Abstract
The success of individuals during the pelagic larval phase is critical to maintaining healthy and viable populations of coral reef fishes; however, it is also the most environmentally sensitive and energetically demanding life stage. Climate change is increasing the frequency and intensity of marine heatwaves, which could have significant effects on the development and survival of larval coral reef fishes. However, little is known about how the larvae of pelagic-spawning coral reef fishes will be affected due to the difficulty of spawning and rearing these species in captivity. In this study, we tested how elevated temperatures, similar to those occurring during a marine heatwave, affected the yolk utilization, growth, and survival of larval, Lutjanus carponotatus, a common mesopredatory fish on Indo-west Pacific coral reefs. Eggs and larvae were reared at a current-day average summer temperature (28.5 °C) and two elevated temperatures (30 °C and 31.5 °C) until 14 d post-hatch (dph). Larvae in the elevated temperatures depleted their yolk reserves 39% faster than at the control temperature. The standard length of larvae was 55% (30 °C) and 92% (31.5 °C) longer in the elevated temperature treatments than the control temperature at 14 dph. Conversely, survival of larvae was 54% (30 °C) and 68% (31.5 °C) lower at elevated temperatures compared with the control temperature. This study provides new insights as to how the early life stages of coral reef fishes could be affected by ocean warming and marine heatwaves, with implications for their population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most marine species have a pelagic larval stage that is important for population replenishment and connectivity, but which tends to have a high and variable rate of mortality (Doherty and Williams 1988; Armsworth 2002; Jones et al. 2009). Consequently, successful recruitment into benthic marine populations is heavily dependent on processes occurring during the larval phase (Caley et al. 1996). This early life period is typically characterized by rapid development, large energy demands, and high sensitivity to environmental conditions (biotic and abiotic) (May 1974; Houde 1989; Blaxter 1991; Post and Parkinson 2001; Stallings et al. 2010). Thus, any changes in environmental conditions during early development can be stressful, energetically costly, and lead to a bottleneck for population replenishment (Doherty et al. 2004; Dahlke et al. 2020). As the majority of marine organisms are ectotherms, environmental temperature is a critical factor during development, with higher temperatures increasing the rate of metabolism (Gillooly et al. 2001; Neuheimer et al. 2011; Schulte 2015). These physiological changes can accelerate developmental rate and increase energetic demands (Houde 1989; Blaxter 1991; Pepin 1991), with implications for the size, condition and number of individuals that survive the pelagic larval stage (O’Connor et al. 2007, Munday et al. 2009).
Varying effects of higher than normal temperatures have been reported in larval fishes. For example, temperatures above the summer average increased larval growth and survivorship in some studies (Green and Fisher 2004; Fielder et al. 2005; Sponaugle et al. 2006; O’Conner et al. 2007), whereas it decreased larval growth and survival in others (McCormick and Molony 1995; Grorud-Colvert and Sponaugle 2011; Pope et al. 2014; Watson et al. 2018). Much of the diversity in thermal sensitivity is attributed to differences in the optimal temperature range of various populations and species (McLeod et al. 2015). For instance, numerous species from higher latitudes exhibit positive effects on growth (Pepin 1991; Steinarsson and Björnsson 1999; Dou et al. 2005) and survival (Fowler and Jennings 2003; Pope et al. 2014) when cohorts experience warmer than average summer temperatures. Conversely, tropical reef fish, which have evolved in relatively warm and stable temperature at lower latitudes, often experience negative effects when exposed to warmer than normal summer temperature. For example, an increase of just 1–2 °C increased energetic demands (Johansen and Jones 2011; McLeod et al. 2013; McLeod and Clark 2016), decreased growth rate (Munday et al. 2008; McLeod et al. 2015; Spinks et al. 2019), and reduced survivorship (Houde 1989; Rankin and Sponaugle 2011), during the early life stage of some coral reef fishes. However, much of the research to date has focused on smaller-bodied demersal spawning reef fish, such as damselfish, that are frequently caught in light traps or easily bred in captivity (McCormick and Molony 1995; Munday et al. 2008; Donelson et al. 2010; Rankin and Sponaugle 2011; Spinks et al. 2019). How increased environmental temperatures affect the early life history development and survival of larger-bodied pelagic-spawning coral reef fishes is largely unknown, in part because of the difficulty in breeding and rearing many of these species in captivity.
A significant number of coral reef fish commence spawning just before, or during, the warmer months of the year (Domeier and Colin 1997; Russell 2001); therefore, larvae develop during periods most susceptible to marine heatwaves (MHW). Climate change is causing average sea surface temperature to rise and increasing the frequency and occurrence MHWs in summer (King et al. 2016; Hoegh-Guldberg et al. 2018; Pörtner et al. 2019). Abnormally warm days in marine environments have increased approximately 50% over the last century (Oliver et al. 2018), with the probability of a MHW occurring currently being ~ 9 times higher than in preindustrial times (Frölicher et al. 2018). As climate change progresses, the probability of a MHW occurring could reach 41 times higher than in preindustrial times and increase in duration by ~ 300% by 2100 (under an RPC 8.5; Hoegh-Guldberg et al. 2014; Frölicher et al. 2018). With the Great Barrier Reef having experienced heatwaves in 4 out of the last 6 summers, which resulted in many reefs experiencing temperatures 1–3 °C above the summer average from days to weeks at a time (2016, 2017, 2020, 2022; AIMS 2017; Hughes et al. 2017; BOM 2020), there is an urgency to understand how extreme temperatures affect the early life stages of coral reef fish. In particular, there is a paucity of research on the effects of elevated temperature on larger predatory and pelagic-spawning species, with some notable exceptions (e.g. Pratchett et al. 2017). This is largely due to the complexities and technical difficulties of successfully spawning and rearing broadcast spawning species in aquaria (Battaglene and Stewart 1997; Moorhead and Zeng 2010). However, these challenges must be overcome to understand how these species may fare with future MHWs, as significant proportion of reef fish are broadcast spawners.
In this study, we investigated how elevated temperatures affect the early life history of the coral reef snapper, Lutjanus carponotatus, a mesopredatory fish that is ecologically important to coral reef ecosystems, and important to recreational and commercial fisheries (GBRMPA 2014). Due to the increased metabolic demands at higher temperatures, we would expect individuals to utilize endogenous resources at a faster rate and experience accelerated growth rates. To test this, we exposed gametes from fertilization to 14 d post-hatch (dph) to either 28.5 °C (the ambient summer conditions), 30 °C or 31.5 °C. The 30 °C and 31.5 °C temperatures were chosen as they are projected to be average reef temperature in the future (mid and end of century) and because these temperatures have already occurred for days to weeks during recent heatwaves (Hughes et al. 2018; Spinks et al. 2019). During this experiment, we assessed how elevated temperatures: (1) affected the utilization of endogenous energetic reserves from 0 to 51 h post-hatching (hph), (2) influenced growth and development at 1 dph and 14 dph by measuring key morphometric and developmental points, and (3) affected hatching and larval survival at 1 dph and 14 dph to better understand the potential impacts of ocean warming on the early life of coral reef fishes.
Methods
Study species
The Spanish Flag snapper, L. carponotatus, is a tropical mesopredator that inhabits coral reefs throughout the Great Barrier Reef (GBR) and Indo-west Pacific (Allen 1985). They are a gonochoristic serial spawner with the peak spawning for the GBR population occurring between October and December (Kritzer 2004). Over this period, the upper SST range is ~ 26.5 to 28.5 °C for the northern GBR where our broodstock fish were collected (the 10-yr average between 2011 and 2021, from Lizard Island and One Tree representing the northern and southern range; AIMS 2017). The pelagic larval phase of L. carponatus is approximately 33–38 d (Quéré and Leis 2010) after which they settle to benthic reef habitat.
Broodstock and spawning
Adult snapper were caught by hand-line fisherman from the northern GBR between November 2018 and September 2019 and transported to the Marine and Aquaculture Research Facility at James Cook University, Townsville. Between six to eight adult fish (28–40 cm standard length) were housed together in each of ten 2500-L tanks (n = 72 adults). The temperature cycle for the adults followed a pattern similar to the average monthly temperatures for the northern GBR, where the winter and summer temperatures were 23 °C and 28.5 °C, respectively. The target water temperature was maintained by an 11-kW heat pump (Toyesi, Titan) that was regulated by an automated controller (Innotech, Omni). Temperatures were maintained within ± 0.1 °C of the target temperature. Adults were fed daily a mixture of Skretting pellets (Spectra SS) and pilchards at ~ 2% body weight. Spawning was allowed to occur naturally without hormonal stimulus, with spawns produced at 28.5 °C. Spawning occurred late at night, and eggs were collected in an overflow egg collector (Moran et al. 2007). For this experiment, four adult tanks were used (n = 32 adults total), as they spawned simultaneously during December 2019–January 2020. The spawns were mixed before being placed into larval rearing tanks to maximize genetic diversity. Spawns were collected on 2 December 2019 for the larval development experiment and spawns from the same 4 tanks a week later were used in the yolk utilization experiment.
Experimental design
The study comprised two experiments, one focused on the yolk utilization of larvae between 0 and 51 hph, and another on larval development from hatching to 14 dph (Table 1). For both the yolk utilization and larval development experiments, the eggs and larvae were reared in three stable temperature treatments of 28.5 °C, 30 °C, and 31.5 °C (Table 2). At ~ 6 h (0600) post-fertilization, eggs mixed from the 4 adult tanks where spawning occurred were transferred to 20-L larval rearing tanks (n = 6 per treatment) set at 28.5 °C. Larval rearing tanks were stocked at ~ 500 eggs per tank for the yolk utilization experiments and ~ 1000 eggs per tank for the larval development experiment. Immediately after stocking with eggs, tanks in the two elevated temperature treatments (30 °C and 31.5 °C) were increased gradually over 2 h until they reached target temperature. To accurately estimate the number of eggs per tank and account for any mortality during handling, three 50-mL samples from each tank 6 h after stocking (1200), the eggs were counted and averaged from each tank and multiplied by the tank volume to estimate total eggs in each tank. To increase the accuracy of sampling, each tank was gently stirred and agitated to homogenize the eggs throughout the tank. The average stocking density at this point varied between tanks by 3–5%. Hatching occurred between ~ 14 and 16 h post-fertilization (1400–1600).
Each larval rearing tank received water flow of ~ 100 mL min−1 allowing for a complete water volume exchange every ~ 3 h. Each temperature treatment received water from a 1000-L sump connected by partial exchange to a central 5000-L sump where filtration (Mechanical bag filters to 25 micron and 400 L protein skimmer) and UV sterilization occurred (Wedeco, B32-PE). The experimental room air temperature was set to 26.5 °C, and the temperature treatment (28.5 °C, 30 °C, and 31.5 °C; Table 2) sumps (1000 L each) were maintained by 3 kW bar heaters (Toyesi) that were automatically controlled and monitored (Innotech, Omni). Each treatment had two replicate sumps. Salinity, alkalinity, pH, and oxygen saturation were all maintained at natural levels (Table 2). Salinity, oxygen (Hach, HD40u) and pH (Mettler, Seven2Go Pro) were measured daily. Alkalinity was measured weekly (Metrohm, 888 Titrando). The photo period was set at 12:12 light/dark (0700–1900) with simulated sunrise and sunset through an hour gradual increase from 0 to 100% (0700–0800) and an hour decrease from 100 to 0% (1900–2000).
Standard green-water methodology was used for rearing larval fishes (Palmer et al. 2007; McMahon et al. 2020). From 1 dph, larval rearing tanks were treated with inert algae (Nano 3600, Reed Mariculture) at a concentration of ~ 230,000 cells mL−1. Larvae in the yolk utilization experiment were reared in green-water, but were not fed. In the larval development experiment, larvae in all treatments were fed enriched rotifers (INVE, Selco S.parkle) three times a day (0800, 1200, and 1600) from 1 dph at a density of ~ 10 1 mL−1. From 7 dph, larvae were fed enriched Artemia nauplii (INVE, Selco S. presso) at a density of 1 mL−1 once per day (0800). Rotifer feeding and green-water treatment were gradually reduced between 9 and 11 dph.
Sampling
In the yolk utilization experiment, eggs were initially sampled from each tank at stocking (0 hph, n = 20). Subsequently, larvae were sampled from each tank (n = 20) once the majority of eggs appeared to have hatched (3 hph) and then every 12 h until 51 hph, when endogenous energy reserves were depleted.
During the larval development experiment, larvae were sampled from the three temperature treatments at 1 dph and 14 dph. To sample each tank, the water was gently stirred to homogenize the larvae and three 50-mL samples were taken. Larvae in each sample were counted, and the total larvae in the tank were extrapolated from the samples. The variation between each tanks triplicate counts were < 7%. Initial survival was measured once most eggs in each tank were either hatched or had died. Survival through the experiment was calculated as a percentage using the number of individuals in each tank at a sampling point compared to initial stocking. Larvae were then placed gently back into the tank except for 10 individuals (n = 60 per treatment), which were euthanized and photographed using a digital camera (Olympus, SC50) fitted to a stereo microscope (Olympus, SZX7). All larvae were euthanized and photographed within 30 min of sampling.
Morphometric data were extracted from digital photographs using ImageJ2 (Rueden et al. 2017) by an observer (SJM) who was blind to the treatments. In the yolk utilization experiment, standard length (SL), muscle depth (MD), yolk area (YA) and oil globule area (OGA) were measured. In the larval development experiment, the larval morphology measured was standard length (SL), total length (TL), body length (BL), muscle depth (MD), fin depth (FD), eye diameter (ED), mandible length (ML), yolk area (YA), oil globule diameter (OGD), head length (HL) and head depth (HD) (Fig. 1). Larvae were also noted as either being pre- or post-flexion, which was defined as a stage of development where the posterior of the notochord bends upward in preparation of caudal fin development. In the larval development experiment, oil globule diameter, rather than area, was measured as the oil globule shape changes from spherical to asymmetrical during consumption.
Morphological measurements from larval Lutjanus carponotatus at a 1 and b 14 d post-hatching. Scale bars represent 1 mm. Standard length (SL), total length (TL), body length (BL), head length (HL), head depth (HD), mandible length (ML), muscle depth (MD), fin depth (FD), eye diameter (ED), yolk area (YA) and oil globule diameter (OGD)
Statistical analysis
To determine whether temperature had an effect on the consumption of yolk and oil globules (YA and OGA), Kruskal–Wallis tests, followed by Dunn tests, were conducted at each time point to determine differences between the temperature treatments. Nonparametric testing was completed due to both YA and OGA having zero values. Specifically, comparisons of YA were conducted from 0 to 27 hph due to YA being depleted in all fish at 39 hph (i.e. data were all zeros > 39 hph), while comparisons of OGA were conducted from 0 to 51 hph. Linear mixed effects models (LME) were conducted, and F-statistics extracted on the morphometric data from the yolk utilization experiment (standard length and muscle depth). Temperature and hph were fixed factors, and tank was included as a random effect. When significant differences were found between temperature treatments, pairwise comparisons were made with estimated marginal means and Tukey’s method of p-value adjustment.
Since growth metrics can be highly correlated, principal component analyses (PCAs) were used on the morphological data from larvae at 1 dph and 14 dph. Using PCA reduced the complexity of the multivariate data and identified the variables accounting for the largest variation in the data. The PCA for 1 dph larvae included: TL, SL, BL, HL, MDV, FD, OGD, and YA. For the PCA of the 14 dph larvae, the measurements used were: TL, SL, BL, HL, HD, ML, ED, MD, and FD. In the 1 dph PCA, three principal components were needed to explain over 70% of the variation, while in the 14 dph PCA 97% of the variation was explained by the first principal component.
To further examine the effects of temperature on morphology, LMEs were applied to the first two principal components and to each of the morphological measurement separately. Temperature was included as a fixed factor, and rearing tank was used as a random effect in these LMEs. Individual generalized linear models (GLM) were used to compare the survival of larvae at 1, 7 and 14 dph between treatments. Initial stocking density was used as a covariate in the model, and proportional survival was tested as the dependant variable. F-statistics were extracted for all LMEs to determine differences between temperature treatments and when significant pairwise comparisons were made with estimated marginal means and Tukey’s method of p-value adjustment.
All analyses were conducted in R (R Core Team 2014) using the nlme and GLM packages (Bates et al. 2012). Post hoc tests were conducted using emmeans. All models met the assumptions of the relevant tests. This was confirmed by accessing the residuals, goodness of fit, and checking dispersion.
Results
Yolk utilization
There was no difference in yolk area among treatments at 0 hph (X2 = 3.56, p = 0.169), and yolk reserves were completely consumed in all treatments by 39 hph (Fig. 2a). However, water temperature had a significant effect on yolk area from 3 to 27 hph (3 hph: X2 = 29.95, p < 0.001; 15 hph: X2 = 40.50, p < 0.001; 27 hph: X2 = 38.05, p < 0.001). Larvae from both warmer treatments had significantly less yolk at each time point between 3 and 27 hph compared with the 28.5 °C control, and at 27 hph there was a smaller yolk area remaining in the 31.5 °C than in the 30 °C larvae (Fig. 2a; all p < 0.001; Table A1). The oil globule of larvae that developed at 28.5 °C was consumed by 63 hph, whereas larvae from both warmer treatments consumed their oil globule by 39 hph (Fig. 2b). There was a significant effect of temperature on oil globule area (all p < 0.05; Table A2), with larvae from the warm treatments using their oil globule ~ 39% faster than the 28.5 °C control. Specifically, larvae that developed in 31.5 °C had a smaller oil globule area at all post-hatching sampling times, while the oil globule of larvae in 30 °C was smaller at all times except 3 hph (Fig. 2b; Table A2).
The standard length of larvae in all treatments increased by ~ 30% between hatching and 15 hph, after which time length remained at around 2.8 mm (Fig. 2c). While standard length differed with both temperature (F2,6 = 17.7, p = 0.003) and time (F5,337 = 1495.90, p ≤ 0.001), there was a significant interaction between temperature and hours post-hatching (F10,337 = 29.3, p < 0.001). This was largely driven by differences between temperature treatments at 3 hph (28.5 vs. 31.5 °C: t = − 9.37, p = 0.003; 30 vs. 31.5 °C: t = − 8.75, p = 0.004) and 15 hph (28.5 vs. 30 °C: t = − 8.68, p = 0.004; 28.5 vs. 31.5 °C: t = − 11.81, p < 0.001), where larvae were longer in warmer treatments. After 15 hph, there was a trend that standard length decreased slightly (~ 3–5%) in the warmer treatments, while the control fish did not (Fig. 2c; 31.5 °C 15 vs. 27, 39 and 51 hph: t = 4.47, t = 4.50, t = 5.44, respectively, all p ≤ 0.001; 30 °C 27 vs. 51 hph: t = 4.44, p = 0.002). There was also a significant interaction between temperature and time for muscle depth (F10,337 = 3.56, p < 0.001). This was largely driven by the warmer treatments tending to have greater muscle depth at 3–15 hph and reduced muscle depth at 51 hph; however, this did not result in any significant temperature differences within a time-point. A general pattern of decreased muscle depth by ~ 20% from 0 to 3 hph (28.5 °C 0 vs. 3 hph: t = 6.80, p < 0.001; 30 °C 0 vs. 3 hph: t = 8.65, p < 0.001) followed by an increase of ~ 30% between 3 and 15 hph (28.5 °C 3 vs. 15 hph: t = − 15.27, p < 0.001; 30 °C 3 vs. 15 hph: t = − 15.13, p < 0.001, 31 °C 3 vs. 15 hph: t = − 14.55, p < 0.001) was observed. Muscle depth then steadily decreased by ~ 10% between 15 and 51 hph (Fig. 2d; 28.5 °C 15 vs. 51 hph: t = 4.04, p = 0.008; 30 °C 15 vs. 51 hph: t = 6.37, p < 0.001, 30 °C 15 vs. 51 hph: t = − 8.44, p < 0.001).
Growth morphometrics
At 1 dph, PC1 (46% of total variation) was largely described by differences in all morphological measurements (standard length, total length, body length, head length, muscle depth, and fin depth) except yolk area and oil globule diameter that were associated with the PC2 axis (19%; Fig. 3a). At 1 dph, there was a significant difference between the control and elevated temperatures (28.5 vs. 30 °C: t = − 4.55, p = 0.009; 28.5 °C vs. 31.5 °C: t = − 5.38, p = 0.004), but not between the two elevated temperatures (30 vs. 31.5 °C: t = − 0.82, p = 0.702). There were significant differences between temperatures for PC2 (F2,6 = 6.12, p = 0.036), and this was found to be driven by the difference between the 30 °C and 31.5 °C treatments (t = 3.49, p = 0.001). The effect of temperature was further supported by the individual analysis of each trait finding that 30 °C larvae were significantly larger in total length (8%), standard length (7%), body length (9%), and head length (14%) compared to control larvae (Fig. 4a; all p < 0.01; Table A3). Larvae from the 31.5 °C treatment also were significantly larger in total length (9%), standard length (6%), body length (7%), head length (15%), and muscle depth (12%) compared with control fish (Fig. 4a; all p < 0.05; Table A3). In addition, oil globule diameter was significantly smaller (14%) in 31.5 °C treated fish than the other two treatments (Fig. 4a; all p < 0.05; Table A3).
PCA of morphometric data for larval Lutjanus carponotatus at A 1 dph and B 14 dph. Rings represent 95% confidence that data fall with for 28.5 °C (blue), 30 °C (orange) and 31.5 °C (red). Morphological measurements are indicated by arrows. Standard length (sl), total length (tl), body length (bl), muscle depth (md), fin depth (fd), eye diameter (ed), mandible length (ml), yolk area (ya), oil globule diameter (ogd), head length (hl) and head depth (hd)
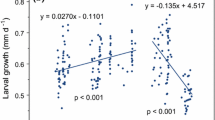
At 14 dph, there was clear morphological distinction between larvae from 28.5 °C and the warmer groups, seen by the lack of overlap in 95% confidence ellipses for PC1, which described 97% of all trait variation (Fig. 3b). All morphometric measurements, and their combination described by PC1, were significantly larger with increasing water temperature (Fig. 3b; all p ≤ 0.001; Table A4). This resulted in morphology being significant between all treatment groups (All p ≤ 0.001; Table A4). Specifically, standard length, total length and body length were ~ 55% longer in the 30 °C treatment and ~ 92% longer in the 31.5 °C treatment, compared to 28.5 °C (Fig. 4b). Head depth was 86% bigger in 30 °C treatment and 146% bigger in the 31.5 °C treatment compared to 28.5 °C (Fig. 4b). Muscle depth was 192% bigger in the 30 °C treatment and 283% bigger in the 31.5 °C treatment compared with the 28.5 °C control (Fig. 4b). In addition, at 14 dph all fish in both the 30 °C and 31.5 °C treatments had undergone flexion, while no individuals had commenced flexion in the 28.5 °C control.
Survival
The hatching success of eggs was significantly higher in the 30 °C (+ 10%) and 31.5 °C (+ 9%) treatments, compared to the 47% hatching success at 28.5 °C (Fig. 5; 28.5 vs. 30 °C: z = − 3.586, p = 0.010; 28.5 vs. 31.5 °C: z = − 3.478, p = 0.015). However, this pattern was reversed in larvae at 7 dph, where survival at 28.5 °C (39%) was 3 and 4 times higher than in the 30 °C and 31.5 °C treatments, respectively (28.5 vs. 30 °C: z = 9.659, p < 0.001; 28.5 vs. 31.5 °C: z = 11.147, p < 0.001). This pattern was maintained at 14 dph, with 15% survival in the control treatment, which was 3 times higher than the 31.5 °C treatment (z = 3.881, p = 0.003). A trend of higher survival in the 28.5 °C treatment was also present at 14 dph when compared to the 30 °C treatment; however, it was not statistically significant (z = 3.067, p = 0.055).
Discussion
We found that under elevated summer temperatures (30 and 31.5 °C) consistent with climate change projections (mid and end of century) and heatwave conditions already occurring on coral reefs on the GBR, larval L. carponotatus had higher hatching success, developed at a faster rate, and grew up to twice as large by 14 dph compared to larvae reared at the current-day control temperature of 28.5 °C. Faster growth could be expected to enhance survival in a natural setting if larger size provides a refuge from predation (Shulman 1985; Schmitt and Holbrook 1999; Kingsford et al. 2022). However, survival during the 14-d experiment was 50–70% lower in the elevated temperature treatments. Larvae in warmer conditions consumed their endogenous energy reserves ~ 20% faster, which could potentially explain reduced survivorship if it meant a reduced time window for which to begin feeding. For this population of L. carponotatus, the potential benefits of higher growth and development at higher temperatures may be negated by decreased survival, highlighting the complexity of determining the effects of environmental stressors on fish early life stages.
Elevated water temperature increased the rate of growth and development during the first 14 d of life. By 14 dph, all morphometric measurements were larger in larvae that developed at 30 and 31.5 °C compared to larvae reared at the current-day control temperature of 28.5 °C. Importantly, it was not just morphology that was increased, but also the rate of development, because all fish in elevated temperatures had undergone flexion at 14 dph, whereas no fish had entered flexion at 28.5 °C. The combination of faster growth and development indicates that the rate of metabolism was increased due to elevated temperature (Fry 1971; Johnston and Dunn 1987; Angilletta et al. 2004). Furthermore, the uniform response between elevated temperature and the change in measured morphology (i.e. the increase in 31.5 °C larvae was approximately 2 times the 30 °C response) are consistent with a direct effect from increased metabolism. These findings suggest L. carponotatus (from the central region of the species range) may be living below its thermal optimum for the larval period, and small increases in water temperature (≤ 3 °C) could enhance growth and development during early life in the wild. However, due to the increased levels of mortality observed at elevated temperatures any benefit in growth would likely be redundant. Interestingly, our findings contrast with previous work on demersal spawning reef fish where warmer temperatures (> 29 °C) reduced larval and juvenile growth (Zarco-Perello et al. 2012; McLeod et al. 2015). While the reason for this difference is unknown, it is possible that the different spawning strategies (i.e. demersal spawning producing fewer but more highly provisioned eggs) are differentially affected as temperature increases. It is important to note that in the current laboratory experiment larvae were provided with ample food to fuel increased metabolism and developmental rate at elevated temperatures, whereas this may not be available in nature (Owen 1989; MacKenzie and Leggett 1991; Hays et al. 2005). Limited and/or patchy food supply in nature could limit any benefits of higher temperature on larval growth and developmental rate (McCormick and Molony 1992; Bochdansky et al. 2005). It is also possible that the faster growth observed in the two elevated treatments could be due in part to selection for faster growing phenotypes (Meekan and Fortier 1996). However, this seems unlikely from our PCA results at 14 dph with 97% of all variation in morphology described by PC1 and all trait vectors clustering together, which implies that larvae are following an ontogenetic developmental path that is simply accelerated in warmer conditions.
Endogenous provisions are critical to the survival of larval fish as they supply the energy needed to grow and develop to the stage they start feeding. They are the only energetic resources available to larval fish until they develop the organs and structures (e.g. gut, stomach, jaw, eyes) to successfully capture and digest their prey (Kamler 2008). Consequently, the amount of endogenous resources provided by mothers, and the rate at which larvae consume them, can be critical to successfully surviving through the pre-feeding stage of early life (Kamler 2002; Tocher 2010). We found that larvae in warmer treatments had a faster rate of yolk utilization from 3 to 27 hph; however, all temperature groups consumed their yolk by 39 hph. Furthermore, the oil globule was used ~ 39% faster and was exhausted by 39 hph in the elevated temperature treatments, whereas control 28.5 °C fish consumed their oil globules by 63 hph. This accelerated usage of yolk and oil is likely due to the additional metabolic costs at higher temperatures (Houde and Zastrow 1993; Rombough 1997; Kamler 2002). Consequently, it appears that the faster usage of energy reserves at elevated temperatures was used to maintain homeostasis, since there was no observed increase in growth between temperature treatments over this timeframe. In fact, there was a slight reduction in length and muscle depth for warmer treatments at 51 hph, although this was not statistically significant. Interestingly, at ~ 1 dph temperature appeared to have contrasting effects on the standard length of larvae in the yolk utilization experiment versus the growth experiment. While the reason for this is unknown, it may have been caused by the use of different spawns in the experiments, which may have been of different quality (growth experiment spawn started 7 d prior to yolk utilization). Additionally, the largest change in growth and development metrics, and between treatments, was seen between 3 and 15 hph, which suggests that this window may encompass a key metamorphic milestone for this species. Critically for larvae, elevated temperature and the increased consumption rate of energetic reserves would result in a shorter window in which to successfully begin feeding, which could reduce survival in a natural setting where food availability can be patchy (Owen 1989; MacKenzie and Leggett 1991; Hays et al. 2005).
Survival during larval development is low for most marine fish, less than 1% on average (Pepin 1991; Houde 1994), and small changes in survival during this life stage can impact successful recruitment of different cohorts to the adult population (Rijnsdorp et al. 2009; Pörtner and Peck 2010; Petitgas et al. 2013). The point in larval development where endogenous reserves are depleted and larvae must switch to feeding is a critical period for survival (Hjort 1914; May 1974; Houde 1997). We observed that elevated water temperature significantly reduced the survival of larval L. carponotatus prior to 7 dph and the effect persisted to 14 dph. Survival in the elevated temperature groups declined markedly within the first week of life to just 10–14% compared to ~ 40% survival in the current-day control temperature. This increase in mortality would coincide with endogenous resources being used (observed at 63 hph in this study) in combination with the additional metabolic costs of elevated temperature when larvae begin to feed, a time when they are known to be inefficient at food acquisition (China and Holzman 2014). Therefore, it is possible that the window for successful first feeding may be reduced at elevated temperatures, and combined with increased metabolic demands, would likely reduce larval survival in nature. Reduced survival and higher temperatures could have detrimental effects on populations if these patterns were to hold in the natural environment, which has been observed in the early life stage of other coral reef fishes (Houde 1989; Rankin and Sponaugle 2011).
This study found that elevated temperatures consistent with recent heatwave conditions on coral reefs and projected to become average summer water temperatures in the future had both positive and negative effects on the early life history of larval L. carponotatus. Increased growth, along with accelerated attainment of developmental milestones, might be seen as advantageous, because it means that larvae more quickly reach a size where they gain some refuge from predation (Shulman 1985; Schmitt and Holbrook 1999; Kingsford et al. 2022). However, we found that faster growth came at the cost of substantially lower survival. Consequently, there is a potential trade-off between more rapid attainment of predatory size thresholds at higher temperature and the direct effects of higher temperature on mortality rate. It seems unlikely that the increased growth observed would occur in all natural settings since food availably is often patchy and limited (Owen 1989; MacKenzie and Leggett 1991; Hays et al. 2005). Yet, natural survival of larvae to settlement in most marine fish is typically no greater than 1%; therefore, the impact of elevated temperature will depend on whether it is additive with natural selection, or simply shifts the phenotypic composition of the surviving cohort. Further research focusing on the long-term effects of heatwaves and warming on larval fish, monitoring growth, development and survival into the juvenile to sub-adult stages, would benefit our understanding of how ocean warming will affect coral reef fish. This study has provided insights as to how the early life stages of a coral reef mesopredator could be impacted by a warming ocean, which will allow us to better understand how these populations will fare as climate change progresses.
Data availability
The data sets is available on JCU’s Topical data hub. https://doi.org/10.25903/77vf-8y27.
References
AIMS (2017) AIMS sea water temperature observing system (AMIS temperature logger program), https://doi.org/10.25845/5b4eb0f9bb848, accessed 25-July-2022
Allen, GR (1985) FAO species catalogue. V. 6: Snappers of the world. An annotated and illustrated catalogue of lutjanid species known to date: FAO
Angilletta MJ Jr, Steury TD, Sears MW (2004) Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integr Comp Biol 44:498–509
Armsworth PR (2002) Recruitment limitation, population regulation, and larval connectivity in reef fish metapopulations. Ecology 83:1092–1104
Bates, D, Maechler, M, Bolker, B, Walker, S, Christensen, RHB, Singmann, H, . . . Scheipl, F (2012) Package ‘lme4’. CRAN. R Foundation for Statistical Computing, Vienna, Austria
Battaglene, S & Fielder, S (1997) The status of marine fish larval-rearing technology in Australia. Live food in aquaculture (pp. 1–5): Springer
Blaxter J (1991) The effect of temperature on larval fishes. Neth J Zool 42:336–357
Bochdansky A, Grønkjær P, Herra T, Leggett W (2005) Experimental evidence for selection against fish larvae with high metabolic rates in a food limited environment. Mar Biol 147:1413–1417
BOM (2020) 2020 marine heatwave on the Great Barrier Reef. Bureau of Meteorology. https://doi.org/10.13140/rg.2.2.12494.48969
Caley M, Carr M, Hixon M, Hughes T, Jones G, Menge B (1996) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
China V, Holzman R (2014) Hydrodynamic starvation in first-feeding larval fishes. P Natl Acad Sci USA 111:8083–8088
Dahlke FT, Wohlrab S, Butzin M, Pörtner H-O (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369:65–70
Doherty P, Dufour V, Galzin R, Hixon M, Meekan M, Planes S (2004) High mortality during settlement is a population bottleneck for a tropical surgeonfish. Ecology 85:2422–2428
Doherty PJ, Williams DM (1988) The replenishment of coral reef fish populations. Oceanogr Mar Biol Annu Rev 26:487–551
Domeier ML, Colin PL (1997) Tropical reef fish spawning aggregations: Defined and reviewed. B Mar Sci 60:698–726
Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401:233–243
Dou S, Masuda R, Tanaka M, Tsukamoto K (2005) Effects of temperature and delayed initial feeding on the survival and growth of Japanese flounder larvae. J Fish Biol 66:362–377
Fielder DS, Bardsley WJ, Allan GL, Pankhurst PM (2005) The effects of salinity and temperature on growth and survival of Australian snapper, pagrus auratus larvae. Aquaculture 250:201–214
Fowler A, Jennings P (2003) Dynamics in 0+ recruitment and early life history for snapper (pagrus auratus, sparidae) in South Australia. Mar Freshwater Res 54:941–956
Frölicher TL, Fischer EM, Gruber N (2018) Marine heatwaves under global warming. Nature 560:360–364
Fry F (1971) The effect of environmental factors on the physiology of fish. Fish Physiol 6:1–98
GBRMPA (2014) Great Barrier Reef outlook report 2014, Great Barrier Reef marine park authority
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Green BS, Fisher R (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299:115–132
Grorud-Colvert K, Sponaugle S (2011) Variability in water temperature affects trait-mediated survival of a newly settled coral reef fish. Oecologia 165:675–686
Hays GC, Richardson AJ, Robinson C (2005) Climate change and marine plankton. Trends Ecol Evol 20:337–344
Hjort J (1914) Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. ICES
Hoegh-Guldberg, O, Cai, R, Poloczanska, ES, Brewer, PG, Sundby, S, Hilmi, K, . . . Stone, DA. (2014). The Ocean. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 1655–1731
Hoegh-Guldberg, O, Jacob, D, Bindi, M, Brown, S, Camilloni, I, Diedhiou, A, . . . Guiot, J (2018) Impacts of 1.5 C global warming on natural and human systems. Global warming of 1.5 C. An IPCC Special Report
Houde E (1997) Patterns and trends in larval-stage growth and mortality of teleost fish. J Fish Biol 51:52–83
Houde ED (1989) Comparative growth, mortality, and energetics of marine fish larvae: Temperature and implied latitudinal effects. Fish B NOAA 87:471–495
Houde ED (1994) Differences between marine and freshwater fish larvae: implications for recruitment. ICES J Mar Sci 51:91–97
Houde ED, Zastrow CE (1993) Ecosystem and taxon specific dynamic and energetics properties of larval fish assemblages. B Mar Sci 53:290–335
Hughes, TP, Kerry, JT, Álvarez-Noriega, M, Álvarez-Romero, JG, Anderson, KD, Baird, AH, . . . Berkelmans, R (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373-377
Hughes, TP, Kerry, JT, Baird, AH, Connolly, SR, Dietzel, A, Eakin, CM, . . . Torda, G (2018) Global warming transforms coral reef assemblages. Nature 556:492-496
Johansen J, Jones G (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Change Biol 17:2971–2979
Johnston I, Dunn J (1987) Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. Symp Soc Exp Biol 41:67–93
Jones G, Almany G, Russ G, Sale P, Steneck R, Van Oppen M, Willis B (2009) Larval retention and connectivity among populations of corals and reef fishes: History, advances and challenges. Coral Reefs 28:307–325
Kamler E (2002) Ontogeny of yolk-feeding fish: An ecological perspective. Rev Fish Biol Fisher 12:79–103
Kamler E (2008) Resource allocation in yolk-feeding fish. Rev Fish Biol Fish 18:143–200
King AD, Black MT, Min SK, Fischer EM, Mitchell DM, Harrington LJ, Perkins-Kirkpatrick SE (2016) Emergence of heat extremes attributable to anthropogenic influences. Geophys Res Lett 43:3438–3443
Kingsford MJ, Krunes EA, Hall AE (2022) Testing the critical size at settlement hypothesis for two species of coral reef fish. Mar Ecol Prog Ser 681:87–101
Kritzer JP (2004) Sex-specific growth and mortality, spawning season, and female maturation of the stripey bass (Lutjanus carponotatus) on the Great Barrier Reef. Fish B NOAA 102:94–107
MacKenzie B, Leggett W (1991) Quantifying the contribution of small-scale turbulence to the encounter rates between larval fish and their zooplankton prey: Effects of wind and tide. Mar Ecol Prog Ser 73:149–160
May, R (1974) Larval mortality in marine fishes and the critical period concept. The early life history of fish, Springer, Berlin, pp 3–19
McCormick M, Molony B (1992) Effects of feeding history on the growth characteristics of a reef fish at settlement. Mar Biol 114:165–173
McCormick MI, Molony BW (1995) Influence of water temperature during the larval stage on size, age and body condition of a tropical reef fish at settlement. Mar Ecol Prog Ser 118:59–68
McLeod IM, Clark TD (2016) Limited capacity for faster digestion in larval coral reef fish at an elevated temperature. PLoS ONE 11:e0155360
McLeod IM, Jones RE, Jones GP, Takahashi M, McCormick MI (2015) Interannual variation in the larval development of a coral reef fish in response to temperature and associated environmental factors. Mar Biol 162:2379–2389
McLeod, IM, Rummer, JL, Clark, TD, Jones, GP, McCormick, MI, Wenger, AS, & Munday, PL (2013) Climate change and the performance of larval coral reef fishes: The interaction between temperature and food availability. Conserv Physiol 1:cot024
McMahon SJ, Parsons DM, Donelson JM, Pether SM, Munday PL (2020) Elevated CO2 and heatwave conditions affect the aerobic and swimming performance of juvenile Australasian snapper. Mar Biol 167:1–12
Meekan MG, Fortier L (1996) Selection for fast growth during the larval life of Atlantic cod, Gadus morhua, on the Scotian shelf. Mar Ecol Prog Ser 137:25–37
Moorhead, JA & Zeng, C (2010) Development of captive breeding techniques for marine ornamental fish: A review. Rev Fish Sci 18(:315–343
Moran D, Smith CK, Gara B, Poortenaar CW (2007) Reproductive behaviour and early development in yellowtail kingfish (Seriola lalandi valenciennes 1833). Aquaculture 262:95–104
Munday PL, Donelson JM, Dixson DL, Endo GGK (2009) Effects of ocean acidification on the early life history of a tropical marine fish. P R Soc B 276:3275–3283
Munday PL, Jones GP, Pratchett MS, Williams AJ (2008) Climate change and the future for coral reef fishes. Fish Fish 9:261–285
Neuheimer A, Thresher R, Lyle J, Semmens J (2011) Tolerance limit for fish growth exceeded by warming waters. Nat Clim Change 1:110–113
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. PNAS 104:1266–1271
Oliver, ECJ, Donat, MG, Burrows, MT, Moore, PJ, Smale, DA, Alexander, LV, . . . Wernberg, T (2018) Longer and more frequent marine heatwaves over the past century. Nat Comm 9:1-12
Owen R (1989) Microscale and finescale variations of small plankton in coastal and pelagic environments. J Mar Res 47:197–240
Palmer PJ, Burke MJ, Palmer CJ, Burke JB (2007) Developments in controlled green-water larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture 272:1–21
Pepin P (1991) Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. C J Fish Aquat Sci 48:503–518
Petitgas, P, Rijnsdorp, AD, Dickey‐Collas, M, Engelhard, GH, Peck, MA, Pinnegar, JK, . . . Nash, RD (2013) Impacts of climate change on the complex life cycles of fish. Fish Oceanogr 22:121-139
Pope, EC, Ellis, R, Scolamacchia, M, Scolding, J, Keay, A, Chingombe, P, . . . Wilson, R (2014) European sea bass, Dicentrarchus labrax, in a changing ocean. Biogeosciences 11:2519-2530
Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J Fish Biol 77:1745–1779
Pörtner, H-O, Roberts, DC, Masson-Delmotte, V, Zhai, P, Tignor, M, Poloczanska, E, & Weyer, N (2019) The ocean and cryosphere in a changing climate. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate
Post JR, Parkinson EA (2001) Energy allocation strategy in young fish: Allometry and survival. Ecology 82:1040–1051
Pratchett, MS, Cameron, DS, Donelson, J, Evans, L, Frisch, AJ, Hobday, AJ, . . . Munday, PL (2017) Effects of climate change on coral grouper (plectropomus spp.) and possible adaptation options. Rev Fish Biol Fisher 27:297-316
Quéré G, Leis JM (2010) Settlement behaviour of larvae of the stripey snapper, Lutjanus carponotatus (teleostei: Lutjanidae). Environ Fish Biol 88:227–238
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rankin TL, Sponaugle S (2011) Temperature influences selective mortality during the early life stages of a coral reef fish. PLoS ONE 6:e16814
Rijnsdorp AD, Peck MA, Engelhard GH, Möllmann C, Pinnegar JK (2009) Resolving the effect of climate change on fish populations. ICES J Mar Sci 66:1570–1583
Rombough, PJ (1997) The effects of temperature on embryonic and larval development. In: Global Warming: Implications for Freshwater and Marine Fish (eds C.M. Wood and D.G. McDonald). Cambridge University Press, Cambridge, pp177– 223
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Imagej 2: Imagej for the next generation of scientific image data. BMC Bioinformatics 18:1–26
Russell, M (2001) Spawning aggregations of reef fishes on the Great Barrier Reef: Implications for management. Great Barrier Reef Marine Park Authority
Schmitt RJ, Holbrook SJ (1999) Settlement and recruitment of three damselfish species: Larval delivery and competition for shelter space. Oecologia 118:76–86
Schulte PM (2015) The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218:1856–1866
Shulman MJ (1985) Recruitment of coral reef fishes: Effects of distribution of predators and shelter. Ecology 66:056–1066
Spinks, RK, Munday, PL, & Donelson, JM (2019) Developmental effects of heatwave conditions on the early life stages of a coral reef fish. J Exp Biol 222:jeb202713
Sponaugle Su, Kirsten G-C, Deanna P (2006) Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida keys. Mar Ecol Prog Ser 308:1–15
Stallings CD, Coleman FC, Koenig CC, Markiewicz DA (2010) Energy allocation in juveniles of a warm-temperate reef fish. Environ Biol Fish 88:389–398
Steinarsson A, Björnsson B (1999) The effects of temperature and size on growth and mortality of cod larvae. J Fish Biol 55:100–109
Tocher DR (2010) Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac Res 41:717–732
Watson, S-A, Allan, BJM, McQueen, DE, Nicol, S, Parsons, DM, Pether, SMJ, . . . Munday, PL (2018) Ocean warming has a greater effect than acidification on the early life history development and swimming performance of a large circumglobal pelagic fish. Glob Change Biol 24:4368-4385
Zarco-Perello S, Pratchett M, Liao V (2012) Temperature-growth performance curves for a coral reef fish, Acanthochromis polyacanthus. Galaxea 14:97–103
Acknowledgements
This project was supported by the ARC Centre of Excellence for Coral Reef Studies (PLM) and GBRMPA Reef Guardians (SJM). We thank Ben Lawes, Simon Wever, and Andrew Thompson (MARFU, JCU) for their invaluable technical support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This project followed animal ethics guidelines at James Cook University (JCU Animal Ethics No. A2522).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McMahon, S.J., Munday, P.L. & Donelson, J.M. Energy use, growth and survival of coral reef snapper larvae reared at elevated temperatures. Coral Reefs 42, 31–42 (2023). https://doi.org/10.1007/s00338-022-02306-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02306-y