edn (Springer Science+Business Media, New York, 1999)
A.B. Kadam, V.K. Mande, S.B. Kadam, R.H. Kadam, S.E. Shirsath, R.B. Borade, J. Alloy. Compd. 840, 155669 (2020)
S. Xavier, S. Thankachan, B.P. Jacob, E.M. Mohammed, J. Nanosci. 2013, 1–7 (2013)
M.A. Almessiere, Y. Slimani, S. Güner, A. Baykal, I. Ercan, J. Rare. Earths. 37, 871–878 (2019)
Y. Slimani, M.A. Almessiere, M. Sertkol, S.E. Shirsath, A. Baykal, M. Nawaz, S. Akhtar, B. Ozcelik, I. Ercan, Ultrason. Sonochem. 57, 203–211 (2019)
S. Akhtar, S. Rehman, M.A. Almessiere, F.A. Khan, Y. Slimani, A. Baykal, Nanomaterials 9, 1635 (2019)
M.A. Almessiere, Y. Slimani, H. Güngüneş, S. Ali, A. Manikandan, I. Ercan, A. Baykal, A.V. Trukhanov, Nanomaterials 9, 820 (2019)
C. Murugesan, M. Perumal, G. Chandrasekaran, Physica B 448, 53–56 (2014)
M. Biswas, P.K. Ojha, C. Durga, N. Gokhale, C.S. Suresh, Mater. Sci. Appl. 03, 110–115 (2012)
M.A. Almessiere, Y. Slimani, M. Sertkol, F.A. Khan, M. Nawaz, H. Tombuloglu, E.A. Al-Suhaimi, A. Baykal, Ceram. Int. 45, 16147–16156 (2019)
D.B. Pawar, P.P. Khirade, V. Vinayak, L.S. Ravangave, S.M. Rathod, Appl. Sci. 2, 1–2 (2020)
N. Shehata, K. Meehan, Potential applications of samarium as a dopant element, 111–135 (2014)
M.A. Almessiere, Y. Slimani, A.V. Trukhanov, A. Baykal, H. Gungunes, E.L. Trukhanova, S.V.C. Trukhanov, V.G. Kostishin, J. Ind. Eng. Chem. 90, 251–259 (2020)
Y. Slimani, B. Unal, M.A. Almessiere, A.D. Korkmaz, S.E. Shirsath, G. Yasin, A.V. Trukhanov, A. Baykal, Results. Phys. 17, 103061 (2020)
E.E. Ateia, G. Abdelatif, F.S. Soliman, J. Mater. Sci: Mater. Electron. 28, 5846–5851 (2017)
E.E. Ateia, B. Hussein, C. Singh, M.M. Arman, Eur. Phys. J.Plus. 137(443), 1–11 (2022)
M.D. Yahya, K.S. Obayomi, M.B. Abdulkadir, Y.A. Iyaka, A.G. Olugbenga, Water. Sci. Eng. 13, 202–213 (2020)
B. Post, X-Ray Spectrom. 4, A18–A18 (1975)
E.E. Ateia, A.T. Mohamed, M. Maged, A. Abdelazim, Appl. Phys. A. (2020). https://doi.org/10.1007/s00339-020-03841-7
E.E. Ateia, K. Meleka, F. Ghobrial, Appl. Phys. A. (2021). https://doi.org/10.1007/s00339-021-04974-z
E.E. Ateia, M.K. Abdelmaksoud, H. Ismail, J. Mater. Sci.: Mater. Electron. 32, 4480–4492 (2021)
R.D. Shannon, Acta. Crystallogr. A 32, 751–767 (1976)
M.A. Ahmed, N. Okasha, R.M. Kershi, J. Magn. Magn. Mater. 320, 1146–1150 (2008)
J.E. Sansonetti, W.C. Martin, J. Phys. Chem. Ref. Data 34, 1559–2259 (2005)
D. Andrault, G. Fiquet, M. Kunz, F. Visocekas, D. Häusermann, Science 278, 831–834 (1997)
S. Švarcová, K. Wiik, J. Tolchard, H.J.M. Bouwmeester, T. Grande, Solid. State. Ionics. 178, 1787–1791 (2008)
Q. Tang, X. Zhu, Nanomaterials (Basel Switzerland) 12, 575 (2022)
D.O. Morais, A. Pancotti, G.S. de Souza, M.V. Saivish, A. Braoios, M.L. Moreli, M.V.B. Souza, V.G. da Costa, J. Wang, J Mater Sci Mater Med 32, 101 (2021)
B. Ghosh, A. Dutta, S. Shannigrahi, T.P. Sinha, J. Mater. Sci. 49, 819–826 (2013)
K. Eid, E.K. Abdel-Khalek, M.A. Motawea, A.A. Mohamed, H.H. El-Bahnasawy Res. Sq. (2022)
M. Sezer, I. Oztoprak, A.Ş. Ahsen, A. Oral, A. Buyukaksoy, J. Electrochem. Soc. 167 (2020) 124508. https://doi.org/10.1149/1945-7111/abaceb
T. Patri, P. Justin, P.D. Babu, A. Ghosh, Appl. Phys. A. (2019). https://doi.org/10.1007/s00339-019-2516-x
M. Muhler, R. Schlögl, G. Ertl, J. Catal. 138, 413–444 (1992)
V.K. Malahalli, K. Kuribayashi, K. Kitazono, J. Am. Ceram. Soc. 92, 903–910 (2009)
S. Singh, P. Kaur, V. Kumar, K.B. Tikoo, S. Singhal, J. Rare Earths 39, 781–789 (2021)
F.-H. Chen, J. Her, S. Mondal, M.-N. Hung, T.-M. Pan, Appl. Phys. Lett. 102, 193515 (2013)
A. Bajorek, B. Liszka, B. Szostak, M. Pawlyta, J. Magn. Magn. Mater. 503, 166634 (2020)
H. Kim, H.J. Yun, B.J. Choi, RSC Adv. 8, 42390–42397 (2018)
G. Barmparis, Z. Łodziana, N. López, I. Remediakis, Beilstein J. Nanotechnol. 6, 361–368 (2015)
SMORF (2014)
S. Farhadi, Z. Momeni, M. Taherimehr, J. Alloy. Compd. 471, L5–L8 (2009)
F.J. Owens, C.P. Poole, The physics and chemistry of nanosolids (Wiley, USA, 2008)
A. Grosman, C. Ortega, Langmuir: ACS J. Surf. Coll. 21, 10515–10521 (2005)
Z. Alothman, Materials 5, 2874–2902 (2012)
K. Ariga, A. Vinu, Y. Yamauchi, Q. Ji, J. Hill, Bull. Chem. Soc. Jpn. 85, 1–32 (2012)
A. Samariya, S.N. Dolia, A.S. Prasad, P.K. Sharma, S.P. Pareek, M.S. Dhawan, S. Kumar, Curr. Appl. Phys. 13, 830–835 (2013)
L. Néel, Proc. Phys. Soc. Sect. A 65, 869–885 (1952)
V. Jagadeesha Angadi, B. Rudraswamy, K. Sadhana, S.R. Murthy, K. Praveena, J. Alloy. Compd. 656, 5–12 (2016)
Y. Matsuura, N. Kitai, S. Hosokawa, J. Hoshijima, J. Magn. Magn. Mater. 411, 1–6 (2016). https://doi.org/10.1016/j.jmmm.2016.03.007
A.H. Morrish, S.P. Yu, Phys. Rev. 102, 670–673 (1956)
A.H. Morrish, S.P. Yu, J. Appl. Phys. 26, 1049–1055 (1955)
J.M. Hastings, L.M. Corliss, Rev. Mod. Phys. 25, 114–119 (1953)
L. John Berchmans, R. Sindhu, S. Angappan, C.O. Augustin, J. Mater. Process. Technol. 207, 301–306 (2008)
E.J.W. Verwey, J.H. de Boer, Recl. Trav. Chim. Pays-Bas 55, 531–540 (1936)
S.S. Ata-Allah, F.M. Sayedahmed, M. Kaiser, A.M. Hashhash, J. Mater. Sci. 40, 2923–2930 (2005)
M.A. Ahmed, A.A. Azab, E.H. El-Khawas, E.A. El Bast, Synth React. Inorg, Metal-Organic, Nano-Metal Chem. 46, 376–384 (2016)
R.M. Kershi, F.M. Ali, M.A. Sayed, J. Adv. Ceram 7, 218–228 (2018)
G. Majeed (2017) 903–908.
K.G. Akpomie, F.A. Dawodu, K.O. Adebowale, Alex. Eng. J. 54, 757–767 (2015)
I.H. Ali, M.K. Al Mesfer, M.I. Khan, M. Danish, M.M. Alghamdi, Processes 7(4), 217 (2019). https://doi.org/10.3390/pr7040217
W.A. Khoso, N. Haleem, M.A. Baig, Y. Jamal, Sci Rep 11, 3790 (2021)
M. Kaur, M. Kaur, D. Singh, A.C. Oliveira, V.K. Garg, V.K. Sharma, Nanomaterials 11(6), 1471 (2021). https://doi.org/10.3390/nano11061471
C.V. Tran, D.V. Quang, H.P. Nguyen Thi, T.N. Truong, D.D. La, ACS. Omega. 5, 7298–7306 (2020)
S.K. Maji, S.-W. Wang, C.-W. Liu, Desalin. Water Treat. 51, 7775–7790 (2013)
F. Haghseresht, G.Q. Lu, Energy. Fuels. 12, 1100–1107 (1998)
N. Ayawei, A.N. Ebelegi, D. Wankasi, J. Chem. 2017, 3039817 (2017)
E. E. Ateia, Kareem Elsayed, Rania Ramadan, Journal of Inorganic and Organometallic Polymers and Materials, published online 28 April 2022
J.Q. Jiang, C. Cooper, S. Ouki, Chemosphere 47, 711–716 (2002)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saeid, Y.A., Ateia, E.E. Efficient removal of Pb (II) from water solution using CaFe2−x−yGdxSmyO4 ferrite nanoparticles. Appl. Phys. A 128, 583 (2022). https://doi.org/10.1007/s00339-022-05718-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05718-3