Abstract
Gorgonians (like corals) are important habitat-forming organisms that support a diversity of macrofauna. This study explored structural attributes of gorgonian gardens formed by rose gorgonians (Leptogorgia sp. nov.) and associated macrofaunal assemblages in Caleta Pichicuy (Central Chile). Hierarchical sampling was conducted at 20 m depth (maximum colony abundances) in order to assess spatial variability in abundance and colony attributes at two spatial scales (among sites and rocky walls). The abundance and composition of the associated vagile and sessile macrofauna were also examined using univariant (Taxa richness and Shannon index (H’e)) and multivariant approaches and were compared with adjacent bare rocky habitats. Our results showed a high abundance of gorgonians (ca. 28.9–36.5 colonies m−2) compared to other gorgonian gardens in the world. For structural attributes, our results showed smaller colonies with thicker holdfasts in more exposed sites, suggesting the influence of hydrodynamic forces on the colony morphology. Taxa richness and H’e of vagile fauna showed threefold and twofold, respectively, higher values in gorgonian gardens compared to bare walls, but no differences were observed for sessile fauna. In addition, PCoA and PERMANOVA evidenced a distinctive assemblages’ composition between habitats for both vagile and sessile fauna. Correlation analyzes and dbRDA showed, however, little association between structural attributes and associated faunal assemblages (R2 = 0.06, and ca. 3–9.4% of the total variation explained, respectively). Our results constitute the first assessment of structural habitat complexity and accompanying fauna in these gorgonian gardens and establish the baseline for understanding possible future changes associated to human activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gorgonians are one of the most common group of benthic organisms in circalittoral seascapes across tropical, temperate and polar ecosystems (Gomez et al. 2018). These organisms play important roles at ecological and ecosystems scales. Gorgonians feature a three-dimensional, arborescent and branching morphology that provides additional complexity to subjacent rocky walls (Jordan et al. 1996). High colony groupings known as gorgonian gardens have a major role in the secondary production of the system (Krieger and Wing 2002; Montseny et al. 2020), including species with commercial interest such as lobsters or spiders crabs have been observed around gorgonian gardens (Chimienti 2020). These gardens provide key ecological services, such as carbon sequestration in long-lived carbonate skeletons (up to 0.73 ± 0.71 g C m−2 yr −1 during decades or centuries; Coppari et al. 2019), and human goods such as new medicines derived from bioactive compounds and economically valuable diving spots (Dias et al. 2015). Given the slow growth rates and long-living periods of gorgonians species ranging from 15 to over 200 years (Wenker and Stevens 2020) it is vital to preserve their integrity, which is currently experiencing an important degradation in different regions such as the Mediterranean and the Atlantic Sea (Cassola et al. 2016; Chimienti et al. 2021) that can trigger cascading effects on associated macrofaunal assemblages (Cerrano et al. 2000). For instance, the loss of the gorgonian Paramuricea clavata due to heat stress causes additional changes on the coralligenous seascape associated to a reduction in the cover of other calcareous species such as bryozoans and coralline algae (Scinto et al. 2010) and an increase in the dominance of turf species (Verdura et al. 2019).
Gorgonian habitats host a great diversity of benthic organisms that might range from habitat generalists to highly specialized species featuring remarkable habitat adaptations (Hixon and Menge 1991; Messmer et al. 2011). For instance, in shallow and temperate areas, the gorgonian Leptogorgia virgulata constitute a key habitat for the gastropod, Neosimnia uniplicata, and the shrimp, Neopontinides beaufortensis. These species dwell firmly attach to gorgonian branches, mimicking its shape and color while the gorgonian benefits for debris-removal (Patton 1972). In the same way, the nudibranch Tritonia wellsii uses the gorgonian Leptogorgia virgulata as hatchery and feeding habitat (Patton 1972). In deep and temperate areas like Portofino (Italy), the kleptoparasit polychaete Haplosyllis chameleon excavates unharming galleries in the coenenchyme of Paramuricea clavata to escape from predation and feeds on preys stolen from polyps (Pola et al. 2020). Besides specific associations, the presence of gorgonians can also determine the nature of associated understorey vegetation due to the shading effect of the erect branching structure, limiting the growth of erect macroalgal communities and favoring the development of coralligenous assemblages (Ponti et al. 2018). Gorgonian gardens also generate a wide range of microhabitats that are used as refuges by a diversity of opportunistic benthic organisms and increase the available surface area for settlement (Ponti et al. 2016). For instance, Valisano et al. (2016) reported higher abundance of crustaceans, gastropods and polychaetes inside gorgonian habitats than outside, evidencing their role in providing protection against benthic predators. Besides, the structural complexity of the habitat may be enhanced by association with bryozoans and hydrozoans species frequently settling on colony branches (Cúrdia et al. 2015). Another example was reported by Calcinai et al. (2013), who observed different associations between encrusting sponges using octocorals, such as Carijoa riisei and/or Alertigorgia hoeksemai, as growth substrates in both shallow and deeper areas of the tropical waters of the Indo-Pacific. In addition, gorgonians in temperate mesophotic ecosystems, play a crucial role in capturing and transporting suspended particles from the water column to the sea floor (Cerrano et al. 2019). Authors also emphasize the role of gorgonians in facilitating coevolutionary processes with other organisms and in reducing water speed, thereby enhancing sediment stability.
Approximately 65% of the subclass Octocorallia, including gorgonians, is found in cold-waters (Roberts et al. 2006; Morris et al. 2012). Gorgonian gardens are well-known to proliferate from 10 to 200 m depth in both shallow and deep waters, depending on the species (Roberts et al. 2006). Bayer (1953) already described the Eastern Pacific Ocean, from Ecuador to South of Chile, as one of the geographic regions with higher diversity of species in the Gorgoniidae family. Yet, only the Ecuador coast has been well documented in regards to gorgonian diversity (Homeier et al. 2008), whereas the taxonomy, distribution and ecological role of gorgonians in other geographic areas of the Eastern Pacific remains unclear (Vargas et al. 2014). At present, the only available information is that the area host abundant gardens of rose cold-water gorgonians (Häussermann and Försterra 2007), but their taxonomic identity along the entire coast is unclear and there are literally no studies on the associated communities of benthic faunal assemblages (Tognelli et al. 2005). Besides, to our knowledge no studies have been conducted on associated assemblages despite this information is essential to improve our understanding on their ecological role and create a baseline for future studies focused on their conservation.
In this context, the present study, conducted in Caleta Pichicuy (Central Chile) was aimed to assess: (1) the abundance of rose gorgonians along the depth gradient, (2) the spatial variability in the abundance and structural colony attributes of rose gorgonians (height, width and surface cover) across sites and sampled rocky walls, (3) the possible association between colony attributes, and the abundance and local diversity indexes of vagile and sessile fauna (i.e., species richness and H’e Shannon–Wiener Index), and the overall community composition and (4) explore the possible contribution of gorgonian gardens to enhance local diversity by comparing the ecological indices and taxa composition of vagile and sessile fauna in rose gorgonian gardens and in adjacent bare rocky walls. Overall, this study provides new valuable information on the population structure and ecological importance of rose gorgonian gardens in the coastline of Chile and represent a first step toward possible management and conservation actions.
Material and methods
Study site
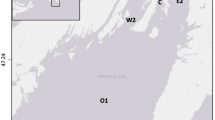
Gorgonian gardens of Caleta Pichicuy are formed by a currently undescribed species of Leptogorgia (Camps-Castella et al. under review). The study was conducted in the Caleta Pichicuy (Chile, NE Pacific) in the region of Valparaíso. In the area, the Humboldt Current System influences the coast with cold and nutrient-rich water (Echevin et al. 2012), although with great temporal variation resulting from differences in wind stress fields and the magnitude of the local upwelling (Tapia et al. 2014). Sampling was conducted in summer 2021 in three study sites of Caleta Pichicuy separated ca. 1 km between them and subjected to contrasting degrees of exposure to local currents: Jardín del Gato (JG: 32° 20′ 51.39″ S, 71°28′14.18″ W), La Isla (LI: 32° 20′51.86″ S, 71° 27′ 45.31″ W) and El Asilo (EA: 32° 20′50.49″ S, 71° 27′ 39.63″ W) (Fig. 1), and three different rocky walls per site (separated ca. 500 m). Jardin del Gato is the most exposed site, followed by La Isla and El Asilo. The degree of exposure was qualitatively assessed using combined information on main local winds and orientation toward the northward flow direction of the Humboldt Current (López et al. 2019). Besides, the presence of near shore emergent rocks in the proximity of La Isla and El Asilo makes these sites comparatively more protected compared than Jardin del Gato. Sampling was conducted with the boat and facilities of the Pichicuy diving center, who provided experienced advice on local differences in hydrodynamic exposure. In the case of LI, one replicate bare rocky wall was near (200 m) a kelp forest of Macrocystis pyrifera. The maximum depth is 40 m in Jardin del Gato, and 35 m in LI and EA.
Preliminary assessment of the depth distribution of rose gorgonians and its associated biodiversity
In order to assess the potential effect of depth in the composition of vagile (includes only slow-moving species, such as cryptic fishes within Bleniidae and Gobiidae families) and sessile fauna, an exploratory analysis was conducted along the depth gradient. Sampling was conducted at 6 depths, where gorgonians are present: 5, 10, 15, 20, 25, and 30 m. At each site and depth, the abundance of gorgonians, and the composition of sessile and vagile fauna was evaluated using random 50 × 50 cm PVC quadrates (N = 3). Sampling was restricted to 30 m due to scuba diving safety rules and dive time constraints. Results of the preliminary assessment show that the highest densities of gorgonians were observed at 20 m depth (Fig. 2). Therefore, we decided to concentrate sampling efforts at 20 m depth to maximize the observation of patterns of associated biodiversity.
Fixed depth assessment (20 m) of the abundance and structural attributes of the rose gorgonian population
At each site the abundance of rose gorgonians was assessed in three different rocky walls (hereafter, A, B and C) separated ca. 500 m using random 50 × 50 cm PVC quadrates (N = 15, per 3 sites, per 3 walls = 135 total) and results expressed in number of colonies per m2. To evaluate the effect of the gorgonian structural complexity on associated organisms, colony height and width was measured in situ to the nearest mm by the same observer. Colony height was estimated as the maximum distance from the base to the tip of the farthest branch. The surface area occupied by gorgonians within quadrat was determined from photographs with the Image J software and used as a proxy of colony biovolume (Cúrdia 2012). Also, the holdfast diameter of N = 5 random gorgonian colonies was determined using Image J software across sites and rocky walls. In each photo, the scale was calibrated to obtain a precision of 1 mm. Photographs were taken with a 12-megapixel digital camera (Olympus TG-5).
Fixed depth assessment (20 m) of biodiversity and ecological indices
At each study site, 50 × 50 cm photo-quadrats were randomly taken to evaluate the influence of the gorgonian habitat in the composition and abundance of associated invertebrates and cryptobenthic fishes associated to gorgonian colonies in the three rocky walls and adjacent rocky walls without gorgonians (N = 15, per 3 sites, per 3 walls with gorgonians, 3 walls without gorgonians = 270 total).
The number of vagile and sessile macroinvertebrates species per replicate quadrat (i.e., taxa richness), was identified to the lowest possible taxonomic level (genus and/or species). Cover was estimated as the total quadrat area occupied by a given taxa using Image J software with adequate calibration. Local diversity per quadrat was calculated using the Shannon–Wiener index (H’e). Also, qualitative information (presence/absence) of large benthic fish species inside gorgonian gardens and in adjacent rocky walls without gorgonians was noted at each of the dives (Buhl‐Mortensen et al. 2010) in order to obtain some preliminary information on possible effects.
Data analysis
Preliminary assessment of depth distribution of rose gorgonians and associated biodiversity
Patterns in the abundance of rose gorgonians among sites (fixed factor, 3 levels) and depths (fixed factor, 6 levels) were investigated with a 2-way factorial ANOVA. Differences in taxa composition across sites (fixed factor, three levels) and depths (fixed factor, 6 levels) were explored with PERMANOVA using Bray–Curtis similarity distances for multivariate analyzes.
Fixed depth assessment (20 m) of abundance and structural attributes of the rose gorgonians
Differences in the patterns of abundance and colony attributes including number of colonies, colony height, width, biovolume and diameter of the base of rose gorgonians among sites (fixed factor, three levels) and rocky walls (fixed factor, three levels, nested in site) were investigated with a 2-way Nested ANOVA.
Fixed depth assessment (20 m) of biodiversity and ecological indices
Differences in vagile and sessile species richness and Shannon–Wiener diversity Index among sites (fixed factor, three levels), habitats (fixed factor, two levels) and rocky walls (fixed factor, three levels, nested in Site × Habitat) was investigated with a 3-way Nested ANOVA.
Fixed depth assessment (20 m) of spatial patterns of macrofaunal assemblages
PERMANOVA with Bray–Curtis similarity distances was used to investigate differences across sites (fixed factor, three levels), habitats (fixed factor, two levels) and rocky walls (fixed factor, three levels, nested in Site × Habitat). Significant factors and interactions were investigated with post hoc pairwise. SIMPER analysis was conducted to identify the relative contribution of each taxon to dissimilarities. Differences in assemblage composition of vagile, sessile and all community were represented by unconstrained ordination plot using the principal coordinate analysis (PCO) based on Bray–Curtis similarities of Log (X + 1) transformed data (vagile and sessile fauna separately), and presence/absence transformed data of all community (matrix of vagile and sessile fauna).
Fixed depth assessment (20 m) of relationship between gorgonian attributes and community composition
The influence of gorgonian structural attributes (height, width, surface area of all gorgonian colonies per quadrat (SC)) in the structure of associated macroinvertebrates (vagile, sessile and the entire community, the later based on presence/absence transformed data) was investigated with a distance-based redundancy analysis (dbRDA). Analyzes were conducted on log transformed (log (x + 1)) data for gorgonian structural attributes matrix of each type of community composition (vagile and sessile fauna), and with presence/absence transformed data for the entire community.
Homogeneity of variances and normality assumptions were tested by Cochran’s test and Kolmogorov–Smirnov distribution fitting test of the residuals, respectively, and transformed when necessary to meet ANOVA assumptions. The critical level of significance was fixed at α = 0.05. Student–Newman–Keuls (SNK) post hoc comparisons were used when necessary to identify significant differences in the interaction between different factors. All multivariate statistical analyzes were conducted using R Software.
Results
Preliminary assessment of depth distribution of rose gorgonians and associated biodiversity
In general, gorgonians were present in all study sites from 5 to 30 m depth. The abundance of gorgonians (Number of colonies per m2) showed significant differences among sites and depths (p < 0.001, Table 1), with the highest abundance of gorgonians being observed at 20 m depth in Jardin del Gato (Fig. 2). A consistent decrease in abundances was observed toward deeper and shallower depths (Fig. 2). There was also a significant Site x Depth interaction resulting from variability in depth abundances across sites (Table 1). In contrast, no depth effect was observed for taxa composition of vagile and sessile fauna or the entire community (Table 2A, B, C).
Fixed depth assessment (20 m) of abundance and structural attributes of rose gorgonians
The abundance of gorgonians (Number of colonies per m2) showed significant differences among sites (Table 3A), with higher values in Jardin del Gato followed by El Asilo and La Isla (mean among rocky walls ± SD: 36.5 ± 2.5, 30.5 ± 1.9 and 28.9 ± 1.8, respectively, Fig. 3a). However, no rocky wall effect was detected (Table 3a). For structural attributes, colony height was significantly higher in El Asilo than La Isla and Jardin del Gato (17.8 ± 1.1 cm, 17.1 ± 1.0 cm and 14.1 ± 0.6 cm, respectively), and there was also a rocky wall effect (Table 3b, Fig. 3b). The same site pattern was observed for width (17.5 ± 1.0 cm, 16.9 ± 0.9 cm and 15.4 ± 0.6 cm, respectively) but differences among rocky walls were not significant (Table 3c, Fig. 3c). In contrast, the surface area of gorgonian colonies per quadrat (SC) did not show any significant effect (Table 3d). The holdfast of the colonies was significantly higher in Jardin del Gato, followed by La Isla and lower in El Asilo (11.4 ± 0.8 cm, 6.0 ± 0.4 cm and 5.3 ± 0.8 cm, respectively), and there was also a significant variability among rocky walls (Table 3e, Fig. 3d). Multiple linear regressions models showed that gorgonian abundance was the variable with the highest (and significant) association to the Shannon-Wiener diversity index of vagile fauna, despite correlation was extremely low (R2= 0.06, Table 4). In contrast, gorgonian abundance showed no significant associations with either the Shannon-Wiener diversity index of sessile taxa (Table 4).
Abundance and structural attributes of gorgonians among sites (JG: Jardin del Gato; LI: La Isla; EA: El Asilo) and rocky walls (A, B, C: nested in Site × Habitat) (5th-95th percentile). A Number of individuals per m2, B Height of gorgonians, C Width of gorgonians and D base of gorgonians. b, c, d: in cm. Boxplots: central line = median, box = upper and lower quartiles, point = mean and errors bars = SE
Fixed depth assessment (20 m) of biodiversity and ecological indices
Large native rocky reef fishes observed only in the gorgonian habitat included the sea chub Medialuna ancietae, Graus nigra and the Semicossyphus darwini. In addition, individuals of chalapo cinid Labrisomus philippii, the sandperch Pinguipes chilensis, the bilagai Cheilodactylus variegatus and the seabass Paralabrax humeralis were observed in both habitats, with and without gorgonians. No species was observed exclusively in rocky walls without gorgonians.
A total of 51 taxa were observed, including fishes, but only 44 were recorded at species level and quantified during the study (Table S1). We found 35 vagile and 9 sessile invertebrates, 38 of which were identified to species level, and the rest to Genera (Incatella sp.), family (Porifera family Suberitiidae) or only Phylum Porifera (Table S1). Mollusca was the most diverse phyla with 21 taxa across study sites, habitats and rocky walls. In gorgonian habitats the gastropod Tegula quadricostata was the species displaying the highest abundance, and the Porifera sp5 had the highest surface cover (m2). In contrast, in habitats without gorgonians the gastropod T. atra was the most abundance vagile species, and the mussel Aulacomya atra was the species with the highest surface cover. In addition, there are some species such as the fish Hypsoblennius sordidus, the catshark Schroederichthys chilensis, the actinia Anthothoe chilensis, the starfish Patiria chilensis and others only observed in gorgonian habitat (Table S1).
For vagile fauna, taxa richness differed significantly among habitats, with higher numbers in the gorgonian habitat (4.6 ± 1.4 taxa) than in adjacent rocky walls without gorgonians (2.5 ± 1.0 taxa) (Table 5a, Fig. 4a). There were also significant effects of site and rocky wall, resulting from species richness being higher in La Isla than Jardin del Gato and El Asilo, and significant spatial variability across rocky walls (Table 5a, Fig. 4a). Also, a significant Site x Habitat interaction was observed, due to higher differences among sites in rocky walls without gorgonians than in gorgonians habitats (Table 5a, Fig. 4a). Similar effects with enhanced diversity in the gorgonian habitat and comparable site patterns were found for the Shannon–Wiener taxa diversity index (H’ e) (1.3 ± 0.5 in the gorgonian habitat and 0.3 ± 0.3 without gorgonians), although a significant effect of rocky walls was also observed (Table 5b, Fig. 4b). There were also significant effect of sites, habitat, rocky walls and Site x Habitat, resulting from total abundance of vagile fauna being higher in bare areas (9.6 ± 4.2 ind m−2) than in gorgonian gardens (7.1 ± 0.7 ind m−2) in the other sites (Fig. 4c). In contrast, for sessile fauna, both taxa richness and Shannon–Wiener diversity index of sessile fauna showed no differences between sites, habitats, rocky walls and the interaction (Table 5d, e, Fig. 4d, e).
Univariate ecological indices across sites (JG: Jardin del Gato; LI: La Isla and EA: El Asilo), habitats (W: with gorgonians, WO: without gorgonians) and rocky walls (A, B, C: nested in Site x Habitat). Bars display the mean (± SE) for: A Species richness of vagile fauna, B Shannon–Wiener diversity index (H’ e) of vagile fauna, C Total abundance of vagile fauna (ind m−2), D Species richness of sessile fauna and E Shannon–Wiener diversity index (H’ e) of sessile fauna
Fixed depth assessment (20 m) of community patterns
PERMANOVA analysis showed that 34.3% of the total variation in the assemblages of vagile fauna was due to habitat type (Table 6a). In contrast, the effect of sites and rocky walls accounted for a lower, although significant proportion (14.2% and 4.7%, respectively) of the total observed variation (Table 6a). There was a significant Site x Habitat interaction which accounted for 16.50% of the total observed variation (Table 6a). PERMANOVA pairwise tests indicated significant differences among all sites (Table. S2a). Pairwise test for the significant Ha x Si interaction showed differences between habitats on La Isla (t = 2.5, p < 0.01) and El Asilo (t = 2.3, p < 0.01) (Table. S2b). Significant differences between sites were only observed in habitats without gorgonians (Jardin del Gato, La Isla: t = 1.9, p = 0.03; La Isla, El Asilo: t = 1.5, p = 0.03) (Table S2c). For sessile assemblages there was also a significant effect of habitat type, which explained ca. 9.7% of total variation (Table 6b). A significant site effect (4.7% of the total variation; Table 6b) resulted from differences between Jardin del Gato and La Isla and between Jardin del Gato and El Asilo (see pairwise t test in Table. S3a). The Ha x Si interaction accounted for 3.5% of the total variation (Table 5b) and was due to differences between habitats in the Jardin del Gato site (Table S3b). Patterns for the entire community (based on presence/absence transformed data of vagile and sessile fauna) showed significant differences across habitats and sites, which accounted for 13.9% and 11.0% of the total observed variation, respectively (Table 6c). Also, there was a significant Habitat x Site interaction that accounted for 4.8% of the total observed variation (Table 6c). Pairwise tests showed significant differences across all sites (see Table S4a). For the Ha x Si interaction, pairwise test results evidenced differences between habitats in La Isla and El Asilo sites (Table S4b). Also, La Isla showed significant differences with the other two sites in the gorgonian habitat whereas in habitats without gorgonians it was El Asilo that was significantly different from the other sites (Table S4c).
SIMPER analysis evidenced habitat dissimilarities of 94.4% in the composition of vagile fauna, with T. atra (more abundant in habitats without gorgonians) and T. quadricostata (more abundant in gorgonian habitats) being the taxa with higher contributions to observed dissimilarities (Table S5d). However, there were also low average similarities within habitats (14.8–21.8%) (Table S5k, l). For sessile fauna, differences in taxa composition were also very important of 95.7% between habitats, with Porifera sp5 (more abundant in gorgonian habitat), the soft coral Incrustatus comauensis, the Porifera sp3 and the mussel Aulacomya atra (more abundant in habitats without gorgonians) being the taxa with higher contributions to dissimilarities (Table S6d). At the level of the entire community, SIMPER results showed average dissimilarities between habitats of 91.2%, with T. atra, O. penicillatus (more abundant in rocky walls without gorgonians) and T. quadricostata (more abundant in gorgonian habitats) being the taxa with highest contributions to observed differences (Table S7d). All full SIMPER results of similarity and dissimilarities among sites and rocky walls can be found in the S1 Appendix.
PCoA showed an overall separation of habitats in the ordination space and evidenced that the 21% of the total variation in vagile fauna assemblages was explained by the first axis (Fig. 5a). Further variability (up to 13.2%) was also explained by the second axis. The data cloud was driven by the gastropods T. atra, T. quadricostata and O. penicillatus, represented by the correlation vectors (Fig. 5a). The T. atra species was strongly associated with assemblage composition in bare rocky walls and the T. quadricostata and starfish O. penicillatus were mostly associated with the presence of gorgonian gardens (Fig. 5a). For sessile fauna, the PCoA showed that the differences between habitats were mostly explained by the first axis (14% of total variation) compared with second axis (9.9% of total variation (Fig. 5b). A. atra and the unidentified benthic biofilm were taxa with highest associations with the first axis in habitats without gorgonians (Fig. 5b). For the entire community, the first and the second axis of the PCoA explained 18.1% and 12.8%, respectively, of the total variation (Fig. 5c). The A. atra, T. atra and the unidentified benthic biofilm were strongly associated with bare rocky walls (Fig. 5c). In contrast, the nudibranch T. challengeriana and T. quadricostata showed an association with the gorgonian habitat (Fig. 5c).
Principal coordinates analyzes (PCO) ordination plot of: A Vagile community, B Sessile community and C all community (based on presence/absence transformed data of pooled matrix) in three sites (Jardin del Gato, La Isla and El Asilo) of the Caleta Pichicuy (Chile) and habitat type (W: with gorgonians filled symbols, WO: without gorgonians empty symbols). Vectors superimposed to plot represent the correlations by the species with the PCO axes. Analysis performed on Bray–Curtis dissimilarities
Fixed depth assessment (20 m) of relationship between gorgonian structural attributes and community composition
The dbRDA indicated that only about 3% of total variation of vagile fauna assemblages was explained by the two ordination axes (44.9% by dbRDA1 and 34.9% by dbRDA2) (Fig. 6a). The large size of width and SC vectors confirm that these gorgonian attributes contribute to the structure of the vagile assemblage’s composition (Fig. 6a). Similar results were found for the sessile assemblages’ composition (Fig. 6b). The dbRDA plot only explained 4% of the total variation (42.4% by dbRDA1 and 25.3% by dbRDA2), being height and SC the larger vectors that contribute the sessile composition structure. For the entire community, results confirmed that abundance and height attributes were influencing variables, although only 9.4% of the total variation was explained (50.4% by dbRDA1 and 27.6% by dbRDA2) (Fig. 6c).
Distance-based redundancy analysis (dbRDA) biplot to investigate the relationships between gorgonian structural attributes and community composition of A Vagile community, B Sessile community) and C All community (based on presence/absence transformed data). Assemblage symbols are as follows; squares: Jardin del Gato; circles: La Isla; triangles: El Asilo; gray color: rocky wall A; red color: rocky wall B; and black color: rocky wall C. Samples are plotted as points using weighted averages of taxa scores in each constrained axis. The vector lines reflect the relationship of the different attributes of gorgonian complexity to the ordination axes. Vectors lengths is proportional to their relative significance
Discussion
Abundances of gorgonians and variability in structural features
The present study constitutes the first assessment of monospecific cold-water rose gorgonian gardens depth distribution, abundance and structural features in Chile and provides the beginnings for understanding key ecological patterns of these habitats. Our results showed a clear pattern of distribution within depth and similar across sites in Caleta Pichicuy (Chile), with abundances peaking at depths of ca. 20 m. A similar pattern was observed by Cúrdia et al. (2013) who reported maximal abundances at 20–25 for Leptogorgia sarmentosa in the Mediterranean. A plausible explanation for the depth distribution observed in Caleta Pichicuy may be related to the presence of dense erect algae (Lessonia trabeculata), and/or filamentous turf-forming algae in the upper level of the sublittoral zone (Stotz et al. 2016), which may effectively outcompete gorgonians due to faster growth (Gili et al. 1989). The influence of other factors such as light exposure, substrate suitability and wave action or currents is also another source of variability as indicated in other species (Gili et al. 1989; Garrabou et al. 2002).
The abundance of gorgonian colonies at ca. 20 m depth was one of the highest reported in the literature, especially in the Eastern Pacific (Cúrdia et al. 2013; Carvalho et al. 2014) with densities ranging from ca. 20–90 in Jardin del Gato to 10–40 colonies per m−2 in La Isla (means of 28.9 ± 1.8, 36.5 ± 2.5 colonies m−2, respectively) (Fig. 7a–f). In the Caribbean Sea, abundances as low as 0.81 ± 0.41 colonies m−2 for Pseudopterogorgia americana (Jordán-Dahlgren 2002) and 0.01 ± 0.01 colonies· m−2 in Antillogorgia acerosa (Manrique Rodríguez et al. 2019) have been reported. In the Mediterranean Sea, densities of 11–53 colonies per m2 (33 ± 14 colonies · m−2) have been indicated for Paramuricea clavata, 1–56 (20 ± 14 colonies · m−2) for Eunicella singularis, and up to 25 colonies · m−2 for Eunicella cavolini (Linares et al. 2008; Gori et al. 2011; Carvalho et al. 2014). Our results may be explained by the large amount of food available through the Chilean coastal upwelling driven by the Humboldt Current System in the study area (Yuras et al. 2005) compared to more oligotrophic systems such as the Mediterranean Sea or the Caribbean Sea. Spatial variability among study sites could be related to the effect of topography on current patterns affecting food supply and wave action controlling the gorgonian populations. In fact, sheltering from wave action also appeared to influence structural attributes of gorgonian colonies. Smaller colonies (14.1 ± 0.6 cm height) with thicker holdfasts (11.4 ± 0.3 cm in diameter), were observed in Jardin del Gato (most exposed) followed by La Isla (17.1 ± 1.0 cm and 6.0 ± 0.4 cm, respectively), whereas those in El Asilo were larger (17.8 ± 1.1 cm) and with thinner holdfasts (5.3 ± 0.8 cm) (shelter by a prominent rocky wall and hosting the lowest abundance of gorgonians). These observations reinforce previous studies pointing that holdfast thickness provide enhanced resistance against wave action (Yoshioka and Yoshioka 1991) and that greater colony sizes can be reached in areas protected from hydrodynamic forces (Dahlgren 1989).
Underwater photographs of the gorgonian gardens and their associated biodiversity. A Gorgonian abundance in rocky walls, B Different associated organisms such as sponges and O. penicillatus, C Detail of gorgonians and the associated nudibranch T. odhneri to them, D Schreoderichtys chilensis associated to gorgonians and E T. challengeriana associated to gorgonians
Habitat patterns and associated biodiversity
Our study evidences that rose gorgonian gardens hosted higher taxa richness (mean of 4.6 vs. 2.5 taxa across sites) and diversity (H’e of 1.3 vs. 0.3) than adjacent bare rocky walls. Besides, the community structure of vagile, sessile and combined faunal assemblage also differed between gorgonian gardens and adjacent rocky walls. Similar patterns of differences in faunal assemblages have been reported in the presence or absence of gorgonians species such as Paramuricea clavata and Savalia savaglia in the Mediterranean Sea (Cerrano et al. 2010; Ponti et al. 2018).
In general, Mollusca, Echinodermata and Chordata, were dominant groups in gorgonian habitats. The Order Nudibranchia was highly represented in gorgonian gardens, with two species of genus Tritonia—well-known to include many octocoral-eating species (Wyeth et al. 2006) T. challengeriana and T. odhneri (Fig. 7c, f). In contrast, opposed habitat abundances were observed for T. quadricostata and T. atra in rocky walls in gorgonian and bare rocky walls, respectively. Disproportionate abundances of T. atra have been reported on rocky substrate with encrusting coralline (Stotz et al. 2016) or kelp species (Vásquez and Buschmann 1997) due to herbivorous feeding habits (Schmitt 1987). Hence, high abundances of T. atra in bare rocky habitat in La Isla appear to be driven by the presence of adjacent unharvested kelp forest of Macrocystis pyrifera, which could have acted as a source of individuals. Among echinoderms, the starfish Odontaster penicillatus was also found (Fig. 7b) predominantly inside gorgonian habitats. According to Mutschke and Mah (2009), the species features preferential predation on sponges as in other close species (Lawrence 2013) and could explain its apparent preference for gorgonian habitats hosting enhanced availability of sponge species such as Clionaopsis platei or Porifera sp5. Although the same taxa could be observed in both habitat types, total abundances yielded higher records in bare walls due to very few taxa (Incatella sp., Crassilabrum and Tegula atra). For the vast majority of taxa gorgonians appear to act as a source of individuals to neighboring systems following an edge effect dynamics allowing the movement of vagile fauna and/propagule dispersal to adjacent, less favorable habitats (Macreadie et al. 2010; Bramwell 2016; Carroll et al. 2019). As has observed for the T. atra abundance, further exchanges and subsidized patterns of productivity may occur in the proximity of other habitats such as kelp forest (Shelamoff et al. 2020) and require further investigation in Chilean waters. For instance, enhanced connectivity between seagrass meadows and rocky substrates facilitates migration of the sea urchin Paracentrotus lividus due to a spillover effect from the rocky habitat featuring higher recruitment (Prado et al. 2009; Boada et al. 2018). Yet, Ceccherelli et al. (2014) reported high spread of invasion algae Caulerpa racemosa at edge habitat of seagrass meadows than inside due to human disturbance on the margins (e.g., anchoring or dredging) of Posidonia oceanica beds in the Mediterranean Sea. In addition, Smith et al. (2011) observed that predation rate pipepish (Sitgmatopora spp.) was higher in the edge habitat (sand areas) than in sand patches inside seagrass or in the middle of seagrass meadows. Hence, increased connection between habitats may have either positive (Carroll et al. 2019) or negative effects for different species (Ceccherelli et al. 2014).
Sessile fauna showed no differences in univariate ecological indices (species richness and diversity) suggesting that the edge effect observed for vagile species was largely inherent to their mobility capacity across benthic habitats. Yet, there were also some differences in community structure between habitats that were mostly due to enhanced surface cover of certain species (the soft coral Incrustatus comaeunsis, Porifera sp3 and the commercial mussel Aulacomya atra) in bare rocky substrates. According to Velasco‐Charpentier et al. (2021), the recruitment of A. atra in the South of Chile is facilitated by microhabitats provided by kelp forests of Lessonia flavicans. Hence, substrate competition with gorgonians during the process of benthic settlement by intercepting settling propagules or producing allelochemicals (Ponti et al. 2014) could explain the distribution of these sessile species including Porifera and soft corals.
The reef fish community associated to gorgonian gardens was only qualitatively evaluated in this work, but such preliminary assessment already revealed clear differences between habitats. The blenny Helcogrammoides cunninghami, was more abundant within gorgonian gardens as expected for a cryptic species (Pérez-Matus et al. 2016). The endemic catshark Schroederichthys chilensis possibly uses gorgonian gardens as nursery habitats and was also dominant in this habitat (Fig. 7d), similarly to reported in other close species (Scyliorhinus canicula or Scyliorhinus rotifer) (Bo et al. 2015). This hypothesis is further supported by observations of catshark eggs attached to colony branches during our samplings. Endemic rocky reef fishes of economic importance, such as Semicossyphus darwini and Graus nigra, were only observed within gorgonian gardens, mainly between the alleys of gorgonian walls. Abundance patterns of these species could be partly associated to preferential feeding on the sea urchin Tetrapygus niger, which is also more abundant on gorgonian gardens (Perez-Matus et al. 2012).
Relationship between gorgonian structural attributes and community composition
Contrary to our expectations the results evidenced a reduced influence of gorgonian attributes on community composition (but see Cúrdia et al. 2015 for significant effects of colony abundance and height on epifaunal assemblages). According to Cúrdia et al. (2015), increased habitat complexity may be scale-dependent and requires the evaluation of heterogeneity as a fractal dimension. Yet, they propose that the presence or absence of gorgonians may enhance species richness and diversity more than colony complexity itself (i.e., the fractal dimension). Also, Pierre and Kovalenko (2014) showed that for ecosystem engineers, heterogeneity is the most important feature fostering biodiversity than overall habitat complexity. Valisano et al. (2016) showed that lower sedimentation inside gorgonian gardens of P. clavata than outside creates a homeostatic effect on the surrounding habitat that enhanced vagile fauna. Besides, sole the presence of gorgonians might increase substrate availability thus enhancing local diversity, compared to adjacent soft bottoms, as indicated for the gold coral Savalia savaglia (Cerrano et al. 2010).
Conclusions and take-home conservation message
Our study evidences a clear distribution pattern of rose gorgonian gardens along the depth gradient, with a peak at 20 m. The large abundance of gorgonian colonies in Pichicuy (up to 90 ind. m−2) was striking and highlight the importance of the Humboldt Current System in the Chilean Coast. Furthermore, our study suggests that presence or absence of gorgonians gardens is a key factor driving community structure and species composition in Caleta Pichicuy, specially for vagile fauna. These gardens clearly host an elevated taxa richness and diversity and appear to act as a source of vagile organisms to adjacent bare rocky walls. The similarity of structural community variables across study sites might have been influenced by the limited spatial scale at which the study was conducted, thus hindering the observation of important differences in taxa richness and diversity and community composition, particularly for sessile fauna. Thus, a large-scale comparison along the Chilean coast or further differences in colony structure might be needed to fully grasp patterns of variability associated to differences in structural complexity and density of rose gorgonian gardens.
Fishes of commercial interest highly threatened by overfishing (Godoy et al. 2010) such as Semicosspyhus darwini, Graus nigra and Medialuna ancietae were associated with gorgonian gardens, highlighting the importance of protecting this habitat. Currently, study sites are included within the Management Areas for the Exploitation of Benthic Resources (AMERB) which allows an intensive extraction of resources and lacks a holistic ecosystem management approach. In contrast, in other world regions such as the Mediterranean Sea or Portugal coasts, gorgonian habitats are strictly protected within MPAs through the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR). Now that a baseline on the ecological importance of rose gorgonian gardens has been established, a similar protection figure should be considered on Chilean species and habitats, along with further studies aimed at understanding key ecosystem processes and dynamics. It is also suggested that future management plans or ecological studies, should include a monitoring plan of the health status of rose gorgonian gardens and associated communities, which so far appear to be unique to the Chilean locality of Caleta Pichicuy.
References
Bayer FM (1953) Zoogeography and Evolution in the Octocorallian Family Gorgoniidae. Bull Mar Sci 3:100–119
Bo M, Bavestrello G, Angiolillo M et al (2015) Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 10:e0119393
Boada J, Farina S, Arthur R et al (2018) Herbivore control in connected seascapes: habitat determines when population regulation occurs in the life history of a key herbivore. Oikos 127:1195–1204
Bramwell NA (2016) Differential responses and sensitivity to edge of seagrass fish and their primary prey faunal groups with respect to two distinct adjacent habitat edge types bordering Posidonia australis beds on the northern shores of Jervis Bay, NSW Australia. University of Technology, Sydney PhD dissertation,
Buhl-Mortensen L, Vanreusel A, Gooday AJ et al (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol 31:21–50. https://doi.org/10.1111/j.1439-0485.2010.00359.x
Calcinai B, Bavestrello G, Bertolino M et al (2013) Sponges associated with octocorals in the Indo-Pacific, with the description of four new species. Zootaxa 3617:1–61. https://doi.org/10.11646/zootaxa.3617.1.1
Carroll JM, Keller DA, Furman BT, Stubler AD (2019) Rough Around the Edges: Lessons Learned and Future Directions in Marine Edge Effects Studies. Curr Landsc Ecol Rep 4:91–102
Carvalho S, Cúrdia J, Pereira F et al (2014) Biodiversity patterns of epifaunal assemblages associated with the gorgonians Eunicella gazella and Leptogorgia lusitanica in response to host, space and time. J Sea Res 85:37–47. https://doi.org/10.1016/j.seares.2013.10.001
Cassola GE, Pacheco MSC, Barbosa MC et al (2016) Decline in abundance and health state of an Atlantic subtropical gorgonian population. Mar Pollut Bull 104:329–334. https://doi.org/10.1016/j.marpolbul.2016.01.022
Ceccherelli G, Pinna S, Cusseddu V, Bulleri F (2014) The role of disturbance in promoting the spread of the invasive seaweed Caulerpa racemosa in seagrass meadows. Biol Invasions 16:2737–2745. https://doi.org/10.1007/s10530-014-0700-7
Cerrano C, Bavestrello G, Bianchi C, n, et al (2000) A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol Lett 3:284–293. https://doi.org/10.1046/j.1461-0248.2000.00152.x
Cerrano C, Danovaro R, Gambi C et al (2010) Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv 19:153–167. https://doi.org/10.1007/s10531-009-9712-5
Cerrano C, Bastari A, Calcinai B et al (2019) Temperate mesophotic ecosystems: gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur Zool J 86:370–388. https://doi.org/10.1080/24750263.2019.1677790
Chimienti G (2020) Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea. Diversity 12:176. https://doi.org/10.3390/d12050176
Chimienti G, De Padova D, Adamo M et al (2021) Effects of global warming on Mediterranean coral forests. Sci Rep 11:20703. https://doi.org/10.1038/s41598-021-00162-4
Coppari M, Zanella C, Rossi S (2019) The importance of coastal gorgonians in the blue carbon budget. Sci Rep 9:13550. https://doi.org/10.1038/s41598-019-49797-4
Cúrdia J, Monteiro P, Afonso CML et al (2013) Spatial and depth-associated distribution patterns of shallow gorgonians in the Algarve coast (Portugal, NE Atlantic). Helgol Mar Res 67:521–534. https://doi.org/10.1007/s10152-012-0340-1
Cúrdia J, Carvalho S, Pereira F et al (2015) Diversity and abundance of invertebrate epifaunal assemblages associated with gorgonians are driven by colony attributes. Coral Reefs 34:611–624. https://doi.org/10.1007/s00338-015-1283-1
Cúrdia J (2012) Gorgonians of the South of Portugal: biology, ecology and conservation. 296
Dahlgren EJ (1989) Gorgonian Community Structure and Reef Zonation Patterns on Yucatan Coral Reefs. Bull Mar Sci 45:678–696
Dias IM, Cúrdia J, Cunha MR et al (2015) Temporal variability in epifaunal assemblages associated with temperate gorgonian gardens. Mar Environ Res 112:140–151. https://doi.org/10.1016/j.marenvres.2015.10.006
Echevin V, Goubanova K, Belmadani A, Dewitte B (2012) Sensitivity of the Humboldt Current system to global warming: a downscaling experiment of the IPSL-CM4 model. Clim Dyn 38:761–774. https://doi.org/10.1007/s00382-011-1085-2
Garrabou J, Ballesteros E, Zabala M (2002) Structure and Dynamics of North-western Mediterranean Rocky Benthic Communities along a Depth Gradient. Estuar Coast Shelf Sci 55:493–508. https://doi.org/10.1006/ecss.2001.0920
Gili JM, Murillo J, Ros J (1989) The distribution pattern of benthic Cnidarians in the Western Mediterranean. Sci Mar 53:19–35
Godoy N, Gelcich S, Vásquez J, Castilla J (2010) Spearfishing to depletion: Evidence from temperate reef fishes in Chile. Ecol Appl Publ Ecol Soc Am 20:1504–1511. https://doi.org/10.1890/09-1806
Gomez CG, Gonzalez A, Guzman HM (2018) Reproductive traits and their relationship with water temperature in three common octocoral (Anthozoa: Octocoralia) species from the tropical eastern Pacific. Bull Mar Sci 94:1527–1541. https://doi.org/10.5343/bms.2017.1051
Gori A, Rossi S, Linares C et al (2011) Size and spatial structure in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, northwestern Mediterranean Sea). Mar Biol 13:
Häussermann V, Försterra G (2007) Large assemblages of cold-water corals in Chile: a summary of recent findings and potential impacts. Bull Mar Sci 81(3):195–207
Hixon MA, Menge BA (1991) Species diversity: Prey refuges modify the interactive effects of predation and competition. Theor Popul Biol 39:178–200. https://doi.org/10.1016/0040-5809(91)90035-E
Homeier J, Werner FA, Gradstein SR, et al (2008) Flora and fungi: composition and function. In: Gradients in a tropical mountain ecosystem of Ecuador. Springer, pp 87–100
Jordan F, DeLeon CJ, McCreary AC (1996) Predation, habitat complexity, and distribution of the crayfishProcambarus alleni within a wetland habitat mosaic. Wetlands 16:452–457. https://doi.org/10.1007/BF03161334
Jordán-Dahlgren E (2002) Gorgonian distribution patterns in coral reef environments of the Gulf of Mexico: evidence of sporadic ecological connectivity? Coral Reefs 21:205–215. https://doi.org/10.1007/s00338-002-0226-9
Krieger KJ, Wing BL (2002) Megafauna associations with deepwater corals (Primnoa spp.) in the Gulf of Alaska. Hydrobiologia 471:83–90. https://doi.org/10.1023/A:1016597119297
Lawrence JM (2013) Starfish: Biology and Ecology of the Asteroidea. JHU Press
Linares C, Coma R, Garrabou J et al (2008) Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J Appl Ecol 45:688–699. https://doi.org/10.1111/j.1365-2664.2007.01419.x
López BA, Macaya EC, Jeldres R et al (2019) Spatio-temporal variability of strandings of the southern bull kelp Durvillaea antarctica (Fucales, Phaeophyceae) on beaches along the coast of Chile—linked to local storms. J Appl Phycol 31:2159–2173. https://doi.org/10.1007/s10811-018-1705-x
Macreadie PI, Connolly RM, Jenkins GP et al (2010) Edge patterns in aquatic invertebrates explained by predictive models. Mar Freshw Res 61:214–218
Manrique Rodríguez N, Agudelo C, Sanjuan-Muñoz A (2019) Gorgonian octocoral community at Varadero coral reef in the Colombian Caribbean: diversity and spatial distribution. Bull Mar Coast Res 48:. https://doi.org/10.25268/bimc.invemar.2019.48.1.757
Messmer V, Jones GP, Munday PL et al (2011) Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92:2285–2298. https://doi.org/10.1890/11-0037.1
Montseny M, Linares C, Viladrich N et al (2020) A new large-scale and cost-effective restoration method for cold-water coral gardens. Aquat Conserv Mar Freshw Ecosyst 30:977–987. https://doi.org/10.1002/aqc.3303
Morris K, Tyler PA, Murton B, Rogers AD (2012) Lower bathyal and abyssal distribution of coral in the axial volcanic ridge of the Mid-Atlantic Ridge at 45 N. Deep Sea Res Part Oceanogr Res Pap 62:32–39
Mutschke E, Mah C (2009) Asteroidea-Estrellas de Mar. Fauna Mar Bentónica Patagon Chil Nat Focus
Patton WK (1972) Studies on the Animal Symbionts of the Gorgonian Coral, Leptogorgia Virgulata (Lamarck). Bull Mar Sci 22:419–431
Perez-Matus A, Pledger S, Diaz FJ et al (2012) Plasticity in feeding selectivity and trophic structure of kelp forest associated fishes from northern Chile. Rev Chil Hist Nat 85:29–48
Pérez-Matus A, Sánchez F, González-But JC, Lamb RW (2016) Understory algae associations and predation risk influence broad-scale kelp habitat use in a temperate reef fish. Mar Ecol Prog Ser 559:147–158
Pierre JIS, Kovalenko KE (2014) Effect of habitat complexity attributes on species richness. Ecosphere 5:art22. https://doi.org/10.1890/ES13-00323.1
Pola L, Calcinai B, Pica D et al (2020) Updating the current knowledge on the relationships between Haplosyllis chamaeleon Laubier, 1960 (Annelida, Syllidae) and Paramuricea clavata (Risso, 1826) (Cnidaria, Plexauridae) in the Mediterranean Sea. Mar Biodivers 50:105. https://doi.org/10.1007/s12526-020-01127-y
Ponti M, Perlini RA, Ventra V et al (2014) Ecological Shifts in Mediterranean Coralligenous Assemblages Related to Gorgonian Forest Loss. PLoS ONE 9:e102782. https://doi.org/10.1371/journal.pone.0102782
Ponti M, Grech D, Mori M et al (2016) The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Mar Biol 163:120. https://doi.org/10.1007/s00227-016-2897-8
Ponti M, Turicchia E, Ferro F et al (2018) The understorey of gorgonian forests in mesophotic temperate reefs. Aquat Conserv Mar Freshw Ecosyst 28:1153–1166. https://doi.org/10.1002/aqc.2928
Prado P, Romero J, Alcoverro T (2009) Welcome mats? The role of seagrass meadow structure in controlling post-settlement survival in a keystone sea-urchin species. Estuar Coast Shelf Sci 85:472–478
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the Deep: The Biology and Geology of Cold-Water Coral Ecosystems. Science 312:543–547. https://doi.org/10.1126/science.1119861
Schmitt RJ (1987) Indirect interactions between prey: apparent competition, predator aggregation, and habitat segregation. Ecology 68:1887–1897
Scinto A, Bertolino M, Huete-stauffer C et al (2010) Effects of the loss of Paramuricea clavata (Risso, 1826) forests on coralligenous assemblages. Rapp Comm Int Mer Médit 39:1
Shelamoff V, Layton C, Tatsumi M et al (2020) Kelp patch size and density influence secondary productivity and diversity of epifauna. Oikos 129:331–345. https://doi.org/10.1111/oik.06585
Smith TM, Hindell JS, Jenkins GP et al (2011) Edge effects in patchy seagrass landscapes: The role of predation in determining fish distribution. J Exp Mar Biol Ecol 399:8–16. https://doi.org/10.1016/j.jembe.2011.01.010
Stotz WB, Aburto J, Caillaux LM, González SA (2016) Vertical distribution of rocky subtidal assemblages along the exposed coast of north-central Chile. J Sea Res 107:34–47. https://doi.org/10.1016/j.seares.2015.11.006
Tapia FJ, Largier JL, Castillo M et al (2014) Latitudinal discontinuity in thermal conditions along the nearshore of central-northern Chile. PLoS ONE 9:e110841
Tognelli MF, Silva-Garcı́a C, Labra FA, Marquet PA, (2005) Priority areas for the conservation of coastal marine vertebrates in Chile. Biol Conserv 126:420–428. https://doi.org/10.1016/j.biocon.2005.06.021
Valisano L, Notari F, Mori M, Cerrano C (2016) Temporal variability of sedimentation rates and mobile fauna inside and outside a gorgonian garden. Mar Ecol 37:1303–1314. https://doi.org/10.1111/maec.12328
Vargas S, Guzman HM, Breedy O, Wörheide G (2014) Molecular phylogeny and DNA barcoding of tropical eastern Pacific shallow-water gorgonian octocorals. Mar Biol 161:1027–1038. https://doi.org/10.1007/s00227-014-2396-8
Vásquez JA, Buschmann AH (1997) Herbivore-kelp interactions in Chilean subtidal communities: a review. Rev Chil Hist Nat 70:41–52
Velasco-Charpentier C, Pizarro-Mora F, Navarro NP, Valdivia N (2021) Disentangling the links between habitat complexity and biodiversity in a kelp-dominated subantarctic community. Ecol Evol 11:1214–1224
Verdura J, Linares C, Ballesteros E et al (2019) Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species. Sci Rep 9:5911. https://doi.org/10.1038/s41598-019-41929-0
Wenker RP, Stevens BG (2020) Sea whip coral Leptogorgia virgulata in the Mid-Atlantic Bight: Colony complexity, age, and growth. PeerJ 8:e8372. https://doi.org/10.7717/peerj.8372
Wyeth RC, Woodward OM, Willows AD (2006) Orientation and navigation relative to water flow, prey, conspecifics, and predators by the nudibranch mollusc Tritonia diomedea. Biol Bull 210:97–108
Yoshioka PM, Yoshioka BB (1991) A comparison of the survivorship and growth of shallow-water gorgonian species of Puerto Rico. Mar Ecol Prog Ser Oldendorf 69:253–260
Yuras G, Ulloa O, Hormazábal S (2005) On the annual cycle of coastal and open ocean satellite chlorophyll off Chile (18–40 S). Geophys Res Lett 32(23).
Acknowledgements
We are grateful to the staff of Buceo Pichicuy for providing us lodging and boat to our sampling work. Also, we are grateful to the people and fisherman of Caleta Pichicuy for their hospitality and for help during the months of work. Specially, Judith Camps is grateful to Patricio Manterola for help with fieldwork sampling. We thank the anonymous reviewers for helpful and valuable comments and suggestions on the manuscript. JCC received financial support from Dirección de Posgrado and Programa de Magíster en Ecología Marina, Universidad Católica de la Santísima Concepción. IAH received financial support from COPAS COASTAL ANID FB210021. The funders had no role in study design, data collection and analysis or preparation of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. JCC received financial support from Dirección de Posgrado and Programa de Magíster en Ecología Marina, Universidad Católica de la Santísima Concepción. IAH received financial support from COPAS COASTAL ANID FB210021.
Author information
Authors and Affiliations
Contributions
IAH and PP contributed to the study conception and design. Material preparation and data collection were performed by JCC. Data analysis were performed by JCC, IAH, PP and JTM. The first draft of the manuscript was written by JCC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The funders had no role in study design, data collection and analysis or preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Camps-Castellà, J., Prado, P., Tena-Medialdea, J. et al. Structural attributes and macrofaunal assemblages associated with rose gorgonian gardens (Leptogorgia sp. nov.) in Central Chile: opening the door for conservation actions. Coral Reefs 43, 201–217 (2024). https://doi.org/10.1007/s00338-023-02463-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02463-8