Abstract
The three-dimensional (3D) structure of habitat-forming corals has profound impacts on reef ecosystem processes. Elucidating coral structural responses to the environment is therefore crucial to understand changes in these ecosystems. However, little is known of how environmental factors shape coral structure in deep and dark waters, where cold-water coral (CWC) reefs thrive. Here, we attempt to infer the influence of current flow on CWC framework architecture, using 3D scanning to quantify colony shape traits (volume compactness and surface complexity) in the reef-building CWC Desmophyllum pertusum from adjacent fjord and offshore habitats with contrasting flow regimes. We find substantial architectural variability both between and within habitats. We show that corals are generally more compact in the fjord habitat, reflecting the prevailing higher current speeds, although differences in volume compactness between fjord and offshore corals are more subtle when comparing the fjord with the more exposed side of the offshore setting, probably due to locally intensified currents. Conversely, we observe no clear disparity in coral surface complexity between habitats (despite its positive correlation with volume compactness), suggesting it is not affected by current speed. Unlike volume compactness, surface complexity is similarly variable within a single colony as it is between colonies within the same habitat or between habitats and is therefore perhaps more dependent than volume compactness on microenvironmental conditions. These findings suggest a highly plastic, trait-specific and functionally relevant structural response of CWCs to current flow and underscore the importance of multiple concurrent sources of hydrodynamic forcing on CWC growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold-water coral (CWC) reefs are biodiversity-rich ecosystems found in a vast range of water depths and habitats, down to over 1000 m of depth on seamounts and continental slopes (Freiwald et al. 2004), and up to 39 m in Norwegian fjords (Fosså et al. 2002). These ecosystems rely on the three-dimensional (3D) skeletal frameworks formed by a few species of azooxanthellate scleractinians such as the widely distributed Desmophyllum pertusum (previously known as Lophelia pertusa), the primary framework builder in the North Atlantic Ocean (Freiwald et al. 2004). The majority of the CWC reef framework consists of dead coral skeleton (devoid of living tissue) (Vad et al. 2017), which supports key ecological functions modulated by its physical branching structure, including habitat provision for most of the numerous reef-associated species (Mortensen et al. 1995) and baffling and retention of suspended sediment and particulate organic matter (Wheeler et al. 2005; Maier et al. 2021; Wang et al. 2021). Skeletal traits of reef-building corals are indeed known to broadly affect not only their individual performance and response to the environment, but also ecosystem processes, i.e. they represent both response and effect traits (Suding et al. 2008; Zawada et al. 2019b). For example, branch spacing in shallow-water Acropora spp. varies in response to light and water motion (Bottjer 1980; Oliver et al. 1983; Kaniewska et al. 2008), influencing in turn abundance and size of associated epifauna (Vytopil and Willis 2001), and can therefore be used to evaluate the impact of disturbance and environmental change on reef functional diversity (e.g. McWilliam et al. 2020). Skeletal traits assume particular significance in a CWC reef, as this is typically constructed by only one or two species at a given location (Freiwald 2002). Hence, knowing how CWC skeletal morphology is controlled by environmental variation is vital to better understand benthic ecosystem functioning in response to future change.
Light and water motion (especially wave stress), as exemplified above, are generally regarded as the most important environmental drivers of shallow-water coral morphology (Todd 2008). Here, we focus only on water motion, because CWCs inhabit deep aphotic waters and lack symbiotic zooxanthellae. In shallow-water corals, water movement is known to prevent sediment smothering, enhance nutrient availability and diffusion (Todd 2008) and favour waste removal (Chamberlain and Graus 1975), but also to cause mechanical stress on their rigid skeletal structure (Graus et al. 1977). A typical response of shallow-water branching corals to increased water motion consists of acquiring a more compact architecture with shorter, thicker and/or more crowded branches (e.g.Veron and Pichon 1976; Bottjer 1980), which grants higher mechanical resistance to flow-induced breakage (Graus et al. 1977) and, by slowing down currents more effectively (Chamberlain and Graus 1975), allows efficient food capture (Sebens et al. 1997). Phenotypic responses of this kind result from plasticity and/or adaptation (Todd 2008) and vary across multiple spatial levels, from within colonies to between geographic regions (Veron 1982).
It is unclear whether water motion affects coral skeletal morphology analogously in deep waters, where light levels and storm wave damage do not play a role but currents have even greater physiological relevance, given that CWCs primarily feed on suspended food sources (e.g. zooplankton and particulate organic matter; Mueller et al. 2014). Computational modelling has recently started to elucidate the fine-scale interactions between flow velocity and CWC growth, highlighting their complex interplay mediated by coral feeding efficiency and energetic reserves (Hennige et al. 2021). Although D. pertusum does seem to follow similar morphological trends to shallow-water species in response to current intensity (De Clippele et al. 2018; Büscher et al. 2019; Sanna and Freiwald 2021), the available evidence is mostly qualitative (e.g. based on discrete morphological categorisations) and very little is known about the mechanisms, magnitude and scales of this presumed hydrodynamic control. The logistic difficulties of working in the deep ocean and the relatively slow growth of CWC colonies (Lartaud et al. 2017) have thus far hampered adequate investigation (either in situ or in aquarium) of their morphological responses to environmental factors. Photogrammetry based on remotely operated vehicle (ROV) video data is an increasingly common methodology for the detailed characterisation of 3D structure and ecology of CWC reef habitats (Price et al. 2019; Lim et al. 2020), but its resolution is still insufficient to capture the fine-scale morphology of CWC colonies. Here, we investigate how CWCs are shaped by habitat-specific and local topography-induced flow regimes, by quantifying the skeletal architecture of physical colony samples of D. pertusum from mid-Norwegian inshore (fjord) and offshore shelf habitats with high-resolution 3D scanning. The hydro-physicochemical affinity of these adjacent habitats provides the rare opportunity to elucidate the long-term effects of hydrodynamic forcing on CWC framework architecture while ruling out other environmental effects as much as possible.
Materials and methods
Study areas
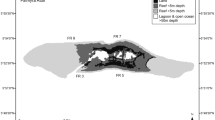
We used a natural experiment involving two postglacial Desmophyllum pertusum reef sites which occur in close proximity, but distinct habitats across the mid-Norwegian continental shelf: the inshore, shallower Nordleksa Reef, and the offshore Sula Reef Complex (Fig. 1). Another mid-Norwegian offshore site, Træna, was included in the study to account for the intracolonial variation of D. pertusum, given the availability of several co-occurring samples from this location.
Study areas. a Location of the reef study sites in mid-Norwegian waters. b 3D bathymetric map of the Sula Reef Complex (10 × vertical exaggeration), with study areas delimited by ellipses. Map based on 5 × 5 m grid bathymetry data by MAREANO (Buhl-Mortensen et al. 2015; ©Kartverket)
The Nordleksa Reef is found at water depths between 145 and 210 m near the island of Nordleksa, at the entrance of the Trondheim Fjord, extending over a length of ~ 1.7 km from west to east with two main reef summits (Juva et al. 2020). The Sula Reef Complex is made up of numerous individual reefs (isolated or coalescent) up to 35 m high, occurring at 240–315 m depth over a distance of ~ 14 km on the Sula Ridge, a narrow southwest–northeast-trending asymmetrical spur with a steep escarpment along the southeast side (Freiwald et al. 2002; Fig. 1b). Large-scale circulation over the Nordleksa Reef and the Sula Reef Complex is dominated by the Norwegian Coastal Current and the underlying, denser North Atlantic Current, both flowing northwards along the Norwegian margin (Freiwald et al. 2002). In another study still to be published, water mass characteristics and hydrodynamics were investigated at Nordleksa (63° 36.45′ N, 09° 22.92′ E at 217 m depth) and Sula (64° 06.66′ N, 08° 07.12′ E at 305 m depth) over one year (summer 2013–2014) by benthic landers. Variations in horizontal flow speed and direction during this period are shown in Fig. 2. Despite their proximity, the flow regimes of these two sites differ markedly: at Nordleksa, bottom currents are under strong tidal control (tidal fluctuations explained ca. 60% of their horizontal velocities) and showed higher mean annual horizontal speed (~ 18 cm s−1) compared to Sula (~ 8 cm s−1) (Fig. 2; Büscher et al. in prep.). Nonetheless, instantaneous horizontal flow velocities can be far higher and reached peak speeds of 104 cm s−1 at Nordleksa and 157 cm s−1 at Sula (Büscher et al. in prep.). At Nordleksa, partially blocked flow conditions promote turbulence (Juva et al. 2020). While flow velocity and direction can vary significantly and rather instantaneously (due to e.g. interaction with complex local topography), other hydrographic parameters vary on smaller scales and more gradually, allowing us to constrain the impact of bottom currents. During the benthic deployment period between 2013 and 2014, the mean annual bottom temperature was slightly warmer at Nordleksa with 7.9 ± 0.3 °C compared to 7.5 ± 0.3 °C at Sula, while mean annual bottom salinity was lower at Nordleksa (34.8 ± 0.2 g kg−1 absolute salinity) compared to Sula (35.1 ± 0.5 g kg−1 absolute salinity) (Büscher et al. in prep.). The pH as measured by distinct water samples collected close to live corals at both sites was 7.99–8.05 at Nordleksa (depending on depth and proximity to corals) and 8.04 at Sula (Büscher et al. 2019).
Bottom flow speed (Umag) (a, b) and average flow speed in north–south horizontal flow direction (c, d; up = north, down = south), measured over one year by benthic landers at Sula (left panels, a + c, blue) and Nordleksa (right panels, b + d, orange). The thicker lines in a and b show the 1-day running mean, and the horizontal direction in c and d is shown with 1-day resolution. The flow data are compared with NorKyst-800-model data (grey) (Albretsen et al. 2011) for the respective sites
To appraise the role of flow regimes driven by local seafloor topography, we selected two distinct areas on opposite sides of Sula Ridge’s long axis, separated by approximately 5 km, one area in the southwest part of the ridge (henceforth, Sula SW) and the other in the northeast part (Sula NE) (Fig. 1b). Sula SW is characterised by higher relief and a steeper ridge escarpment (Thorsnes et al. 2015), which presumably expose the corals to stronger bottom currents due to topographic acceleration of flow (Thorsnes et al. 2016).
The Træna site consists of elongated reefs found at 300–330 m depth in the Træna Deep (Wehrmann et al. 2009), a trough located north of Nordleksa and Sula (Fig. 1a).
Study material
We focused on the dead skeletal framework of D. pertusum because, in addition to its high ecological significance, it represents a mature colony stage that was exposed to the surrounding environmental conditions throughout its development (unlike still-growing live colony layers). Since sampling entire colonies (capable of reaching a diameter of a couple of metres) from the seabed is logistically and ecologically problematic, we used a collection of dead coral blocks retaining a unitary 3D structure, fist-sized or larger (up to ~ 30 cm in height) and comprising at least 30 corallites (i.e. the skeletal cups of individual polyps), so as to be representative of the 3D coral framework (Fig. 3a). When fragmentation appeared to have altered the original framework structure (e.g. forming large concavities), we either discarded the specimen or, whenever possible, further fragmented it into a unitary piece of suitable size by removing peripheral isolated branches. Samples were also cleaned from sediment and large epizoans (e.g. sponges, bivalves) using a brush and tweezers, to prevent inaccurate representation of the skeletal surface.
Low statistical sample sizes are usually unavoidable when working with large CWC fragments. Our study represents a convenient exception in this sense (see also Büscher et al. 2019), as we managed to incorporate a relatively high number of suitable samples (n = 58; Supplementary Information, Table S1), collected by research cruises during repeated surveys of the study areas over a time span of 20 years (1994–2013). At Nordleksa and Sula, samples had been collected across the reefs mostly with mobile gear (dredge and submersible), considerably reducing the probability of sampling the same colony multiple times. Another indication of their origin from different colonies was their wide variety of preservation states. Conversely, Træna samples had all been collected from the same spot on a reef top with a single box core (50 × 50 × 60 cm), and hence, they were most likely part of the same colony, with negligible bathymetric variation. All samples showed the typical extra-calcification induced by the symbiotic polychaete worm Eunice norvegica (Freiwald and Wilson 1998), albeit to varying extents.
3D shape quantification
Digital 3D surface models of the coral samples were produced using the structured-light 3D scanner Artec Space Spider with the software Artec Studio 15 (Artec 3D, Luxembourg). Since 3D surface scanning only captures the external morphology of objects, artefacts might develop when working with densely branched specimens, due to self-shading (Reichert et al. 2016). To minimise this effect, we performed several individual scans of each specimen with different orientations. Noise was removed from individual scans with the Eraser tool. Watertight triangle meshes with a resolution of 0.2 mm (Fig. 3b) were created via the Sharp fusion algorithm, and, when necessary, further cleaned from artefacts using the Small-object filter, Eraser and Defeature brush tools. Another consequence of surface-based scanning was that corallites, as well as encrusted E. norvegica tubes, despite being at least partially hollow, were generally modelled as completely solid elements, which may not reflect the actual mechanical properties of colonies. However, this is not problematic within the scope of our study, as we focused on how the CWC skeleton is spatially distributed in the environment.
We characterised coral architecture on the basis of two morphological traits of high functional relevance, volume compactness and surface complexity, related to fundamental geometric properties (volume and surface area) and both affecting habitat provision for reef-associated organisms. Moreover, volume compactness is particularly indicative of structural robustness and interstitial space available for sediment accumulation, whereas surface complexity is more closely linked to the density of living tissue biomass (Zawada et al. 2019a, b). To quantify these traits, we calculated for each digital model two 3D shape variables, compactness and packing, representing volume compactness and surface complexity, respectively. Compactness measures how different parts of an object fill the space surrounding it and is the ratio of object volume to the volume of its convex hull (Merks et al. 2003), with the convex hull being the smallest convex region that encloses the object. The same variable is called proportion occupied in Doszpot et al. (2019) and convexity in Zawada et al. (2019a, b). Packing measures how the surface area of an object is packed within the object bulk and is the ratio of object surface area to convex hull surface area (Zawada et al. 2019b); it was shown to be strongly correlated with fractal dimension (Zawada et al. 2019a), another important metric of coral complexity (Reichert et al. 2017). Such dimensionless shape variables are independent of size and orientation, unlike other metrics of coral morphology like ratio of surface area to volume (Doszpot et al. 2019) and first moments of volume and surface area (Zawada et al. 2019a). This allowed us to focus exclusively on shape and makes the two variables particularly suitable for our samples, consisting of colony portions of various sizes, rather than whole colonies. Both variables were obtained from volume and surface area measures of the 3D meshes and respective convex hulls (Fig. 3b), computed in MeshLab v2020.12 (Cignoni et al. 2008).
Statistical analysis
We evaluated the association between CWC volume compactness (described by compactness) and surface complexity (described by packing) using Spearman’s rank correlation (as compactness had a non-normal distribution). To comparatively assess the effects of habitat-specific and local topography-induced flow regimes on CWC architecture, differences between Nordleksa, Sula SW and Sula NE were tested using one-way ANOVA with Tukey’s post hoc test and Cohen’s d effect size (calculated with the R package effsize; Torchiano 2020) on both shape variables, log10-transformed to fulfil the normality assumption. Træna was not included in this part of the analysis, as it was representative of only a specific depth and, most likely, a single colony. To better understand the spatial scales and patterns of architectural variation, we compared intercolonial (between and within habitats) and intracolonial disparity in shape traits using the coefficient of variation (CV), a standardised measure of relative variability equal to the standard deviation divided by the mean, and often expressed as percentage. Specifically, we tested for differences in the CV of compactness and packing between study areas, before and after including Træna samples (thus accounting for intracolonial variation), using the asymptotic test of Feltz and Miller (1996) included in the R package cvequality (Marwick and Krishnamoorthy 2019). All analyses were performed in R version 4.1.1 (R Core Team 2021), with an alpha level of 0.05. Plots were produced using the R packages ggplot2 (Wickham 2016), ggbeeswarm (Clarke and Sherrill-Mix 2017) and ggpubr (Kassambara 2020).
Results
Compactness and packing were positively correlated (Spearman coefficient rs = 0.57; P < 0.00001) (Fig. 4). Average values and CV of compactness and packing for each study area are shown in Table 1. ANOVA indicated statistically significant differences in compactness among Nordleksa, Sula SW and Sula NE (F2,43 = 4.11; P = 0.023). These were further explored with Tukey’s test, which showed that pairwise differences were statistically significant only between Sula NE and Nordleksa (adjusted P = 0.036; Fig. 5), while they were non-significant between Sula SW and Nordleksa (adjusted P = 0.058) and between Sula NE and Sula SW (adjusted P = 0.96). Cohen’s d (95% confidence interval) indicated that the largest difference in compactness was between Sula NE and Nordleksa (d = 0.99, ‘large’ effect), followed by the difference between Sula SW and Nordleksa (d = 0.78, ‘medium’ effect) and between Sula NE and Sula SW (d = 0.11, ‘negligible’ effect). Differences in packing, on the other hand, were not statistically significant among areas (F2,43 = 1.12; P = 0.33) and had lower effect sizes than compactness between habitats (dSula NE–Nordleksa = 0.54, ‘medium’ effect; dSula SW–Nordleksa = 0.42, ‘small’ effect; dSula NE–Sula SW = 0.15, ‘negligible’ effect). The asymptotic test indicated no statistically significant differences in the CV of compactness among Nordleksa and the two Sula areas (P = 0.42), while it yielded a statistically significant result (P = 0.042) when including intracolony samples from Træna. Differences in the CV of packing were statistically non-significant, both with (P = 0.14) and without the inclusion of Træna samples in the analysis (P = 0.089). Patterns of variation in volume compactness and surface complexity among study areas in relation to flow speed can be visually compared in the log10-transformed data of Fig. 5.
Architectural variation of Desmophyllum pertusum colony samples between study areas with different flow regimes (Umean = mean annual horizontal flow speed; Umax = peak horizontal flow speed). Volume compactness (a) and surface complexity (b) are described by log10-transformed compactness and packing, respectively, as used in the statistical analysis. Variation is summarised by boxplots indicating median (horizontal line enclosed by box), 1st and 3rd quartiles (lower and upper box hinges) and values within 1.5 × interquartile range (whiskers). Intracolonial samples from Træna were not included in the analysis, but are shown here for comparison
Discussion
Patterns and mechanisms of CWC architectural variation
We observed higher coral compactness in the higher-flow-speed fjord habitat at Nordleksa, in agreement with Büscher et al. (2019), but this difference was statistically significant only with respect to the deeper sector of the Sula offshore habitat (Sula NE). The more exposed Sula SW showed a relatively closer affinity to Nordleksa, which can be explained by the presence of more colonies at the high end of the volume compactness spectrum (Fig. 5a). Hence, there appears to be a joint effect of both sources of current speed variation considered here, i.e. habitat and local bathymetry, suggesting a strong hydrodynamic control on CWC architecture. However, this effect is seemingly restricted to volume compactness, because surface complexity did not differ significantly between habitats or offshore areas, despite the obvious geometric constraints that positively correlate the two traits (compactness and packing are both forms of convexity; Zawada et al. 2019a). Furthermore, while intracolonial variation in volume compactness was considerably lower than variation between and within habitats, surface complexity varied more consistently at all spatial scales analysed (Table 1 and Fig. 5b), as demonstrated by the asymptotic tests. Such contrasting patterns of variation seem to denote a trait-specific response of CWC colonies to flow speed, involving the architectural trait with the higher hydromechanical relevance, i.e. volume compactness.
We hypothesise that this decoupled trait response is enabled by the relatively simple colonial organisation of Desmophyllum pertusum, compared to that of many shallow-water scleractinians (Coates and Jackson 1987). In fact, D. pertusum colonies are characterised by a fasciculate organisation with spaced corallites that represent the only modular level (Rosen 1986) and are free to grow rather independently due to such relaxed structural constraints. This low colony integration, along with intrinsic ontogenetic constraints (Gass and Roberts 2011), leads to high morphological disparity between individual corallites (Sanna and Freiwald 2021) and could therefore explain the high intracolonial variability in surface complexity. Volume compactness, on the contrary, is likely governed by the density of polyps and hence by their budding rate (Sanna and Freiwald 2021).
The high within-habitat and within-colony variation in both architectural traits (Table 1; Fig. 5) is probably contributed by phenotypic plasticity. Clonal propagation, in addition to asexual colony growth, may in fact constitute an important reef development mechanism for D. pertusum, with a few clones capable of having a broad spatial distribution within reefs (Dahl et al. 2012), although the extent of clonality can vary dramatically among different sites (Morrison et al. 2011; Becheler et al. 2017). Plasticity, in particular, likely plays a role in the comparable structural responses to water flow at Sula and Nordleksa, as they are in contrast with the marked genetic differentiation between the two populations (Le Goff-Vitry et al. 2004). This apparent architectural plasticity provides further evidence of a generally high acclimatisation potential of CWCs to environmental change (Form and Riebesell 2012), especially in the light of the trait-specific response we observed. Nevertheless, some adaptive morphological variation due to genetic differentiation is probably present within this species, perhaps not between the neighbouring fjord and offshore habitats analysed here, but rather at larger (e.g. interregional) scales, where morphotypes with strikingly different geometries occur (Sanna and Freiwald 2021).
Environmental influence on CWC architecture
Our results suggest a positive association between current speed and CWC framework compactness, which is in line with the responses known for zooxanthellate scleractinians and reflects trophic and mechanical factors with important implications for organismal fitness. Accelerated flow promotes enhanced food transport to CWC colonies, increasing encounter rates with food particles through their advection and resuspension (Genin et al. 1986; Davies et al. 2009). At the same time, a tightly branched framework effectively reduces the collateral hydromechanical stress (also through secondary branch fusion; Stetson et al. 1962) and slows flow down to speeds that are more favourable for polyp food capture (speeds < 7 cm s−1 are optimal for D. pertusum; Purser et al. 2010; Orejas et al. 2016), resulting in higher feeding efficiency and higher energy budget for growth and reproduction (Hennige et al. 2021). Strong currents have indeed been linked to higher budding rates in D. pertusum (Chapron et al. 2020), as well as in the shallow-water scleractinian Tubastraea spp. (Tanasovici et al. 2022), which is also non-photosynthetic. Along with long-term flow regimes, CWC architecture could be affected by isolated strong current events. These are manifested at Sula by instantaneous flow speeds above 150 cm s−1 (see Fig. 2a in November 2013, for instance), which might contribute to the occurrence of highly compact colonies in this location (Fig. 5a), despite a considerably lower average flow speed than at Nordleksa. However, since growth of D. pertusum colonies entails tens to hundreds of years (Pons-Branchu et al. 2005; Brooke and Ross 2014), long-term flow conditions are probably more relevant for overall framework architecture.
Food supply, in the case of local topographic relief (addressed here by including two distinct Sula areas), may be enhanced not only by accelerated currents but also by increased vertical flux of particulate organic matter from the sea surface (Maier et al. 2021). In the more exposed Sula SW area, individual reefs are indeed larger and more abundant (Thorsnes et al. 2015, 2016), further suggesting higher food supply therein. It is important to note, however, that a compact architecture does not necessarily reflect optimal conditions for CWC growth. De Clippele et al. (2018), for instance, observed this at the Tisler Reef (Hvaler area, Norway): the proportion of short-branched D. pertusum colonies with a compact (“cauliflower”) morphology was higher on the northwest side of the reef, which was exposed to a higher frequency of stronger currents (including the highest recorded speed at this site), while coral cover was much higher on the southeast side, where downwelling and food supply occurred more frequently in addition to more hard substrate being available. Strong currents can be a source of food, but also of mechanical damage for these relatively delicate branching colonies (Wilson 1979; Lim et al. 2020), and high branch density in response to high current speeds may lead to considerable food depletion in inner colony portions (Chamberlain and Graus 1975; Chang et al. 2009). Ultimately, CWC architecture probably represents a trade-off between adequate nutrition and structural integrity.
The local flow regime of a CWC reef habitat is influenced not only by seabed topography, but also by the corals themselves, which are capable of significantly altering the surrounding flow field with their complex structure, deflecting near-bed flow and decreasing turbulence and current speed in their wake (Mienis et al. 2019; Bartzke et al. 2021; Hennige et al. 2021; Corbera et al. 2022). This suggests that CWC colonies can mutually influence each other’s morphology, by inducing heterogeneous flow patterns that may increase local architectural variability. In particular, highly compact colonies, by acting as a tight physical barrier, are likely to reduce the exposure to fast flow and resources of sheltered downstream colonies, which as a result would grow with a less compact architecture. Nevertheless, this effect must be somewhat modulated by larger-scale changes in flow direction (Hennige et al. 2021), especially at Nordleksa, where regular tidal oscillations and turbulent flow are important components of the benthic current regime and food supply mechanisms (Juva et al. 2020). Flow direction has indeed shown to be an important driver of morphology in deep-water octocorals of the genus Paragorgia, which develop a concave fan shape when exposed to strong unidirectional currents, maximising food intake and resistance to hydrodynamic forces, as opposed to non-concave colonies occurring in different flow conditions (e.g. turbulent flow closer to the bottom) (Mortensen and Buhl-Mortensen 2005; Morato et al. 2021). Variation in overall colony shape in response to currents was suggested to also occur in D. pertusum (which can acquire an asymmetrical fan shape too; Sanna and Freiwald 2021) and would represent a morphological response complementary to the architectural strategies observed here.
Fine-scale flow patterns at the colony level might actually represent a key driver of surface complexity, because they modulate differential resource acquisition among polyps, which is closely linked to surface area (Kim and Lasker 1998). This would explain the high intracolonial variability in surface complexity and would imply that the effect of water motion on CWC architecture is not only trait-specific, but also scale-dependent, with surface complexity being affected exclusively at the microhabitat scale. Characterisation of in situ reef flow patterns at the colony and patch scale (cm to m), which are still poorly known (Mienis et al. 2019), is needed to advance our understanding of the effects of water motion on CWC morphology. A more detailed quantification of the relationship between ambient currents and CWC architecture could enable, for instance, the use of compactness as a proxy for flow speed, also in the fossil record, with implications for palaeoenvironmental reconstruction. High local architectural variability and the use of average reef flow speed prevent such quantitative inferences in this study, although very high compactness values (e.g. > 0.30) could arguably be considered a reliable indicator of a CWC habitat with relatively strong currents.
Although limitedly divergent between the studied habitats, other hydrological parameters could influence framework architecture by interacting in complex ways with hydrodynamics, given the fact that coral calcification is jointly affected by several environmental factors (Tambutté et al. 2011). Flow patterns, for instance, can considerably alter bottom water temperature (Guihen et al. 2012). Denser colony growth at Nordleksa might be further promoted by its slightly higher temperature, as laboratory experiments have shown that D. pertusum growth rates increase in response to a combination of higher temperatures and higher food supply (Büscher et al. 2017), and an analogous combination leads to increased growth and budding rates in the Mediterranean scleractinian Cladocora caespitosa (Rodolfo-Metalpa et al. 2008). Moreover, while average physicochemical parameters are rather consistent between Nordleksa and Sula, they show large seasonal variability within both sites (Büscher et al. in prep.), which, along with variability at broader temporal scales, might be related to local architectural diversity. Interspecific interactions can also alter D. pertusum skeletal morphology (Freiwald and Wilson 1998; Beuck et al. 2007), although the signs of ubiquitous presence of the important symbiont Eunice norvegica in our samples suggest a minor influence on colony architectural traits. Nevertheless, environmental (abiotic or biotic) factors that have limited influence on the architecture of CWCs may still be highly relevant for their structural integrity, by affecting internal skeletal properties (e.g. porosity) and thus the ultimate framework strength (Hennige et al. 2020; Wolfram et al. 2022). Therefore, the interplay between multiple factors should be considered when trying to predict CWC net responses to environmental change.
Ecosystem significance of CWC architectural variation
The marked architectural diversity of D. pertusum at local scales has important implications for CWC reef ecosystems, suggesting partitioning of the coral’s ecological functions not solely among different reef zones (living coral, dead framework and coral rubble; Freiwald et al. 2004), but also between different portions of the dead framework. In terms of habitat provision, this architectural variability may induce locally heterogeneous distribution patterns of reef-dwelling organisms that thrive in different framework configurations, contributing to complex local structuring of CWC reef communities (Henry et al. 2013). Architectural differences between fjord and offshore reefs could partly underpin the recognised differences in community composition between these habitats, but it is difficult to establish a causal link. For instance, Mortensen and Fosså (2006) observed higher diversity of coral-associated invertebrates in mid-Norwegian inshore reefs (including Nordleksa) compared to reefs at Sula. This pattern is consistent with the higher surface complexity of Nordleksa corals (although statistically non-significant) but in contrast with their higher volume compactness (Table 1; Fig. 5), because generally surface complexity creates niches for other organisms while compactness reduces them (Zawada et al. 2019b). A possible reason for the diversity pattern observed by Mortensen and Fosså (2006) might be the preference of some invertebrate species for tightly branched coral hosts, which ensure better protection, especially to smaller individuals (Vytopil and Willis 2001), but there is no doubt that several other ecological determinants of community assembly are involved (Pearson 1980). Even with regard to coral structures alone, habitat provision could also be affected by aspects not considered in this study, such as colony size, framework continuity and whole-reef geometry.
Local architectural variation might also play a role in CWC reef and mound formation, by regulating baffling, settling and interstitial accumulation of sediment in the coral framework. In principle, more compact D. pertusum colonies intercept sinking and transported sediment more effectively and require a lower amount of infill, becoming buried more rapidly and thus enhancing reef and mound growth rates under suitable sediment supply conditions (Wang et al. 2021). CWC growth, hydrodynamics and sedimentation are mutually influenced at multiple scales, with development of colonies, reefs and mounds enhancing flow, food supply to living colonies and sediment baffling by dead framework, and thus further growth (Masson et al. 2003; Davies et al. 2009; Buhl-Mortensen et al. 2016; van der Kaaden et al. 2021; Corbera et al. 2022). The high sensitivity of CWC architecture to water flow may further boost this positive feedback mechanism, with exposure of elevated colonies to stronger currents (and enhanced food supply) resulting in higher compactness, which would in turn facilitate post-mortem sedimentary infilling. This hypothesis is supported by observations of morphological variation matching reef zonation at Sula (Mortensen et al. 1995; Freiwald et al. 2002) and at the Stjernsund Reef (Finnmark, Norway) (Freiwald et al. 1997). Enhanced sediment baffling by more compact framework would also increase reef nutrient cycling, as the fine baffled sediment includes particulate organic matter (de Froe et al. 2019) which contributes significantly to carbon turnover in CWC reefs (De Clippele et al. 2021). This effect might be further modulated by architecture-driven changes in diversity and abundance of the framework-dwelling organisms, also involved in nutrient (re)cycling (Maier et al. 2021).
In order to improve our comprehension of the full spectrum of effects that global environmental change can have on CWC ecosystem functioning and services, we argue it is crucial to further investigate not only the response of CWC morphological traits to the environment, but also their effects on reef ecosystem processes.
References
Albretsen J, Sperrevik AK, Staalstrøm A, Sandvik AD, Vikebø F, Asplin L (2011) NorKyst-800 report no. 1: User manual and technical descriptions. In: IMR Res Rep Ser Fisken og Havet 2/2011. Institute of Marine Research, Bergen
Bartzke G, Siemann L, Büssing R, Nardone P, Koll K, Hebbeln D, Huhn K (2021) Investigating the Prevailing Hydrodynamics Around a Cold-Water Coral Colony Using a Physical and a Numerical Approach. Front Mar Sci 8:663304. https://doi.org/10.3389/fmars.2021.663304
Becheler R, Cassone AL, Noël P, Mouchel O, Morrison CL, Arnaud-Haond S (2017) Low incidence of clonality in cold water corals revealed through the novel use of a standardized protocol adapted to deep sea sampling. Deep Sea Res II 145:120–130. https://doi.org/10.1016/j.dsr2.2015.11.013
Beuck L, Vertino A, Stepina E, Karolczak M, Pfannkuche O (2007) Skeletal response of Lophelia pertusa (Scleractinia) to bioeroding sponge infestation visualised with micro-computed tomography. Facies 53:157–176. https://doi.org/10.1007/s10347-006-0094-9
Bottjer DJ (1980) Branching morphology of the reef coral Acropora cervicornis in different hydraulic regimes. J Paleontol 54:1102–1107
Brooke S, Ross SW (2014) First observations of the cold-water coral Lophelia pertusa in mid-Atlantic canyons of the USA. Deep Sea Res II 104:245–251. https://doi.org/10.1016/j.dsr2.2013.06.011
Buhl-Mortensen L, Buhl-Mortensen P, Dolan MFJ, Holte B (2015) The MAREANO programme – A full coverage mapping of the Norwegian off-shore benthic environment and fauna. Mar Biol Res 11:4–17. https://doi.org/10.1080/17451000.2014.952312
Buhl-Mortensen P, Buhl-Mortensen L, Purser A (2016) Trophic Ecology and Habitat Provision in Cold-Water Coral Ecosystems. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots. Springer, Cham, pp 1–26
Büscher JV, Form AU, Riebesell U (2017) Interactive Effects of Ocean Acidification and Warming on Growth, Fitness and Survival of the Cold-Water Coral Lophelia pertusa under Different Food Availabilities. Front Mar Sci 4:101. https://doi.org/10.3389/fmars.2017.00101
Büscher JV, Wisshak M, Form AU, Titschack J, Nachtigall K, Riebesell U (2019) In situ growth and bioerosion rates of Lophelia pertusa in a Norwegian fjord and open shelf cold-water coral habitat. PeerJ 7:e7586. https://doi.org/10.7717/peerj.7586
Chamberlain JJA, Graus RR (1975) Water Flow and Hydromechanical Adaptations of Branched Reef Corals. Bull Mar Sci 25:112–125
Chang S, Elkins C, Alley M, Eaton J, Monismith S (2009) Flow inside a coral colony measured using magnetic resonance velocimetry. Limnol Oceanogr 54:1819–1827. https://doi.org/10.4319/lo.2009.54.5.1819
Chapron L, Le Bris N, Durrieu de Madron X, Peru E, Galand PE, Lartaud F (2020) Long term monitoring of cold-water coral growth shows response to episodic meteorological events in the NW Mediterranean. Deep Sea Res I 160:103255. https://doi.org/10.1016/j.dsr.2020.103255
Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F, Ranzuglia G (2008) MeshLab: an open-source mesh processing tool. In: Scarano V, De Chiara R, Erra U (eds) Sixth Eurographics Italian Chapter Conference. The Eurographics Association, Eindhoven, pp 129–136
Clarke E, Sherrill-Mix S (2017) ggbeeswarm: Categorical Scatter (Violin Point) Plots. R package version 0.6.0. https://cran.r-project.org/package=ggbeeswarm
Coates AG, Jackson JBC (1987) Clonal growth, algal symbiosis, and reef formation by corals. Paleobiology 13:363–378. https://doi.org/10.1017/S0094837300008988
Corbera G, Lo Iacono C, Simarro G, Grinyó J, Ambroso S, Huvenne VAI, Mienis F, Carreiro-Silva M, Martins I, Mano B, Orejas C, Larsson A, Hennige S, Gori A (2022) Local-scale feedbacks influencing cold-water coral growth and subsequent reef formation. Sci Rep 12:20389. https://doi.org/10.1038/s41598-022-24711-7
Dahl MP, Pereyra RT, Lundälv T, André C (2012) Fine-scale spatial genetic structure and clonal distribution of the cold-water coral Lophelia pertusa. Coral Reefs 31:1135–1148. https://doi.org/10.1007/s00338-012-0937-5
Davies AJ, Duineveld GCA, Lavaleye MSS, Bergman MJN, van Haren H, Roberts JM (2009) Downwelling and deep-water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef Complex. Limnol Oceanogr 54:620–629. https://doi.org/10.4319/lo.2009.54.2.0620
De Clippele LH, Huvenne VAI, Orejas C, Lundälv T, Fox A, Hennige SJ, Roberts JM (2018) The effect of local hydrodynamics on the spatial extent and morphology of cold-water coral habitats at Tisler Reef, Norway. Coral Reefs 37:253–266. https://doi.org/10.1007/s00338-017-1653-y
De Clippele LH, van der Kaaden AS, Maier SR, de Froe E, Roberts JM (2021) Biomass Mapping for an Improved Understanding of the Contribution of Cold-Water Coral Carbonate Mounds to C and N Cycling. Front Mar Sci 8:721062. https://doi.org/10.3389/fmars.2021.721062
de Froe E, Rovelli L, Glud RN, Maier SR, Duineveld G, Mienis F, Lavaleye M, van Oevelen D (2019) Benthic Oxygen and Nitrogen Exchange on a Cold-Water Coral Reef in the North-East Atlantic Ocean. Front Mar Sci 6:665. https://doi.org/10.3389/fmars.2019.00665
Doszpot NE, McWilliam MJ, Pratchett MS, Hoey AS, Figueira WF (2019) Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient. Diversity 11:44. https://doi.org/10.3390/d11030044
Feltz CJ, Miller GE (1996) An asymptotic test for the equality of coefficients of variation from k populations. Stat Med 15:647–658. https://doi.org/10.1002/(SICI)1097-0258(19960330)15:6%3C647::AID-SIM184%3E3.0.CO;2-P
Form AU, Riebesell U (2012) Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob Chang Biol 18:843–853. https://doi.org/10.1111/j.1365-2486.2011.02583.x
Fosså JH, Mortensen PB, Furevik DM (2002) The deep-water coral Lophelia pertusa in Norwegian waters: distribution and fishery impacts. Hydrobiologia 471:1–12. https://doi.org/10.1023/A:1016504430684
Freiwald A (2002) Reef-Forming Cold-Water Corals. In: Wefer G, Billett D, Hebbeln D, Jørgensen BB, Schlüter M, van Weering TCE (eds) Ocean Margin Systems. Springer, Berlin, Heidelberg, pp 365–385
Freiwald A, Wilson JB (1998) Taphonomy of modern deep, cold-temperate water coral reefs. Hist Biol 13:37–52. https://doi.org/10.1080/08912969809386571
Freiwald A, Henrich R, Pätzold J (1997) Anatomy of a deep-water coral reef mound from Stjernsund, West Finnmark, northern Norway. In: James NP, Clarke JAD (eds) Cool-water carbonates. Society for Sedimentary Geology, Tulsa, pp 141–162
Freiwald A, Hühnerbach V, Lindberg B, Wilson JB, Campbell J (2002) The Sula Reef Complex, Norwegian shelf. Facies 47:179–200. https://doi.org/10.1007/BF02667712
Freiwald A, Fosså JH, Grehan A, Koslow T, Roberts JM (2004) Cold-water coral reefs: out of sight – no longer out of mind. UNEP-WCMC, Cambridge
Gass SE, Roberts JM (2011) Growth and branching patterns of Lophelia pertusa (Scleractinia) from the North Sea. J Mar Biol Assoc UK 91:831–835. https://doi.org/10.1017/S002531541000055X
Genin A, Dayton PK, Lonsdale PF, Spiess FN (1986) Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 322:59–61. https://doi.org/10.1038/322059a0
Graus RR, Chamberlain JJA, Boker AM (1977) Structural Modification of Corals in Relation to Waves and Currents. In: Frost SH, Weiss MP, Saunders JB (eds) Studies in Geology 4: Reefs and Related Carbonates-Ecology and Sedimentology. The American Association of Petroleum Geologists, Tulsa, pp 135–153
Guihen D, White M, Lundälv T (2012) Temperature shocks and ecological implications at a cold-water coral reef. Mar Biodivers Rec 5:e68. https://doi.org/10.1017/S1755267212000413
Hennige SJ, Wolfram U, Wickes L, Murray F, Roberts JM, Kamenos NA, Schofield S, Groetsch A, Spiesz EM, Aubin-Tam ME, Etnoyer PJ (2020) Crumbling Reefs and Cold-Water Coral Habitat Loss in a Future Ocean: Evidence of “Coralporosis” as an Indicator of Habitat Integrity. Front Mar Sci 7:668. https://doi.org/10.3389/fmars.2020.00668
Hennige SJ, Larsson AI, Orejas C, Gori A, De Clippele LH, Lee YC, Jimeno G, Georgoulas K, Kamenos NA, Roberts JM (2021) Using the Goldilocks Principle to model coral ecosystem engineering. Proc Royal Soc B 288:20211260. https://doi.org/10.1098/rspb.2021.1260
Henry LA, Moreno-Navas J, Roberts JM (2013) Multi-scale interactions between local hydrography, seabed topography, and community assembly on cold-water coral reefs. Biogeosciences 10:2737–2746. https://doi.org/10.5194/bg-10-2737-2013
Juva K, Flögel S, Karstensen J, Linke P, Dullo WC (2020) Tidal Dynamics Control on Cold-Water Coral Growth: A High-Resolution Multivariable Study on Eastern Atlantic Cold-Water Coral Sites. Front Mar Sci 7:132. https://doi.org/10.3389/fmars.2020.00132
Kaniewska P, Anthony KRN, Hoegh-Guldberg O (2008) Variation in colony geometry modulates internal light levels in branching corals, Acropora humilis and Stylophora pistillata. Mar Biol 155:649–660. https://doi.org/10.1007/s00227-008-1061-5
Kassambara A (2020) ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.4.0. https://cran.r-project.org/package=ggpubr
Kim K, Lasker HR (1998) Allometry of resource capture in colonial cnidarians and constraints on modular growth. Funct Ecol 12:646–654. https://doi.org/10.1046/j.1365-2435.1998.00228.x
Lartaud F, Galli G, Raza A, Priori C, Benedetti MC, Cau A, Santangelo G, Iannelli M, Solidoro C, Bramanti L (2017) Growth Patterns in Long-Lived Coral Species. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots. Springer, Cham, pp 595–626
Le Goff-Vitry MC, Pybus OG, Rogers AD (2004) Genetic structure of the deep-sea coral Lophelia pertusa in the northeast Atlantic revealed by microsatellites and internal transcribed spacer sequences. Mol Ecol 13:537–549. https://doi.org/10.1046/j.1365-294X.2004.2079.x
Lim A, Wheeler AJ, Price DM, O’Reilly L, Harris K, Conti L (2020) Influence of benthic currents on cold-water coral habitats: a combined benthic monitoring and 3D photogrammetric investigation. Sci Rep 10:19433. https://doi.org/10.1038/s41598-020-76446-y
Maier SR, Mienis F, de Froe E, Soetaert K, Lavaleye M, Duineveld G, Beauchard O, van der Kaaden AS, Koch BP, van Oevelen D (2021) Reef communities associated with ‘dead’ cold-water coral framework drive resource retention and recycling in the deep sea. Deep Sea Res I 175:103574. https://doi.org/10.1016/j.dsr.2021.103574
Marwick B, Krishnamoorthy K (2019) cvequality: Tests for the Equality of Coefficients of Variation from Multiple Groups. In: R package version 0.2.0. https://cran.r-project.org/package=cvequality
Masson DG, Bett BJ, Billett DSM, Jacobs CL, Wheeler AJ, Wynn RB (2003) The origin of deep-water, coral-topped mounds in the northern Rockall Trough, Northeast Atlantic. Mar Geol 194:159–180. https://doi.org/10.1016/S0025-3227(02)00704-1
McWilliam M, Pratchett MS, Hoogenboom MO, Hughes TP (2020) Deficits in functional trait diversity following recovery on coral reefs. Proc Royal Soc B 287:20192628. https://doi.org/10.1098/rspb.2019.2628
Merks R, Hoekstra A, Kaandorp J, Sloot P (2003) Models of coral growth: spontaneous branching, compactification and the Laplacian growth assumption. J Theor Biol 224:153–166. https://doi.org/10.1016/S0022-5193(03)00140-1
Mienis F, Bouma TJ, Witbaard R, van Oevelen D, Duineveld GCA (2019) Experimental assessment of the effects of cold-water coral patches on water flow. Mar Ecol Prog Ser 609:101–117. https://doi.org/10.3354/meps12815
Morato T, Dominguez-Carrió C, Mohn C, Ocaña Vicente O, Ramos M, Rodrigues L, Sampaio Í, Taranto GH, Fauconnet L, Tojeira I, Gonçalves EJ, Carreiro-Silva M (2021) Dense cold-water coral garden of Paragorgia johnsoni suggests the importance of the Mid-Atlantic Ridge for deep-sea biodiversity. Ecol Evol 11:16426–16433. https://doi.org/10.1002/ece3.8319
Morrison CL, Ross SW, Nizinski MS, Brooke S, Järnegren J, Waller RG, Johnson RL, King TL (2011) Genetic discontinuity among regional populations of Lophelia pertusa in the North Atlantic Ocean. Conserv Genet 12:713–729. https://doi.org/10.1007/s10592-010-0178-5
Mortensen PB, Buhl-Mortensen L (2005) Morphology and growth of the deep-water gorgonians Primnoa resedaeformis and Paragorgia arborea. Mar Biol 147:775–788. https://doi.org/10.1007/s00227-005-1604-y
Mortensen PB, Fosså JH (2006) Species diversity and spatial distribution of invertebrates on deep-water Lophelia reefs in Norway. In: Suzuki Y (ed) Proceedings of the Tenth International Coral Reef Symposium, 2004. Japanese Coral Reef Society, Tokyo, pp 1849–1868
Mortensen PB, Hovland M, Brattegard T, Farestveit R (1995) Deep water bioherms of the scleractinian coral Lophelia pertusa (L.) at 64° n on the Norwegian shelf: structure and associated megafauna. Sarsia 80:145–158. https://doi.org/10.1080/00364827.1995.10413586
Mueller CE, Larsson AI, Veuger B, Middelburg JJ, van Oevelen D (2014) Opportunistic feeding on various organic food sources by the cold-water coral Lophelia pertusa. Biogeosciences 11:123–133. https://doi.org/10.5194/bg-11-123-2014
Oliver JK, Chalker BE, Dunlap WC (1983) Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. I. Long-term growth responses of Acropora formosa (Dana 1846). J Exp Mar Biol Ecol 73:11–35. https://doi.org/10.1016/0022-0981(83)90003-5
Orejas C, Gori A, Rad-Menéndez C, Last KS, Davies AJ, Beveridge CM, Sadd D, Kiriakoulakis K, Witte U, Roberts JM (2016) The effect of flow speed and food size on the capture efficiency and feeding behaviour of the cold-water coral Lophelia pertusa. J Exp Mar Biol Ecol 481:34–40. https://doi.org/10.1016/j.jembe.2016.04.002
Pearson TH (1980) Macrobenthos of fjords. In: Freeland HJ, Farmer DM, Levings CD (eds) Fjord Oceanography. Plenum Press, New York, London, pp 569–602
Pons-Branchu E, Hillaire-Marcel C, Deschamps P, Ghaleb B, Sinclair DJ (2005) Early diagenesis impact on precise U-series dating of deep-sea corals: Example of a 100–200-year old Lophelia pertusa sample from the northeast Atlantic. Geochim Cosmochim Acta 69:4865–4879. https://doi.org/10.1016/j.gca.2005.06.011
Price DM, Robert K, Callaway A, Lo lacono C, Hall RA, Huvenne VAI (2019) Using 3D photogrammetry from ROV video to quantify cold-water coral reef structural complexity and investigate its influence on biodiversity and community assemblage. Coral Reefs 38:1007–1021. https://doi.org/10.1007/s00338-019-01827-3
Purser A, Larsson AI, Thomsen L, van Oevelen D (2010) The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J Exp Mar Biol Ecol 395:55–62. https://doi.org/10.1016/j.jembe.2010.08.013
R Core Team (2021) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Reichert J, Schellenberg J, Schubert P, Wilke T (2016) 3D scanning as a highly precise, reproducible, and minimally invasive method for surface area and volume measurements of scleractinian corals. Limnol Oceanogr Methods 14:518–526. https://doi.org/10.1002/lom3.10109
Reichert J, Backes AR, Schubert P, Wilke T (2017) The power of 3D fractal dimensions for comparative shape and structural complexity analyses of irregularly shaped organisms. Methods Ecol Evol 8:1650–1658. https://doi.org/10.1111/2041-210X.12829
Rodolfo-Metalpa R, Peirano A, Houlbrèque F, Abbate M, Ferrier-Pagès C (2008) Effects of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora caespitosa. Coral Reefs 27:17–25. https://doi.org/10.1007/s00338-007-0283-1
Rosen BR (1986) Modular growth and form of corals: A matter of metamers? Philos Trans R Soc Lond B Biol Sci 313:115–142. https://doi.org/10.1098/rstb.1986.0029
Sanna G, Freiwald A (2021) Deciphering the composite morphological diversity of Lophelia pertusa, a cosmopolitan deep-water ecosystem engineer. Ecosphere 12:e03802. https://doi.org/10.1002/ecs2.3802
Sebens KP, Witting J, Helmuth B (1997) Effects of water flow and branch spacing on particle capture by the reef coral Madracis mirabilis (Duchassaing and Michelotti). J Exp Mar Biol Ecol 211:1–28. https://doi.org/10.1016/S0022-0981(96)02636-6
Stetson TR, Squires DF, Pratt RM (1962) Coral banks occurring in deep water on the Blake Plateau. Am Mus Novit 2114:1–39
Suding KN, Lavorel S, Chapin FS III, Cornelissen JHC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas ML (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Change Biol 14:1125–1140. https://doi.org/10.1111/j.1365-2486.2008.01557.x
Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté É, Zoccola D, Allemand D (2011) Coral biomineralization: From the gene to the environment. J Exp Mar Biol Ecol 408:58–78. https://doi.org/10.1016/j.jembe.2011.07.026
Tanasovici RM, Dias GM, Kitahara MV, Vieira EA (2022) Enduring regardless the conditions: Plasticity in modular growth as a strategy to cope with hydrodynamic variation by the invasive sun-coral (Tubastraea spp.). Mar Environ Res 174:105563. https://doi.org/10.1016/j.marenvres.2022.105563
Thorsnes T, Bellec V, Baeten N, Plassen L, Bjarnadóttir L, Ottesen D, Elvenes S, Rise L, Longva O, Bøe R, Lepland A, Buhl-Mortensen P, Buhl-Mortensen L, Dolan MFJ (2015) Mid-Norwegian continental shelf and slope. In: Buhl-Mortensen L, Hodnesdal H, Thorsnes T (eds) The Norwegian Sea Floor – New Knowledge from MAREANO for Ecosystem-Based Management. MAREANO, Bergen, pp 93–115
Thorsnes T, Bellec VK, Dolan MFJ (2016) Cold-water coral reefs and glacial landforms from Sula Reef, mid-Norwegian shelf. Geol Soc Lond Mem 46:307–308. https://doi.org/10.1144/M46.74
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337. https://doi.org/10.1111/j.1469-185X.2008.00045.x
Torchiano M (2020) effsize: Efficient Effect Size Computation. R package version 0.8.1. https://doi.org/10.5281/zenodo.683128
Vad J, Orejas C, Moreno-Navas J, Findlay HS, Roberts JM (2017) Assessing the living and dead proportions of cold-water coral colonies: implications for deep-water Marine Protected Area monitoring in a changing ocean. PeerJ 5:e3705. https://doi.org/10.7717/peerj.3705
van der Kaaden AS, Mohn C, Gerkema T, Maier SR, de Froe E, van de Koppel J, Rietkerk M, Soetaert K, van Oevelen D (2021) Feedbacks between hydrodynamics and cold-water coral mound development. Deep Sea Res I 178:103641. https://doi.org/10.1016/j.dsr.2021.103641
Veron JEN (1982) The species concept in ‘Scleractinia of Eastern Australia.’ In: Gomez ED, Birkeland CE, Buddemeier RW, Johannes RE, Marsh JA, Tsuda RT (eds) Proceedings of the Fourth International Coral Reef Symposium, 1981, vol 2. Marine Sciences Center, University of the Philippines, Quezon City, pp 183–186
Veron JEN, Pichon M (1976) Scleractinia of Eastern Australia, I: families Thamnasteriidae, Astrocoeniidae, Pocilloporidae. Australian Institute of Marine Science, Townsville
Vytopil E, Willis B (2001) Epifaunal community structure in Acropora spp. (Scleractinia) on the Great Barrier Reef: implications of coral morphology and habitat complexity. Coral Reefs 20:281–288. https://doi.org/10.1007/s003380100172
Wang H, Titschack J, Wienberg C, Korpanty C, Hebbeln D (2021) The Importance of Ecological Accommodation Space and Sediment Supply for Cold-Water Coral Mound Formation, a Case Study From the Western Mediterranean Sea. Front Mar Sci 8:760909. https://doi.org/10.3389/fmars.2021.760909
Wehrmann LM, Knab NJ, Pirlet H, Unnithan V, Wild C, Ferdelman TG (2009) Carbon mineralization and carbonate preservation in modern cold-water coral reef sediments on the Norwegian shelf. Biogeosciences 6:663–680. https://doi.org/10.5194/bg-6-663-2009
Wheeler AJ, Kozachenko M, Beyer A, Foubert A, Huvenne VAI, Klages M, Masson DG, Olu-Le Roy K, Thiede J (2005) Sedimentary processes and carbonate mounds in the Belgica Mound province, Porcupine Seabight, NE Atlantic. In: Freiwald A, Roberts JM (eds) Cold-Water Corals and Ecosystems. Springer, Berlin, Heidelberg, pp 571–603
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer, New York
Wilson JB (1979) ‘Patch’ development of the deep-water coral Lophelia pertusa (L.) on Rockall Bank. J Mar Biol Assoc UK 59:165–177. https://doi.org/10.1017/S0025315400046257
Wolfram U, Peña Fernández M, McPhee S, Smith E, Beck RJ, Shephard JD, Ozel A, Erskine CS, Büscher J, Titschack J, Roberts JM, Hennige SJ (2022) Multiscale mechanical consequences of ocean acidification for cold-water corals. Sci Rep 12:8052. https://doi.org/10.1038/s41598-022-11266-w
Zawada KJA, Dornelas M, Madin JS (2019a) Quantifying coral morphology. Coral Reefs 38:1281–1292. https://doi.org/10.1007/s00338-019-01842-4
Zawada KJA, Madin JS, Baird AH, Bridge TCL, Dornelas M (2019b) Morphological traits can track coral reef responses to the Anthropocene. Funct Ecol 33:962–975. https://doi.org/10.1111/1365-2435.13358
Acknowledgements
This work was supported by the German Research Foundation (DFG), through the MARUM Cluster of Excellence “The Ocean Floor – Earth’s Uncharted Interface” (Project No. 390741603), Research Unit RECORDER Theme 3. Sampling of corals collected during cruise POS455 was enabled by the German Federal Ministry of Education and Research (BMBF) project BIOACID II (Grant No. FKZ 03F0655A) and conducted with permission of the Norwegian Directorate of Fisheries, and CITES export and import permits were issued by the Norwegian Environment Agency and the German Federal Agency for Nature Conservation (BfN). We are grateful to captains, crews and scientists of RV Johan Hjort, Littorina and Poseidon for collecting the study samples. We would also like to thank Corinna Anderssohn, Karl-Heinz Baumann, Elda Miramontes and Max Wisshak for helping us retrieve the samples, Jürgen Titschack and Lydia Beuck for useful feedback on study design, and Nicol Mahnken for the photograph of Fig. 3. Finally, we acknowledge the Topic Editor and two anonymous reviewers for their constructive comments which helped us improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Research funded by the German Research Foundation (DFG; Project No. 390741603) and the German Federal Ministry of Education and Research (BMBF; Grant No. FKZ 03F0655A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Data availability
3D models and morphological data generated and analysed during the current study are available from Figshare: https://doi.org/10.6084/m9.figshare.20278602.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanna, G., Büscher, J.V. & Freiwald, A. Cold-water coral framework architecture is selectively shaped by bottom current flow. Coral Reefs 42, 483–495 (2023). https://doi.org/10.1007/s00338-023-02361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02361-z