Abstract
Predicted sea-level rise and increased storm frequency caused by climate change drastically threaten low-lying inhabited coral atolls. Coral reef frameworks are the atolls’ primary defence from these changes. The growth and integrity of these frameworks is reflected in their carbonate budgets, a dynamic balance between biogenic accretion through coral growth and erosional forces, both of which are affected by factors acting at different spatial scales. We explored how carbonate budgets, estimated using the ReefBudget methodology, vary between three inhabited atolls that face differing anthropogenic stressors in the Lakshadweep Archipelago in the Northern Indian Ocean. We surveyed ten reefs, at two depths each, across the three islands. Overall, net carbonate budgets of reefs across all atolls were below optimal production rates needed to continue protecting shorelines (5 G measured on healthy reefs). This was a result of repeated mass bleaching events as well as local impacts. Carbonate production was influenced by a recent mass bleaching event in 2016 and a cyclone in 2018, and varied between depths and exposures, potentially due to differential recovery and mortality dynamics. Erosional processes were locally mediated with both urchin and parrotfish density showing large differences between islands, possibly linked to nutrient outflow and fishing intensity. We also find that by the year 2100, a large proportion of shallow sites will experience an increase in water depth above half a metre under moderate and high emissions scenarios, but none will breach this threshold under a low emissions scenario. Our results show that patterns of carbonate production were largely mediated by the history of global/regional disturbances, while erosional rates were much more dependent on local factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a warming world, storms and rising sea levels are expected to impact more than 400 million people across the planet, potentially leading to the displacement of up to 187 million people (Li et al. 2009; Nicholls et al. 2011). Low-lying islands are likely to be among the worst impacted, with coral atolls facing the greatest risk due to their low elevation, sandy nature and limited fresh water resources (Roy and Connell 1991; Barnett and Adger 2003). Coral atoll islands are already witnessing rapid changes due to flooding and erosion, and it is feared that between sea-level rise and frequent storms, they could see even more extreme erosion and the contamination of limited freshwater lenses (Yang et al. 2020; Duvat et al. 2021). Many atoll islands are thus at risk of becoming uninhabitable well before 2100 (Storlazzi et al. 2018). To add to these global risks, reefs of heavily populated atolls also have to cope with a host of additional stressors, including sedimentation, pollution, overfishing and developmental pressures which can reduce coral health and slow reef recovery (Carden 2013; Graves et al. 2021).
Whether atoll islands are able to buffer the impacts of climate change depends, in large part, on the positive growth of their coral-built frameworks and their ability to keep up with predicted sea-level rise (SLR). Reef structures are an important primary defence against rising sea levels and coastal erosion, contributing to coastal stability by attenuating wave energy, which becomes particularly crucial during high-intensity weather events (Ferrario et al. 2014; Costa et al. 2016; Elliff and Silva 2017). Reef growth can be represented as carbonate budgets, which reflect the net accumulation of biogenic carbonate deposition. On a healthy reef, coral and coralline algae are typically able to generate enough calcium carbonate to outpace erosional processes and to sustain atoll frameworks (Perry et al. 2011). The ability of reefs to maintain positive growth depends on a variety of factors including coral cover, coral community composition and species-specific growth rates, the abundance and activity of internal and external bioeroders, the history of physical disturbances like storms and cyclones as well as local water conditions (Tomascik 1990; Perry et al. 2012).
With the widespread degradation of reefs across the tropics, natural reef accretion processes are being disrupted, leading to major reductions in carbonate budgets (Perry et al. 2018b). Studies indicate that reefs need to sustain a carbonate production rate of roughly 5 G (CaCO3 m−2 yr−1) to provide optimal shoreline protection (Vecsei 2004; Perry et al. 2018b; Roik et al. 2018). Carbonate budgets for many reefs in the Caribbean and in the Pacific Ocean fall considerably short of this baseline (Perry et al. 2013; Molina-Hernández et al. 2020). Evaluations of carbonate production in Indian Ocean atolls show that repeated mass bleaching events have had a major impact, with some reefs in the Maldives and the Chagos Archipelago showing net negative carbonate budgets following the 2016 mass bleaching event, indicating that the physical structure of the reef is eroding away (Januchowski-Hartley et al. 2017; Perry and Morgan 2017; Lange and Perry 2019). Many of these reefs are on a pathway of recovery, but it is likely that due to repeated bleaching events, they will struggle to obtain optimal carbonate budgets (Lange et al. 2022).
Accretion rates can be highly variable and dynamic, changing dramatically between locations, and with different disturbance histories (de Bakker et al. 2019; Ryan et al. 2019; Brown et al. 2021). Different species of calcifying coral vary considerably in their susceptibility to temperature stress, physical breakage and local stressors such as water quality, making them differentially resilient to mass bleaching events and cyclonic events (Ryan et al. 2019). These global and regional drivers are also mediated by a host of locational factors including depth, wave exposure and temperature that can lead to considerable variability in disturbance responses and post-disturbance recovery patterns (Williams et al. 2013; Lange et al. 2021). Erosional processes on the reef are also influenced by local factors such as nutrient and sediment runoff, pH change and overfishing (Bellwood et al. 2012; Barkley et al. 2015). These can have major impacts on carbonate budgets both directly and indirectly (Rice et al. 2020). Separating global factors, such as mass bleaching events, and regional factors, such as cyclones, from local factors contributing to variability in reef accretion is seldom easy because they dynamically interact to influence the accretion potential of reefs. Yet they hold very different implications for the management of critical reef functions and for the long-term habitability of atoll islands.
We used the census-based ReefBudget methodology developed by Perry et al (2012) and updated for the Indo-Pacific (Perry et al. 2018a) to explore how carbonate budgets varied across three atolls in the Lakshadweep archipelago, a densely populated atoll system in the western Indian Ocean. All of these reefs have been impacted by successive mass bleaching events (Table 1, Yadav et al. 2018), but each island differs in their local stressors, including population density and fishing pressure. This variation allows us to explore the impacts of local and global forces on carbonate budgets. We also explore how maximum vertical reef accretion potential (RAPmax in mm yr−1) (Perry et al. 2013, 2015b) of the shallow reefs compare to projected SLR under three climate scenarios.
Methods
Study area
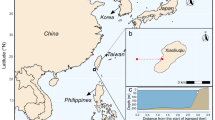
The Lakshadweep Archipelago is a group of ten inhabited atolls, and a number of other islands, islets and sand banks located off the southwest coast of India. It is the northernmost group of islands in the Chagos–Maldives–Laccadive ridge and, as such, shares many geomorphic features with other atoll groups along the ridge. The islands are surrounded by reefs that form protective rims around shallow lagoons. The last national census recorded 64,471 people living on the permanently inhabited islands (Census Commission of India 2011) although the population has since grown considerably. By official estimates, the population density of the archipelago is 2013 people per square kilometre, making it one of the most densely populated subdivisions in India (Census Commission of India 2011). The islands are urbanising rapidly, placing significant strain on island resources, particularly on limited fresh groundwater supply (Najeeb et al. 2021).
We assessed reef carbonate budgets on ten reefs around three atolls, Kavaratti, Kadmat and Agatti. The islands within the atoll each lie on a roughly north–south axis, with islands typically situated on the easternmost fringe of the atoll, leaving a large lagoon to the west. The incoming southwest monsoon affects the western aspect disproportionately, creating distinct exposed (western) and sheltered (eastern) aspects to the reef that strongly influence coral composition and recovery patterns in Lakshadweep (Arthur et al. 2006). The islands also face varying anthropogenic pressures (Table 1). Kavaratti is the capital of the territory and is the most urbanised of all islands in the archipelago. Agatti is much less urbanised and has a lower population density. However, it is a major fishing hub and has the largest fishing fleet of the atolls sampled, including a recently growing reef-based fishery (Jaini et al. 2018). At around 5400 individuals, Kadmat has the half the population of Kavaratti and is the most rural of the three selected atolls although it also has an active fishery (James and Arthur, in press) (Fig. 1).

Modified from Yadav et al. (2018)
A map showing the study area and the study sites.
Over the last two decades, reefs across these atolls have been affected by repeated mass bleaching events and large-scale coral mortality in 1998, 2010 and 2016. Long-term monitoring data from these atolls started in 1998 indicate that the impact of the last two major bleaching events was higher on reefs in Kadmat and Agatti compared to Kavaratti (Yadav et al. 2018). However, in 2018, an unusually large tropical weather system, severe cyclonic storm Ockhi strongly affected the eastern reefs of Kavaratti resulting in mass coral mortality (Riyas et al. 2020), leaving the other atolls relatively undisturbed.
Estimating carbonate budgets
All surveys were conducted between January and March of 2022. At each selected atoll, we conducted rapid surveys to allow us to select sites to sample. We surveyed eight sites across the atolls’ outer reefs where we evaluated the reef based on its dominant carbonate producers and eroders. Based on these rapid surveys, we chose two sites on the sheltered and two on the exposed aspects on Kavaratti and Agatti. On Kadmat, we were able to sample only one sheltered and one exposed site (total number of sampled sites: 10). These sites were selected to be as representative of the atoll as possible. At each site, we sampled at two different depths: shallow (4–6 m) and deep (9–11 m). We used two different depths to explore the impacts of wave action across the depth profile of the reef. To measure the carbonate budgets, we used the ReefBudget methodology developed by Perry et al. (2012) and later modified for the Indo-Pacific (Perry et al. 2018a). The ReefBudget is a census-based approach that allows for a rapid assessment of net carbonate production. Carbonate budgets are estimated as the difference between carbonate production by coral and calcifying algae, and bioerosion by parrotfish, urchins, and other reef bioeroders.
Carbonate production
To estimate the carbonate production of the reef, we laid three 10-m line transects (n = 3 per depth at each site) along which all benthic groups including coral genera and growth form, bare substrate, filamentous and calcifying algae were measured to the nearest cm along the contour of the reef using a separate flexible measuring tape. Transects were placed at least 20 m from each other. Carbonate production was then calculated for each genus using genera- and growth-form-specific calcium production rates from published sources in the Indo-Pacific region (Perry et al. 2018a and references therein). Similarly, carbonate production for calcifying algae was calculated using calcification rates from published studies in the Indo-Pacific (Perry et al. 2018a and references therein). These data were then summed for each transect and averaged across three replicate transects to estimate gross carbonate production at each location.
Carbonate bioerosion
To estimate bioerosion by parrotfish, we conducted three replicate 50 × 5 m belt transects at the same depth as the coral transects which were placed at least 20 m apart (n = 3 per depth at each site). On these transects, we quantified abundances of all parrotfish, recording species, size class and life stage. All these factors impact bioerosion rates to differing degrees (Bruggemann et al. 1996; Lellys et al. 2019). We then used Indo-Pacific size, life phase and species-specific averages for parrotfish bioerosion rates (Perry et al. 2018a, b and references therein) to estimate bioerosion for each transect, which was then averaged across the three transects. Urchin erosion was estimated by first quantifying urchin abundance along 10 × 1 m belt transects along the same transect used to measure benthic composition. Urchin species and test size, to the nearest cm, was also recorded. We then used the relationships between urchin size and bioerosion rates published by Perry et al. (2018a) to estimate total bioerosion for the transect which was averaged across three replicates for a reef-wide rate of urchin bioerosion. The bioerosion rates for the other of the bioeroders, which includes sponges, bivalves and other macro- and micro-bioeroders, were estimated using a relationship between available substrate and observed rates of bioerosion in the Indo-Pacific (Perry et al. 2018a and references therein).
Net carbonate production
Net carbonate production is the difference between carbonate production (gross coral and calcifying algae production) and total bioerosion (gross sea urchin erosion, gross parrotfish erosion and estimated sponge and other macro- and micro-bioerosion), measured in kg CaCO3 m−2 yr−1 (G).
Projecting increased water depth by 2100 based on RAPmax
We used the method developed by Perry et al. (2013, 2015b; 2018b) to calculate reef accretion potential (RAPmax in mm yr−1), an estimate of maximum vertical reef growth rates based on the carbonate budgets. For this analysis, we only considered data from shallow sites, which have the highest potential to protect shorelines. We transformed current net carbonate production to RAPmax, accounting for sediment infilling by parrotfish (25%) and other bioeroders (50%), and framework porosity by different coral growth forms (30% for massive coral-dominated assemblages; 70% for branching/tabular coral-dominated assemblages; 50% for mixed coral assemblages). The method also accounts for background physical export of sediments (exposed sites: 50% and sheltered sites: 20%). We used the projected SLR for Lakshadweep for three SSPs (shared socioeconomic pathways) from the IPCC AR6 (Sixth Assessment Report of the Intergovernmental Panel on Climate Change) (Garner et al. 2021; Fox-Kemper et al. 2021, Garner in prep). The three scenarios included are: SSP1 1.6, a best-case scenario where temperatures are limited to within 1.5 °C, SSP 2 4.5 m, a middle of the road scenario in which temperature rise by 2.1–3.5 °C by 2100, and SSP5 8.5, a business-as-usual scenario which sees temperature increases between 3.3 and 5.5 °C by 2100. We then compared the median, 17th and 83rd percentile of the SLR projections of the three scenarios to RAPmax on the reef. We used the 17th and 83th percentile as the likely range of SLR. The increase in water depth above the reef was calculated by comparing estimated SLR in 2100 to the projected reef height.
Analysis
To estimate the effect of different factors (island, exposure and depth) in contributing to the overall carbonate production and erosion, we used generalised linear mixed effects models (GLMM) with a gamma distribution and linear mixed effects model (LMM) depending on the type of data. We used site as a random effect nested within island (fixed factor with three levels), wave exposure (fixed factor with two levels: exposed and sheltered) and depth (fixed factor with two levels: shallow and deep) for all the models. For carbonate production and urchin erosion, we used generalised linear mixed effects models (GLMM) with a gamma distribution. The parrotfish data were log transformed (after adding a small constant to remove zeros) and then analysed with a linear mixed effects model (LMM), and net carbonate production was directly analysed using an LMM as these data were normally distributed when assessed visually. All analyses were conducted using the lme4 package in R (Bates et al. 2015). The GLMMs were analysed using the function glmer, while the LMMs were analysed with the function lmer.
To analyse differences in the contribution of coral genera and functional groups to carbonate production, we divided sites into three broad categories based on carbonate production: high production sites (> 5 G), moderate production sites (between 3.5 and 5 G) and low production sites (< 3.5 G). We chose these classes to have a similar number of sites in each group for comparison, but also because carbonate budgets of 5 G represents carbonate budgets in healthy reefs (Vecsei 2004; Perry et al. 2018b). We then tested whether the proportion of production from the four coral functional groups, outlined by Darling et al. (2012), differed between the production categories using Kruskal–Wallis tests. Kruskal–Wallis tests were used as the data were not normally distributed. The classification of coral life histories by Darling et al. (2012) includes four categories, competitive, stress tolerant, generalist and weedy, depending on their strategy. All analysis was conducted in R (version 4.0.2, R CoreTeam 2020).
Results
Across the three atolls surveyed, net carbonate production averaged at 2.25 G (± 2.85 G, SD). Net carbonate production was significantly lower at sites around Kavaratti compared to Kadmat and Agatti (Table S1, Fig. 3a). Exposed shallow sites showed the highest net carbonate production, driven by a combination of generally high coral cover and lower rates of parrotfish erosion (Table S1, Fig. 4a).
Carbonate production differences in sites were driven mostly by large differences in live coral cover across reefs; CCA contributed only 2.1% on average to total carbonate production in the studied sites. Sites in Kavaratti had the lowest average carbonate production, with values typically between 2 and 5 G (Mean ± SD, 3.57 G ± 2.46 G) (Figs. 2, 3b). However, both the highest and lowest carbonate production sites were located in Kavaratti. Reefs in Agatti and Kadmat were more similar to each other in carbonate production rates averaging 5.17 G (± 1.54 G SD). There was no significant difference in carbonate production between islands as most differences occurred at the site-level (Fig. 3a, Table S2). The interaction between depth and exposure had a significant impact of carbonate production (Fig. 4b, Table S2). Although production rates did not vary much between the two exposure regimes in deeper sites, shallow exposed reefs had nearly twice the production rates as shallow sheltered sites (Fig. 4b).
Variability of a net carbonate production, b carbonate production, c parrotfish erosion and d urchin erosion between sites sampled across the three islands. W indicates sites on the western aspect, while E indicates sites on the eastern aspect. Y-axis shows either production or erosion of calcium carbonate in G (kg CaCO3 m−2 yr−1). Error bars represent the standard errors
Parrotfish were the dominant bioeroders in Lakshadweep, contributing 47% of the total bioerosion, while urchins and endolithic borers together contributed around 25% each. Chlorurus strongylocephalus was the most important parrotfish bioeroder followed by Scarus rubroviolaceus accounting for 68.9% and 10.7% of parrotfish bioerosion, respectively. Bioerosion by parrotfish varied significantly between islands and was consistently low in Agatti, where rates of bioerosion were more than 9 times lower than those at other surveyed atolls (Table S4, Fig. 3d). This difference was also reflected in the densities of parrotfish which were also much lower in Agatti. Parrotfish erosion tended to concentrate in deeper sites and was the highest on exposed deep sites (Table S4, Fig. 4d).
The sea urchin Echinostrephus molaris was the only species recorded on our transects, save for a single recorded individual of Echinometra mathaei, across all sites. Urchin erosion was consistently low in Kadmat and Agatti, but was significantly higher on the reefs of highly urbanised Kavaratti (Table S3, Fig. 3c). Neither depth nor wave exposure influenced rates of urchin erosion (Fig. 4c, Table S3).
Low carbonate production sites (with carbonate production rates less than 3.5 G) had significantly higher relative contributions by competitive taxa than moderate (3.5–5 G) and high (> 5 G) carbonate production sites (H(2) = 8.93, p = 0.01) (Fig. 5a). A major contributor to this difference were corals of the genus Pocillopora (Fig. 5b). In Lakshadweep’s outer reefs Pocillopora verrucosa, a competitive species is most often encountered. Though stress-tolerant taxa such as Porites were greater contributors at high production sites, this was not a statistically significant difference.
Carbonate production at high, moderate and low carbonate production sites divided by a coral functional groups based on Darling et al. (2012), b the six most important coral genera. Carbonate production values are in G (kg CaCO3 m−2 yr−1)
In the year 2100, the average expected increase in water depth above the reefs for SSP1 1.9 is 0.28 m (17–83th percentile, − 0.07 to 0.56 m), for SSP2 4.5, it is 0.42 m (0.10–0.76 m) and for SSP5 8.5, it is 0.67 m (0.33–1.07 m). None of our sampled sites (0–70%) are projected to show an increased depth of above 0.5 m in 2100 under SSP1 1.9. However, under SSP2 4.5, this number rose to 30% (0–100%), and under SSP5 8.5, every sampled site breached the half metre benchmark (5–100%) (Fig. 6). This benchmark is considered important as models suggest that depth increases beyond this level would lead to significant changes in wave energy regimes (Siegle and Costa 2017; Perry et al. 2018a, b). The large uncertainties indicate the variation in climate models and show that even under optimistic scenarios, there is a possibility that a majority of reefs will cross this 0.5 m threshold.
Increased water depth in 2100 for each island using the median predicted SLR and three SSPs (shared socioeconomic pathways): the three scenarios included are SSP1 1.6, a best-case scenario where temperatures are limited to within 1.5C, SSP2 4.5, a middle of the road scenario, and SSP5 8.5, a business-as-usual scenario. Only shallow transects were considered for these estimates
Discussion
The difficulty in facing the impacts of the climate crisis is that the scales of drivers are much vaster than traditional scales of management. Local actions have limited ability to protect atolls from extreme heat events, cyclones or SLR. Yet how reefs respond to these large-scale events are highly contingent upon geography and local anthropogenic stressors. Unpacking the variability in carbonate budgets on Lakshadweep reefs, the signature of these local forces is evident, even as the history of disturbances has reduced the overall carbonate budgets of many of these reefs to suboptimal levels. We found that bioerosion rates vary between the three atolls, suggesting that each island’s developmental trajectories impose differential anthropogenic pressures on its reefs. Understanding the source of these pressures could form the basis of reef management. We also observed variability in the budget and its components within an atoll suggesting that they are strongly influenced by sub-atoll variations in benthic status, post-disturbance recovery trajectories and distributional differences in fish and urchin numbers.
Carbonate production and coral bleaching
The impact of global stressors is clear when looking at carbonate production. Repeated mass bleaching events in 1998, 2010 and 2016 have filtered the coral community, strongly altering the composition of Lakshadweep reefs (Yadav et al 2018). One way this is visible is through the loss of Acropora from many of these reefs. As a result, we found that competitive genera, such as Pocillopora and Acropora, were higher contributors in low production sites as compared to medium and high production sites. On healthy, relatively undisturbed reefs in the Indo-Pacific, the main contributors to carbonate production and framework building are competitive species with faster growth rates, typically those in the genus Acropora (Perry et al. 2015b; De Bakker et al. 2016). However, these competitive species are also particularly sensitive to heat stress and breakage and have been among the earliest casualties of climate change. Our results indicate that across the Lakshadweep, those taxa have been undergoing a declining role as the dominant structural framework building corals on shallow water reefs, while stress-tolerant taxa are taking up this function. A similar pattern has been documented in the Chagos archipelago, where the loss of Acropora from reefs has led to a severe, but seemingly temporal, decline in carbonate budgets (Lange and Perry 2019). Over the last 6 years, isolated reefs in the Indian Ocean have been recovering, and both coral cover and carbonate production have increased to 40–80% of pre-bleaching levels (Lange et al. 2022). However, with bleaching events becoming more frequent, it is likely that stress-tolerant species will increasingly dominate as the most important carbonate producers on the reefs of the near future (Hughes et al. 2018; Lange and Perry 2019). Whether these typically slower-growing species can contribute sufficiently to carbonate budgets to keep up with SLR will need further exploration.
Carbonate production and the influence of storms
The severe cyclonic storm Ockhi hit Lakshadweep in December 2017 devastating the eastern reefs of Kavaratti (Riyas et al. 2020), the only atoll we sampled in the path of the cyclone. While one western site (protected from the cyclone) had the highest carbonate production seen across all atolls, the reefs on Kavaratti’s eastern aspect showed low carbonate production rates (Fig. 3b). Major storms and cyclones can have big impacts on both individual colony mortality, which can be as high as 100% for some species, and on total live coral cover (Dollar and Tribble 1993; Cheal et al. 2017; Baird et al. 2018). Cyclone Ockhi interrupted the slow recovery trajectory Kavaratti’s sheltered reefs were on in the wake of the 2016 mass bleaching event. This resulted in Kavaratti’s budgets being, on average, lower than the other two atolls, despite being less impacted by bleaching during the 2016 event (Yadav et al. 2018; Riyas et al. 2020). Extreme storms are rare in the Arabian Sea but are projected to get far more common and intense in the coming decades due to anthropogenic climate change (Murakami et al. 2017). These changing meteorological patterns will continue to disrupt recovery trajectories.
Carbonate production and wave exposure
Carbonate production was the highest on exposed shallow reefs compared to deep or sheltered reefs (Fig. 4b). Previous studies in Lakshadweep have found that wave exposed reefs have better rates of post-disturbance recovery linked to higher recruit survival (Arthur et al. 2006; Yadav et al. 2016). Western aspects experience the full force of the annual monsoon winds; post-disturbance, coral rubble and debris are rapidly washed from the reefs leaving stable substrates for new coral recruits to settle on, resulting in faster recovery at these sites (Yadav et al. 2016). On sheltered reefs in contrast, many dead coral structures persist after a major mortality. These structures, such as large table corals, are preferentially settled on by coral recruits but become unstable through time and collapse, leading to reduced recovery on sheltered reefs (Arthur et al. 2006; Yadav et al. 2016). Previous studies have found varying results of the effects of wave action on carbonate production (Perry et al. 2015b; Lange et al. 2021). In the Chagos Archipelago, the lower carbonate production rates on exposed reefs compared to sheltered reefs prior to bleaching was due to the greater presence of Acropora on sheltered reefs, as these species are more susceptible to wave damage (Williams et al. 2013; Perry et al. 2015b). However, the decline of Acropora from Lakshadweep’s reefs due to repeated bleaching over the last two decades could explain the patterns we see (Yadav et al. 2018). Wave energy attenuates rapidly with depth, which is likely why differences in carbonate production between exposure regimes is not evident for deeper reefs (Siegle and Costa 2017; Perry et al. 2018b).
Erosion rates and fishing
The erosional components of the carbonate budget in Lakshadweep are likely driven by local anthropogenic stressors, specific to each island. Though we do not evaluate the impact of for these stressors directly, previous work has shown that anthropogenic stressors such as fishing pressure and nutrient enrichment can impact parrotfish and urchin abundances (McClanahan and Muthiga 1988; Bellwood et al. 2012). We recorded remarkably low rates of parrotfish erosion (0.18 G ± 0.14 G) across Agatti’s reefs. Commercial reef fishing is a relatively recent development in Lakshadweep, and Agatti has the largest fishing fleet across the archipelago, representing 40% of the fleet between the three atolls we studied. While the bulk of this fishery targets groupers and snappers, at Agatti, large parrotfish (Chlorurus strongylocephalus, Scarus ghobban, and others) are also caught in large numbers to be sold locally or to collector boats (unpublished data). These species contribute the majority of total parrotfish erosion, and their removal through overfishing could be leading to significantly lower rates of erosion in Agatti. While this means that net carbonate production in Agatti was the highest across all three atolls, it comes at the cost of declining parrotfish populations. Apart from their role as bioeroders, parrotfish are central to several other important reef processes including reducing algal cover and promoting coral recruitment (Mumby 2009). Bioerosion by parrotfish also plays an important role in sediment transport and island dynamics which can be disrupted when parrotfish numbers decline (Morgan and Kench 2016; Perry 2015a).
Erosion rates and pollution
Another instance of how local pressures could be impacting the carbonate budget is the differences in urchin bioerosion. Urchin erosion across the atolls surveyed was dominated by a single burrowing species, Echinostrephus molaris. At Kavaratti densities of this species averaged at 12.7 indv m−2, compared to an average of 2.9 indv m−2 for the other two islands. The archipelago wide average of 6.8 indv m−2 is an order of magnitude higher than other regions in the Indo-Pacific such as Madagascar (Nadon et al. 2007), Sri Lanka (Gayashan and Jayakody 2014) and the Pacific Islands (Bak 1990) where densities are all below 0.5 indv m−2. The atoll-wide proliferation of E. molaris at Kavaratti hints at high levels of land-based nutrient inputs from this urbanised island. E. molaris is a filter feeder, and its recruitment success, survival and growth may be linked to higher nutrient regimes as has been reported for other sea urchins (Cardona et al. 2013). As the administrative capital, there has been a surge of developmental activities on Kavaratti over the last few decades which is likely to continue (MHUA). Past work has shown that carbonate budgets can be strongly influenced by local factors such as pollution (de Bakker et al. 2019), and the increased runoff from domestic, industrial and construction purposes in Kavaratti could be influencing coral recovery negatively (Birrell et al. 2005; Zaneveld et al. 2016). These links require confirmation as it is unclear whether nutrient runoff can impact outer reefs, which are often better flushed. Further studies may also be needed to discount other factors such as overfishing of predators that may contribute to higher urchin numbers at Kavaratti (Dee et al. 2012; Dunn and Hovel 2019).
In the calculation of urchin erosion, we assume that urchins are continuously eroding, and this erosion rate proportionally increases with urchin size (Perry et al. 2018a). However, the habits of E. molaris indicate that it is a burrowing animal that seldom ventures out, hinting at a potential overestimation of the erosion rates (Campbell et al. 1973). Despite this, the high density of urchins indicates that their erosion is likely to play a large role in the carbonate budgets of these reefs.
Lakshadweep in context
Compared with an optimal of 5–10 G reported from high coral cover reefs (Vecsei 2004; Perry et al. 2018b; Roik et al. 2018), net carbonate budgets across Lakshadweep were generally low, reflecting a history of successive climate disturbances over the last quarter of a century. However, there was considerable variation in carbonate budgets between reefs, with some sites accreting at rates higher than 5 G, while others were already net eroding, below 0 G. By our estimates, carbonate budgets of Lakshadweep’s reefs generally tend to be negative if coral cover falls below 10.2%, a threshold similar to the 12% estimated in the Maldives and the 10% threshold estimated in the Caribbean (Perry et al. 2013; Perry and Morgan 2017). As others have suggested, 10–12% of live coral cover may be a universal minimum to keep reefs net accreting. Lakshadweep confirms a global trend—that atoll reefs are in a poor state, with low carbonate production rates that could threaten island life and livelihoods in the near future (Perry et al. 2018b). However, these results also reflect significant recovery of Lakshadweep’s reefs since 2016, a finding also reflected in a recent study of reefs in the Chagos archipelago (Lange et al. 2022). This potential for a significant recovery over a relatively short period of 3–4 yr indicates that Lakshadweep reefs may still retain some resilience to global disturbances, given enough time between events. However, with the increasing frequency of El Niño events, it is likely that recovery times will decrease (Cai et al. 2014). If Lakshadweep experiences frequent bleaching events, carbonate budgets in the archipelago could remain in a permanently low production state (Perry et al. 2008).
Future projections and RAPmax
By the year 2100, water depth above almost half the reefs in Lakshadweep will increase by more than 0.5 m under SSP2 4.5, while all reefs will breach this threshold under SSP5 8.5. Beyond half a metre, the reefs capacity to protect shores will likely change drastically (Siegle and Costa 2017; Perry et al. 2018a, b). These results support trends observed for reefs across the world, showing that accretion in many locations will be unlikely to track SLR (Perry et al. 2018a, b). This would imply major changes for the Lakshadweep under a business-as-usual scenario, with increased salination of freshwater and inundation likely in the coming decades.
We caution that these results need to be interpreted with care. The conversion of carbonate budgets to RAPmax is not precise and does not account for numerous other process that influence the growth of the reef crest. These projections also assume that carbonate budgets on the reefs will remain the same in the coming decades and are not degraded further. However, it is likely that both carbonate budgets will vary depending on the climate change scenario, recovery dynamics and how anthropogenic pressure shifts. Under higher emissions scenarios, coral cover is likely to reduce even further due to increased coral mortality through bleaching, leading to even lower budgets (Hoegh-Guldberg et al. 2007; Woesik et al. 2018).
Local management and island futures
Our results indicate that understanding the drivers of critical reef functions can help identify where local efforts can still be effective. Importantly, while El Niño events and extreme storms are global in their causes, their impacts are mediated by local factors linked to reef location and anthropogenic stressors. Managing these stressors remains the best way to try to protect these functions. In the case of Lakshadweep, this could mean exploring ways to control land-based pollution as the islands rapidly urbanise and regulating overfishing of key reef species. Whether these efforts will be sufficient to buffer atoll systems from the effects of global climate change is an open question. Of course, the fate of atoll inhabitants ultimately lies in countries, both in the developed and developing world, urgently reducing greenhouse gases. Until then though, the best strategy for atoll islanders is to ensure that local actions help maintain functionally resilient reef frameworks in the face of an increasingly uncertain future.
References
Arthur R, Done TJ, Marsh H, Harriott V (2006) Local processes strongly influence post-bleaching benthic recovery in the Lakshadweep Islands. Coral Reefs 25:427–440
Baird A, Álvarez-Noriega M, Cumbo V, Connolly S, Dornelas M, Madin J (2018) Effects of tropical storms on the demography of reef corals. Mar Ecol Prog Ser 606:29–38
Bak R (1990) Patterns of echinoid bioerosion in two Pacific coral reef lagoons. Mar Ecol Prog Ser 66:267–272
Barkley HC, Cohen AL, Golbuu Y, Starczak VR, DeCarlo TM, Shamberger KEF (2015) Changes in coral reef communities across a natural gradient in seawater pH. Sci Adv 1:
Barnett J, Adger WN (2003) “Climate dangers and atoll countries”: Climate change. Clim Chang 61:321–337
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48
Bellwood DR, Hoey AS, Hughes TP (2012) Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc R Soc B Biol Sci 279:1621–1629
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. 51:408–414
Brown KT, Bender-Champ D, Achlatis M, van der Zande RM, Kubicek A, Martin SB, Castro-Sanguino C, Dove SG, Hoegh-Guldberg O (2021) Habitat-specific biogenic production and erosion influences net framework and sediment coral reef carbonate budgets. Limnol Oceanogr 66:349–365
Bruggemann J, van Kessel A, van Rooij J, Breeman A (1996) Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride:implications of fish size, feeding mode and habitat use. Mar Ecol Prog Ser 134:59–71
Cai W, Borlace S, Lengaigne M, Van Rensch P, Collins M, Vecchi G, Timmermann A, Santoso A, Mcphaden MJ, Wu L, England MH, Wang G, Guilyardi E, Jin FF (2014) Increasing frequency of extreme El Niño events due to greenhouse warming. Nat Clim Chang 4:111–116
Campbell AC, Dart JKG, Head SM, Ormond RFG (1973) The feeding activity of Echinostrephus molaris (de Blainville) in the central red sea. Mar Behav Physiol 2:155–169
Carden YR (2013) Solid Waste-level Rise on Atoll Nation States: A Less Publicised Environmental Issue in the Republic of Kiribati. 10:35–45
Cardona L, Moranta J, Reñones O, Hereu B (2013) Pulses of phytoplanktonic productivity may enhance sea urchin abundance and induce state shifts in Mediterranean rocky reefs. Estuar Coast Shelf Sci 133:88–96
Census Commission of India (2011) Census of India 2011
Cheal AJ, MacNeil MA, Emslie MJ, Sweatman H (2017) The threat to coral reefs from more intense cyclones under climate change. Glob Chang Biol 23:1511–1524
Costa MBSF, Araújo M, Araújo TCM, Siegle E (2016) Influence of reef geometry on wave attenuation on a Brazilian coral reef. Geomorphology 253:318–327
Darling ES, Alvarez-Filip L, Oliver TA, Mcclanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386
De Bakker DM, Meesters EH, Bak RPM, Nieuwland G, Van Duyl FC (2016) Long-term Shifts in Coral Communities On Shallow to Deep Reef Slopes of Curaçao and Bonaire: Are There Any Winners? Front Mar Sci 3:247
de Bakker DM, van Duyl FC, Perry CT, Meesters EH (2019) Extreme spatial heterogeneity in carbonate accretion potential on a Caribbean fringing reef linked to local human disturbance gradients. Glob Chang Biol 25:4092–4104
Dee LE, Witman JD, Brandt M (2012) Refugia and top-down control of the pencil urchin Eucidaris galapagensis in the Galápagos Marine Reserve. J Exp Mar Bio Ecol 416–417:135–143
Dollar SJ, Tribble GW (1993) Recurrent storm disturbance and recovery: a long-term study of coral communities in Hawaii. Coral Reefs 12:223–233
Dunn RP, Hovel KA (2019) Experiments reveal limited top-down control of key herbivores in southern California kelp forests. Ecology 100:e02625
Duvat VKE, Magnan AK, Perry CT, Spencer T, Bell JD, Wabnitz CCC, Webb AP, White I, McInnes KL, Gattuso JP, Graham NAJ, Nunn PD, Le Cozannet G (2021) Risks to future atoll habitability from climate-driven environmental changes. Wiley Interdiscip Rev Clim Chang 12:e700
Ferrario F, Beck MW, Storlazzi CD, Micheli F, Shepard CC, Airoldi L (2014) The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat Commun 5
Fox-Kemper B, Hewitt HT, Xiao C, Aðalgeirsdóttir G, Drijfhout SS, Edwards TL, Golledge NR, Hemer M, Kopp RE, Krinner G, Mix A, Notz D, Nowicki S, Nurhati IS, Ruiz L, Sallée J-B, Slangen ABA, Yu Y (2021) Ocean, Cryosphere and Sea Level Change. In: Masson-Delmotte V., Zhai P., Pirani A., Connors S.L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M.I., Huang M., Leitzell K., Lonnoy E., Matthews J.B.R., Maycock T.K., Waterfield T., Yelekçi O., Yu R., Zhou B. (eds) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. pp 1211–1362
Garner GG, Hermans T, Kopp RE, Slangen ABA, Edwards TL, Levermann A, Nowikci S, Palmer MD, Smith C, Fox-Kemper B, Hewitt HT, Xiao C, Aðalgeirsdóttir G, Drijfhout SS, Edwards TL, Golledge NR, Hemer M, Kopp RE, Krinner G, Mix A, Notz D, Nowicki S, Nurhati IS, Ruiz L, Sallée J-B, Yu Y, Hua L, Palmer T, Pearson B (2021) IPCC AR6 Sea-Level Rise Projections. Version 20210809. PO.DAAC, CA, USA. Dataset accessed 2021–03–07.
Garner GG, Kopp RE, Hermans T, Slangen ABA, Koubbe G, Turilli M, Jha S, Edwards TL, Levermann A, Nowikci S, Palmer MD, Smith C Framework for Assessing Changes To Sea-level (FACTS). Geoscientific Model Development, in prep. Geosci Model Dev
Gayashan M, Jayakody S (2014) Diversity and density of sea urchins populations in rocky shores off Nilwella in Southern province of Sri Lanka. Sri Lanka J Aquat Sci 17:35
Graves CA, Powell A, Stone M, Redfern F, Biko T, Devlin M (2021) Marine water quality of a densely populated Pacific atoll (Tarawa, Kiribati): Cumulative pressures and resulting impacts on ecosystem and human health. Mar Pollut Bull 163:111951
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science (80- ) 359:80–83
Jaini M, Advani S, Shanker K, Oommen MA, Namboothri N (2018) History, culture, infrastructure and export markets shape fisheries and reef accessibility in India’s contrasting oceanic islands. Environ Conserv 45:41–48
James S, Arthur R Breaching the bounds: Urbanisation on a low-lying coral atoll. In: Nagendra H., Mundoli S. (eds) Urban sustainability challenges in India. Orient Blackswan, India,
Januchowski-Hartley FA, Graham NAJ, Wilson SK, Jennings S, Perry CT (2017) Drivers and predictions of coral reef carbonate budget trajectories. Proc R Soc B Biol Sci 284:
Lange ID, Perry CT (2019) Bleaching impacts on carbonate production in the Chagos Archipelago: influence of functional coral groups on carbonate budget trajectories. Coral Reefs 38:619–624
Lange ID, Benkwitt CE, McDevitt-Irwin JM, Tietjen KL, Taylor B, Chinkin M, Gunn RL, Palmisciano M, Steyaert M, Wilson B, East HK, Turner J, Graham NAJ, Perry CT (2021) Wave exposure shapes reef community composition and recovery trajectories at a remote coral atoll. Coral Reefs 40:1819–1829
Lange ID, Perry CT, Stuhr M (2022) Recovery trends of reef carbonate budgets at remote coral atolls 6 years post-bleaching. Limnol Oceanogr
Lellys NT, De Moura RL, Bonaldo RM, Francini-Filho RB, Gibran FZ (2019) Parrotfish functional morphology and bioerosion on SW Atlantic reefs. Mar Ecol Prog Ser 629:149–163
Li X, Rowley RJ, Kostelnick JC, Braaten D, Meisel J, Hulbutta K (2009) GIS Analysis of Global Impacts from Sea Level Rise. Photogramm Eng Remote Sensing 75:807–818
McClanahan TR, Muthiga NA (1988) Changes in Kenyan coral reef community structure and function due to exploitation. Hydrobiol 1988 1663 166:269–276
MHUA Smart Cities Mission. http://smartcities.gov.in/content/innerpage/what-is-smart-city.php
Molina-Hernández A, González-Barrios FJ, Perry CT, Álvarez-Filip L (2020) Two decades of carbonate budget change on shifted coral reef assemblages: are these reefs being locked into low net budget states? Proceedings Biol Sci 287:20202305
Morgan KM, Kench PS (2016) Parrotfish erosion underpins reef growth, sand talus development and island building in the Maldives. Sediment Geol 341:50–57
Mumby PJ (2009) Herbivory versus corallivory: Are parrotfish good or bad for Caribbean coral reefs? Coral Reefs 28:683–690
Murakami H, Vecchi GA, Underwood S (2017) Increasing frequency of extremely severe cyclonic storms over the Arabian Sea. Nat Clim Chang 7:885–889
Nadon M, Griffiths D, Doherty E, Harris AR (2007) The Status of Coral Reefs in the Remote Region of Andavadoaka, Southwest Madagascar. West Indian Ocean J Mar Sci 6:207–218
Najeeb KM, Vinayachandran N, Balakrishnan K (2021) Water Resources Development and Conservation in Lakshadweep Islands, India. 0:38–47
Nicholls RJ, Marinova N, Lowe JA, Brown S, Vellinga P, De Gusmão D, Hinkel J, Tol RSJ (2011) Sea-level rise and its possible impacts given a beyond 4C world in the twenty-first century. Philos Trans R Soc A Math Phys Eng Sci 369:161–181
Perry CT, Morgan KM (2017) Bleaching drives collapse in reef carbonate budgets and reef growth potential on southern Maldives reefs. Sci Rep 7:1–9
Perry CT, Spencer T, Kench PS (2008) Carbonate budgets and reef production states: A geomorphic perspective on the ecological phase-shift concept. Coral Reefs 27:853–866
Perry CT, Kench PS, Smithers SG, Riegl B, Yamano H, O’Leary MJ (2011) Implications of reef ecosystem change for the stability and maintenance of coral reef islands. Glob Chang Biol 17:3679–3696
Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ (2012) Estimating rates of biologically driven coral reef framework production and erosion: A new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31:853–868
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun 4:1–7
Perry CT, Kench PS, O’Leary MJ, Morgan KM, Januchowski-Hartley F (2015a) Linking reef ecology to island building: Parrotfish identified as major producers of island-building sediment in the Maldives. Geology 43:503–506
Perry CT, Murphy GN, Graham NAJ, Wilson SK, Januchowski-Hartley FA, East HK (2015b) Remote coral reefs can sustain high growth potential and may match future sea-level trends. Sci Rep 5:18289
Perry CT, Alvarez-Filip L, Graham NAJ, Mumby PJ, Wilson SK, Kench PS, Manzello DP, Morgan KM, Slangen ABA, Thomson DP, Januchowski-Hartley F, Smithers SG, Steneck RS, Carlton R, Edinger EN, Enochs IC, Estrada-Saldívar N, Haywood MDE, Kolodziej G, Murphy GN, Pérez-Cervantes E, Suchley A, Valentino L, Boenish R, Wilson M, MacDonald C (2018b) Loss of coral reef growth capacity to track future increases in sea level. Nature 558:396–400
Perry C, Lange I, Januchowski-Hartley F (2018a) ReefBudget Indo Pacific: online resource and methodology.
Rice MM, Maher RL, Correa AMS, Moeller HV, Lemoine NP, Shantz AA, Burkepile DE, Silbiger NJ (2020) Macroborer presence on corals increases with nutrient input and promotes parrotfish bioerosion. Coral Reefs 39:409–418
Riyas CA, Idreesbabu KK, Marimuthu N, Sureshkumar S (2020) Impact of the tropical cyclone Ockhi on ecological and geomorphological structures of the small low-lying Islands in the Central Indian Ocean. Reg Stud Mar Sci 33:100963
Roik A, Röthig T, Pogoreutz C, Saderne V, Voolstra CR (2018) Coral reef carbonate budgets and ecological drivers in the central Red Sea - A naturally high temperature and high total alkalinity environment. Biogeosciences 15:6277–6296
Roy P, Connell J (1991) Climatic Change and the Future of Atoll States. J Coast Res 7:1057–1075
Ryan EJ, Hanmer K, Kench PS (2019) Massive corals maintain a positive carbonate budget of a Maldivian upper reef platform despite major bleaching event. Sci Reports 2019 91 9:1–11
Siegle E, Costa MB (2017) Nearshore Wave Power Increase on Reef-Shaped Coasts Due to Sea-Level Rise. Earth’s Futur 5:1054–1065
Storlazzi CD, Gingerich SB, Van Dongeren A, Cheriton OM, Swarzenski PW, Quataert E, Voss CI, Field DW, Annamalai H, Piniak GA, McCall R (2018) Most atolls will be uninhabitable by the mid-21st century because of sea-level rise exacerbating wave-driven flooding. Sci Adv 4:eaap9741
Team RC (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Tomascik T (1990) Growth rates of two morphotypes of Montastrea annularis along a eutrophication gradient, Barbados, W.I. Mar Pollut Bull 21:
Vecsei A (2004) A new estimate of global reefal carbonate production including the fore-reefs. Glob Planet Change 43:
Williams GJ, Smith JE, Conklin EJ, Gove JM, Sala E, Sandin SA (2013) Benthic communities at two remote pacific coral reefs: Effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 2013:e81
Woesik R van, Köksal S, Ünal A, Cacciapaglia CW, Randall CJ (2018) Predicting coral dynamics through climate change. Sci Rep 8:17997
Yadav S, Rathod P, Alcoverro T, Arthur R (2016) “Choice” and destiny: the substrate composition and mechanical stability of settlement structures can mediate coral recruit fate in post-bleached reefs. Coral Reefs 35:211–222
Yadav S, Alcoverro T, Arthur R (2018) Coral reefs respond to repeated ENSO events with increasing resistance but reduced recovery capacities in the Lakshadweep archipelago. Coral Reefs 37:1245–1257
Yang H, Kagabu M, Okumura A, Shimada J, Shibata T, Pinti DL (2020) Hydrogeochemical processes and long-term effects of sea-level rise in an uplifted atoll island of Minami-Daito. Japan J Hydrol Reg Stud 31:100716
Zaneveld JR, Burkepile DE, Shantz AA, Pritchard CE, McMinds R, Payet JP, Welsh R, Correa AMS, Lemoine NP, Rosales S, Fuchs C, Maynard JA, Thurber RV (2016) Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat Commun 7:203–208
Acknowledgements
We received invaluable assistance from the Department of Science and Technology in Lakshadweep, the Lakshadweep Forest Department and numerous local coordinators and boat operators. We thank Chris Perry for his advice on the ReefBudget method. We also thank the projection authors for developing and making the sea-level rise projections available, multiple funding agencies for supporting the development of the projections, and the NASA Sea-Level Change Team for developing and hosting the IPCC AR6 Sea-Level Projection Tool. We would also like to thank the people of Lakshadweep without whom this work would not have been possible.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was made possible due to the funding and assistance from the National Centre for Biological Sciences (NCBS), The Spanish National Research Council (CSIC), the Centre for Wildlife Studies (CWS), Shri A.M.M. Murugappa Chettiar Research Centre (MCRC), Cholamandalam Investment and Finance Company, Azim Premji University Grants, SERB-Department of Science and Technology (EMR 2017/004014) and Rohini Nilekani Philanthropies. The authors have no competing interests to declare that are relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Divan Patel, F., Pinto, W., Dey, M. et al. Carbonate budgets in Lakshadweep Archipelago bear the signature of local impacts and global climate disturbances. Coral Reefs 42, 729–742 (2023). https://doi.org/10.1007/s00338-023-02374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02374-8