Abstract
This study was carried out to examine the mechanical properties of hypereutectic Al–16% Si alloy with the introduction of zinc oxide (ZnO) nanoparticles (NPs) synthesized by simple sol–gel method. The microstructure of the synthesized ZnO NPs was characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The microstructure of the synthesized ZnO NPs reflects the formation of the wurtzite-type ZnO and the average crystallite sizes around 35 nm. Hypereutectic Al–16% Si with varies concentration of ZnO NPs (0.5, 1, 1.5 and 3%) was synthesized utilizing permanent mold casting technique. Energy-dispersive spectrometer (EDS) and scanning electron microscopy (SEM) of hypereutectic Al–16 wt% Si alloys after the dispersion of these nanoparticles were analyzed. The results revealed that an addition of ZnO nanoparticle to hypereutectic Al–16wt % Si alloy enhanced the tensile strength, impact energy and the hardness. The maximum tensile strength, impact energy and hardness of this alloy was 200 MPa, 5.38 J, 60 BHN, respectively, which occurred at 3 wt% of ZnO NPs. This enhancement in mechanical properties can attributed to the decomposition and dissolution of the nano-oxide particles in aluminum alloy melt and grain growth restriction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypereutectic Al–Si alloys have a good castability, high stiffness, best temperatures strength, high electrical conductivity, good corrosion, and wear resistance so was most widely utilized in the industrial applications especially in the engine components such as head cylinder, valves, engine block, pistons and in electrical applications such as the electrical transmission lines [1,2,3,4,5]. Hypereutectic Al-Si alloys have a wide range of use and therefore we must work to improve the mechanical properties. The examination of addition of nanoparticles on the microstructure and the mechanical properties of this alloy is important. Among nanoparticles, zinc oxide (ZnO) is considered a semiconductor material, self-activated crystal, and multi-functional because of low dielectric constant, high catalytic activity, more economical and wide band gap around 3.37 eV with high exciting binding energy [6,7,8]. Therefore, this is used in field of nanotechnology science to improve material operation and machines in the nanometer scale by various techniques; in addition, it is used in solar cell, solid-state gas sensor, nanowire, electronic nanodevices, UV lasing diode, nanoresonators, and piezoelectric transducers [9,10,11,12]. Raghukiran et al. [13] studied the effect of the scandium addition on the mechanical and wear behavior of Al–x%Si–0.8Sc alloys. The results show the Al–x%Si–0.8Sc alloys give higher tensile strength and flow stress, and an improvement in the wear behavior at low concentration ratio of silicon for ternary alloys. In addition, the effect of scandium on hardening and composition of the Al–Ca eutectic alloys were investigated by Blow et al. [14]. It was found the scandium addition to this alloy does not leave an influence on the strengthening. Hengcheng et al. [15] investigated the effect of an addition of strontium on grain structure of Al–13 wt% Si alloy. It was observed that the eutectic grain structure was refined with an addition in Sr and the faster cooling rate leads to the increasing in eutectic nucleation rate. El-Mahallawi et al. [16] used three types of nanoparticles such as (Al2O3), titanium dioxide (TiO2) and zirconia (ZrO2) to improve the properties of A356 aluminum alloys. There is an enhancement in the mechanical properties with an addition of nanoparticles of Al2O3, TiO2, and ZrO2 at stirring temperature and speed 600 °C, 1500 rpm, respectively. The microstructure of aluminum alloy (A356) is transformed to more fine structure and improved mechanical properties due to introduction nano-oxide particles (ZnO,~ 20 nm in size) [17]. Qasim et al. [18] investigated the mechanical properties of Al 356 casting alloys filled by ZnO nanorods. It was indicated that mechanical properties such as tensile strength, hardness, and ductility were good enhanced significantly after adding the nanorods ZnO to Al alloys. Jabeera et al. [19] investigated the metallurgical properties of the aluminum zinc alloy anodes reinforced with the ZnO nanoparticles. An enhancement in the galvanic performance and metallurgical properties of the Al alloy anodes was observed. The improvement in the mechanical properties depends on the dispersion of NPs through Al matrix. The main reason for the improvement of the mechanical properties of aluminum alloys due to the introduction of nanoparticles can be attributed to the restriction of aluminum growth during solidification [20]. This work focuses on studying the mechanical properties of hypereutectic Al–16% Si alloy based on zinc oxide (ZnO) NPs synthesized by simple sol–gel method with varied concentrations 0.5, 1, 1.5, and 3 wt%. In addition, the dependency of aluminum growth on ZnO NP concentration was examined. The correlation between mechanical properties and the microstructure is investigated through study.
2 Experimental procedure
2.1 Zinc oxide nanocrystalline synthesis
ZnO nanocrystalline powder has been synthesized using sol–gel mechanism. Materials used in synthesization include zinc acetate dehydrate [Zn(CH3COO)2⋅H2O], isopropanol and diethanolamine [HN(CH2-CH2OH)2, DEA]. The reagents, provided in analytical grade, were utilized without further purification. First, solution which consists of 6.585 g of Zn(CH3COO)2⋅H2O and 75 mL of isopropanol was stirred at 60 °C for 1 h. Then DEA with molar ratio three times that of Zn(CH3COO)2⋅H2O was added gradually to the obtained solution and kept on the magnetic stirrer for another hour at the same temperature. The solution becomes clear and homogeneous gel after 2 min from adding DEA. With a view to achieve the final ZnO nanocrystalline powder, the obtained gel was refluxed for 1 h at 140 °C and then the result was calcined at 600 °C for 6 h.
The crystal structure of the synthesized ZnO powder was examined by means of X-ray diffraction (XRD) using Philips computerized diffractometer of type XPERT-MPDUC PW 3040 with CuKα radiation source of wavelength λ = 0.15406 nm, at a power of 1600 W (40 kV and 40 mA). The microstructure of the synthesized ZnO NP powder was investigated by high-resolution transmission electron microscope HR-TEM of model JEM-2100 with acceleration voltage up to 200 kV.
2.2 Preparation of nanocomposite
Hypereutectic Al–16% Si alloy was utilized as a base material in this study. Zinc oxide nanopowders were used as reinforcement phase at different weight concentration from 0.5 to 3%. The chemical composition analysis of the base material is summarized in Table 1 [20].
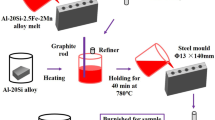
Permanent mold casting technique (PM) was used to produce the hypereutectic Al–16 Si %. The more details about the casting procedure and the cooling conditions were presented in the previous work [20]. The concentrations of ZnO NPs in investigated alloy were 0.5, 1.0, 1.5 and 3 wt%. The desired amount Al–16% Si alloy and synthesized ZnO NPs were in total mass 1 kg. The wanted mass of hypereutectic Al–16% Si alloy was placed into the copper crucible of the electrical furnace after cutting it into small pieces and primates to melt inside the furnace up to 710 °C. Then ZnO nanoparticles with different weight percent was added to the Al–16 Si % alloy in the liquid or semisolid state at 750 °C and mixing with Al–16 Si alloy molten into the copper crucible and stirred manually for 5 min after the addition of the ZnO nanoparticles. Finally, the melt was pouring manually inside the metal mold. Al–Si alloy specimens before and after ZnO nanoparticles addition samples were cutting according to ASTM standard.
The microstructure of the hypereutectic Al–16% Si alloy with and without ZnO nanoparticles was analyzed using a JEOL 6060 scanning electron microscope coupled with an energy-dispersive spectroscopy (EDS) chart.
2.3 Mechanical testing
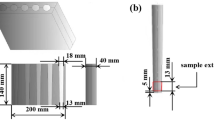
Tensile test was performed using the universal testing machine (TQSM 1000), according to ASTM D638 standard to determine the tensile strength of the hypereutectic Al alloy without and with ZnO nanoparticles addition from 0.5 to 3 wt% at room temperature. The dimension of the sample was 165 mm length; 19 mm width and 5 mm thickness, Fig. 1. For accurate results, four measurements were done for each sample and the average final tensile strength was calculated.
Brinell hardness testing machine utilizing a 10-mm-diameter hardened steel ball and 125 kgf applied load carried out the hardness test. The toughness of Al alloy reinforced by ZnO nanoparticles was obtained by examining the impact energy of this alloy using Charpy impact tester according to ASTM D256 standard. The specimen used in this test was (15 × 15), thickness 4 mm and the pre-crack length was 2 mm.
3 Results and discussion
3.1 Structure of the obtained ZnO NPs
Figure 2 shows XRD pattern of the synthesized ZnO powder. The XRD pattern of ZnO powder detects the formation of pure ZnO with wurtzite structure type based on standard crystallographic data in the reference pattern (JCPDS 36-1451). The crystallite size (D) of the synthesized powder was evaluated from X-ray line broadening using Scherrer’s equation [21, 22]: \(D\,=\,0.9\,\lambda \,/\beta \,\cos \,\theta\), where λ, β and θ are the X-ray wavelength, the full width at half maximum (FWHM) of the diffraction peak and the Bragg diffraction angle, respectively. The average-evaluated D values for all recorded diffraction lines is 32.5 nm and the wurtzite lattice parameters a and c are 0.3242 nm and 0.5195 nm, respectively, which are close to the standard data (a = 0.32488 nm and c = 0.52066 nm) card no JCPDS-36-1451.
Figure 3 shows TEM image of the synthesized ZnO NPs. Figure 3 reflects that the obtained shape of the synthesized ZnO is hexagonal shape with large uniformity.
3.2 Microstructure
Figure 4a–d shows the influence of zinc oxide nanoparticle addition on the microstructure of hypereutectic Al–16% Si alloy, analyzed by the SEM micrographs. It was found that from Fig. 4a, there are large voids, and cracks in the base material (without ZnO nanoparticles). The introduction of ZnO NPs into hypereutectic Al matrix may reduce these voids and cracks in this alloy. The introduction of ZnO NPs in Al–16% Si alloy leads to decrease the grain size of primary Al as shown in Fig. 4a–d. This decrease in grain size was highly observed at high concentration of NPs. The Al–16 Si % alloy was refined in the microstructure by reaction between ZnO NPs and Al, so the strength of the grain boundaries may be enhanced. NPs with high surface area have high reaction with aluminum due to high interfacial zone between them. For high concentration of ZnO NPs (3%), the reaction reaches high value so this sample has highest refinement in microstructure. The refinement of Al by addition of varied elements can be attributed to growth restriction factor and heterogeneous nucleation [17, 18]. More deeply, the addition of ZnO NPs to solution of Al–16 Si % alloy at high temperature leads to decomposition of ZnO NPs to Zn and O. Almost all oxygen after decomposed from ZnO NPs was combined with aluminum oxide. The presence of oxygen decomposed from ZnO NPs at interface between liquid and solid of Al through growth of primary aluminum alloy leads to restrict growth. The restriction of aluminum growth during solidification can be attributed to diffusion of solute Zn at interfacial between solid and liquid of aluminum. In addition, both oxygen and Zn resulted from decomposition are considerable for heterogeneous nucleation of primary aluminum grain.
Figure 5a, b shows SEM and EDX for sample with 3% ZnO NPS. The formation of Al2O3, found in eutectic region, indicates the restriction of growth. The presence of zinc metal and absence of zinc oxide from EDX chart are a clear evidence of decomposition of zinc oxide.
3.3 Mechanical behavior
Mechanical behavior of the hypereutectic Al–16% Si alloy after adding the varying percentages of ZnO nanoparticles (0.5, 1, 1.5 and 3 wt%) was investigated by tensile, impact strength and hardness tests and is summarized in Table 2.
Tensile strength and impact energy of hypereutectic Al–16 Si alloy were enhanced significantly after ZnO nanoparticle addition as shown in Fig. 6 and Table.2. It was observed that the largest value of the tensile strength and impact energy of this alloy was reached at 200 MPa and 5.38 J, respectively, at 3% ZnO nanoparticle addition. Further, the increase in tensile strength with concentration of ZnO nanoparticles attributed to refinement of grain size, discussed in previous section, and the strong interface zone between the nanoparticles and Al matrix due to uniform distribution of NPs inside the Al alloy matrix would lead to an improvement in tensile strength of Al alloy. The highest enhancement in tensile strength was occurred at 3% of ZnO NPs because high refinement of aluminum alloy which means smaller grain size of aluminum.
Also, the impact energy of hypereutectic Al alloy increases with the ZnO nanoparticle concentration increase from 0.5 to 3 wt%. The maximum impact energy occurs with 3 wt% of ZnO nanoparticles. This increase in the impact energy attributed to the good dispersion of the nanoparticle ZnO in Al matrix.
Figure 7 shows the effect of zinc oxide nanoparticle addition on the hardness value (BHN) of hypereutectic Al–16 Si alloy. The hardness value of base Al–16 Si % alloy (0% nanoparticle ZnO) was 46.3 BHN. It has been observed that the hardness value increased with an increasing in ZnO nanoparticle addition. It can be attributed to the refinement of aluminum alloy and the presence of harder and strong zinc oxide nanoparticles, which can withstand the deformation under the effect of indentation loads. The hardness of the Al–16% Si alloy at 3 wt% addition of nanoparticle ZnO was reached to maximum value at 60 BHN. The hardness of the Al–16 Si alloy with addition 1.50% ZnO nanoparticles was 15% higher than that the base Al–16 Si alloys.
4 Conclusions
The effect of ZnO NP content from 0 to 3% on the microstructure and mechanical characteristic of hypereutectic Al–16 Si % alloy were studied. The refinement of this alloy was detected due to ZnO NP introduction and maximum refinement was observed at 3% NP concentration. Decomposition of ZnO NPs through Al–16 Si% alloy leads to refinement and small grain size of Al alloy. Mechanical characteristics of the hypereutectic Al–16 Si % alloy were enhanced after zinc oxide nanoparticle addition in weight percent from 0.5 to 3%. Enhancement in mechanical properties reaches to highest value at 3% NP concentration.
References
S.N. Grigoriev, A.N. Krasnovskii, Study of the triboengineering characteristics of ultra dispersed composite powder materials. J. Frict. Wear 32(3), 164–166 (2011)
D.K. Dwivedi, Wear behavior of cast hypereutectic aluminum silicon alloys. Mater. Des. 27(7), 610–616 (2006)
S. Manasijevic, Z. Acimovic–Pavlovic, K. Raic, R. Radisa, V. Kvrgic, Optimization of cast pistons made of Al–Si piston alloy. I J. Cast Met. Res. 26(5), 255–261 (2013)
L. Hua-qing, F. Hong-xun, X. Xiang, Study and application of hypereutectic Al–Si alloys. Autom. Technol. Mater. 35(10), 21–24 (2001)
D. Codd, Advanced lightweight materials development and technology for increasing vehicle efficiency. (KVA Inc., Escondido, 2008)
P.M. Aneesh, K.A. Vanaja, M.K. Jayaraj, Synthesis of ZnO nanoparticles byhydrothermal method. in Proc. SPIE 6639: 66390J, 2007, pp. 1–7
J.J. Reinosaa, T.P. Lere, C.M. Álvarez-Docio, A.D. Campo, J.F. Fernández, Enhancement of UV absorption behavior in ZnO–TiO2 composites. boletín de la sociedad española de cerámica y vidrio. 55, 55–62 (2016)
E. Galoppinin, J. Rochford, H. Chen, G. Saraf, Y. Lu, A. Hagfeldt, G. Bosochloo, Fast electron transport in metal organic vapor deposition grown dye-sensitized ZnO nanorod solar cells. J. Phys. Chem. B 110(33), 16161–18159 (2006)
Y. Hong, J.H. Li, L.L. Chen, D.Q. Liu, H.Z. Li, Y. Zheng, Synthesis surface modification and photocatalytic property of ZnO nanoparticles. J Ding. Powd. Technol. 189, 426–432 (2009)
X. Zhao, R. Zhou, Q. Hua, L. Dong, R. Yu, C. Pan, Recent progress in ohmic/schottky-contacted ZnO nanowire sensors. J. Nanomater. Hindawi 20, 20–29 (2015)
J. Al-Sabahi, T. Bora, M. Al-Abri, J. Dutta, Controlled defects of zinc oxide nanorods for efficient visible light photocatalytic degradation of phenol. Mater. MDPI 9(4), 238–247 (2016)
Z.L. Wang, Zinc oxide nanostructures: growth, properties, and applications. J. Phys. Condens. Matter 16, R829–R858 (2004)
N. Raghukiran, R. Kumar, Effect of scandium addition on the microstructure, mechanical and wear properties of the spray formed hypereutectic aluminum–silicon alloys. Mater. Sci. Eng. A 641, 138–147 (2015)
N. Belov, A. Naumova, N. Alabin, A. Matveeva, Effect of scandium on structure and hardening of AleCa eutectic alloys. J. Alloy Compd. 646, 741–747 (2015)
L. Hengcheng, B. Juanjuan, Z. Min, D. Ke, J. Yunfeng, C. Mingdong, Effect of strontium and solidifi cation rate on eutectic grain structure in an Al-13 wt% si alloy. China Foundry 6(3), 226–231 (2009)
I.S. El-Mahallawi, A.Y. Shash, A.E. Amer, Nanoreinforced cast Al-Si Alloys with Al2O3, TiO2 and ZrO2 nanoparticles. Metals 5, 802–821 (2015)
J.H. Shin, J.H. Jeon, D.H. Bae, Microstructure refining of aluminum alloys using aluminothermic reaction with ZnO nanoparticles. Elsevier Mater. Lett. 151, 96–99 (2015)
Z.S. Qasim, M.A. Jabbar, J.J. Hassan, Enhancement the mechanical properties of aluminum casting alloys (A356) by adding nanorods structures from zinc oxide. J Mater. Sci. Eng. 6(2), 2–5 (2017)
B. Jabeera, T.S. Anirudhan, S.A. Shibli, Nano zinc oxide for efficient activation of aluminium zinc alloy sacrificial anode. J. New Mater. Electroch. Syst. 8, 291–297 (2005)
M.M. Meiz, M.S. El-Wazery, S.M. Khafagy, R.A. Elsad, Effect of solidification rate on mechanical and electrical behaviour of eutectic and hypereutectic al-si alloys. I J. Adv. Eng. Glob. Technol. 6(2), 2141–2156 (2018)
P. Scherrer, Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr Ges Wiss Göttingen, 26, 1918
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Cryst. 11, 102–112 (1987)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
El-Wazery, M.S., Elsad, R.A., Khafagy, S.M. et al. Enhancement of microstructure and mechanical properties of hypereutectic Al–16%Si alloy by ZnO nanocrystallites. Appl. Phys. A 124, 736 (2018). https://doi.org/10.1007/s00339-018-2160-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2160-x