Abstract
During the first year of life, organisms are faced with competing demands for energy between growth and storage. Most research on energy allocation in young fishes has focused on cold-temperate species which are subjected to strong seasonal fluctuations in productivity, while few studies have considered those at lower latitudes where seasonality is less pronounced. Gag (Mycteroperca microlepis) of the northeastern Gulf of Mexico settle in coastal seagrass beds in the spring as juveniles and emigrate to offshore reefs in the fall. Upon settlement, these young fish grow at remarkably fast rates, but their growth slows considerably before emigration. Slowed growth can be explained by one of three hypotheses: (1) size-specific emigration times; (2) reduced feeding efficiency associated with declines in primary and secondary productivity; or (3) energetic shifts in allocation from growth to storage. Gag emigrate essentially as a cohort, so slowed growth does not result from differential emigration patterns based on fish size. They also emigrate before seasonal declines in primary and secondary productivity; thus, food remains abundant and feeding efficiency constant. The more plausible hypothesis is that there is an energetic shift from growth to storage. The liver serves as the primary site of lipid storage and the hepatosomatic index of juvenile gag increases coincident with reduced growth. The overall effect of increased energy stores is presumably for use during offshore migration and/or for overwinter survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms at all life stages have limited energy budgets (Glazier 1999; Kozlowski and Teriokhin 1999) and thus allocate resources among competing demands for maintenance, growth, reproduction, and storage (Perrin 1992; Reznick and Yang 1993; McManus and Travis 1998). When energetic demands shift, there is a trade-off: energy diverted to reproduction, for instance, is no longer available for growth. Reproduction is not the only developmental trading point for young fish. Indeed, young-of-year (YOY) fishes must balance demands for energy between growth and storage to overcome two major sources of mortality during their first year: predation and starvation. Vulnerability to gape-limited and size-dependent predators decreases with size, so selection for fast somatic growth is common among young fishes (Sogard 1997; Biro et al. 2005). However, many species must also endure periods of low food resource availability at some point during their first year (e.g., due to seasonal declines in productivity, movement into food depauperate habitats) by relying on stored energy to avoid starvation.
One strategy used by YOY fishes to meet these competing demands is to allocate energy to growth initially and then divert that allocation, at least in part, to storage in anticipation of seasonal resource shortages (Post and Parkinson 2001). This strategy allows fish to attain a size above that at which they are most vulnerable to predators while building reserves for future periods when food is lacking. Achieving a large size before food becomes scarce is also advantageous because per mass metabolic demands decrease with increasing size (Shuter and Post 1990; Schultz and Conover 1999). Competing demands of growth and storage are therefore strongest for the smallest, youngest fish (Post and Parkinson 2001). Most of the evidence for fast growth followed by increased storage comes from species at high (cold-temperate) latitudes where productivity varies greatly on seasonal scales. Far less is known about energy allocation of juvenile fishes at lower (warm-temperate) latitudes, where growing seasons are longer, periods of low winter resources are shorter, and temporal patterns of productivity are less variable.
Gag (Mycteroperca microlepis; Serranidae) are warm-temperate groupers inhabiting coastal waters in the Gulf of Mexico and western Atlantic Ocean. These long-lived (up to 30 years) fish undergo several ontogenetic habitat shifts (Ross and Moser 1995) (Fig. 1). Metamorphosing larvae settle in shallow, polyhaline seagrass beds in late spring where they remain for several months until moving onto shallow, offshore reefs in the fall (Keener et al. 1988; Ross and Moser 1995; Koenig and Coleman 1998). Growth of juvenile gag is rapid during most of their seagrass phase (≥1 mm day−1; Ross and Moser 1995; Mullaney and Gale 1996), possibly due to the combined effects of abundant prey available to them in seagrass (Hemminga and Duarte 2000; Gillanders 2006), their high ecological efficiency (Adams 1976a), and their voracious feeding behavior (Adams 1976b; Nelson 1979). However, growth rates of juvenile gag appear to slow markedly just prior to emigration (Ross and Moser 1995; our observations). The reasons for the apparent decline in growth rate have never been explicitly examined, but have been attributed to decreased water temperature, early emigration of fast growing individuals, and reduced feeding efficiency (Ross and Moser 1995).
Life history of Gag showing a settlement to seagrass followed by migration to b shallow reefs (modified from Heppell et al. 2006)
The first hypothesis that decreasing water temperature may lead to declines in growth rates (Ross and Moser 1995) is based on the well-studied effects of ambient temperature on metabolic rates of poikilotherms (Houlihan et al. 1993). However, Koenig and Coleman (1998) observed late season declines in growth of juvenile gag from three Florida seagrass beds despite no significant changes in water temperature (range: 28–31°C). We therefore dismiss the temperature hypothesis as an explanation for decreased growth, at least for gag inhabiting coastal regions in the northeastern Gulf of Mexico.
The second hypothesis suggests that reported slowed growth may represent a sampling artifact caused by early emigration of fast growing individuals. Studies documenting declines in growth of late seagrass-stage juvenile gag used cohort-level growth rates to measure the change in mean size of gag between temporally separated sampling events. Within-cohort settlement to seagrass occurs over a relatively short period of time (Fitzhugh et al. 2005), hence, young-of-year in a local seagrass population are nearly equivalent in age and size. It is also accepted that juvenile gag undergo mass emigration from seagrass meadows to offshore reefs, thus supporting use of cohort-level measurements. However, there is evidence that some individuals move out of the seagrass before the mass emigration (Hastings 1979; Ross and Moser 1995; Koenig and Coleman 1998). If early emigration occurs and is composed of large (i.e., fast growing) individuals, the use of cohort-level growth rates might be obscured by comparing the mean size of the cohort before loss of large individuals through early emigration (equaling the “inflated” mean size) with the mean size of the remaining cohort after early emigration. This would suggest an apparent rather than actual decline in growth.
The third hypothesis that feeding efficiency of juvenile gag may decrease late in their seagrass stage is based on the observation that productivity, and therefore potential prey availability, declines in the late summer and autumn months. No prior study has directly examined feeding efficiency of juvenile gag relative to changes in growth but our own observations in seagrass meadows in the northeaster Gulf of Mexico suggest that prior to emigration, productivity and potential prey availability are not limiting.
This leads us to the objectives of this study, which were to more carefully examine the second and third hypotheses, and posit a fourth which has not been previously suggested: that energy allocation just prior to egress shifts from growth to storage. Specifically, we ask the following questions: (1) Do growth rates of late seagrass-stage juvenile gag decline relative to their earlier stages? and (2) if declines in growth rates are detected, are they due to changes in feeding efficiency, energy allocation, or both?
Methods
Field sampling
We conducted this study on the West Florida Shelf within three seagrass beds of St. George Sound near the Florida State University Coastal and Marine Laboratory (FSUCML; Fig. 2). All three sites were similar in that they were shallow, polyhaline, and continuous seagrass beds composed of turtle grass (Thalassia testudinum), manatee grass (Syringodium filiforme), and shoalgrass (Halodule wrightii) (Koenig and Coleman 1998).
Juvenile gag were collected using a 5 m otter trawl towed for 150 m at a standard rate of 1.8 km h−2. Collections occurred at 1 week to 2 week intervals from May through October 1991, encompassing roughly the entire seagrass stage for juvenile gag. Stomach analyses indicated juvenile gag in seagrass beds feed at consistently high rates during daylight hours compared to nocturnal ones, so sampling was restricted to diurnal periods. Upon capture, gag were measured for standard length, placed on ice, and immediately brought back to the FSUCML.
Growth
We determined growth rates of juvenile gag from standard length-at-age data. Ages were determined using lapilli otoliths from 149 individuals ranging in size from 29 mm to 215 mm, with collections of smaller individuals occurring early in the season and larger ones occurring late in the season (i.e., variation in individual sizes at time t did not reflect the full range of sizes observed across collection events). Otoliths were removed from fish, polished, and increment counts made using a compound microscope. We added six increments to the total count for each otolith because lapilli in gag underestimate ages derived from sagittae by 4 days and increment deposition begins 2 days to 3 days after fertilization (following Brothers and McFarland 1981; Keener et al. 1988). We assumed that increments formed daily.
We used three different functions to model cohort-level growth: (1) ordinary least squares regression (SL∼age; R lm package), assuming linear growth over the juvenile stage, (2) the nonlinear Gompertz function [SL∼lo * exp(g * (1−exp(-k * age))) (Ricker 1975); R nls package], which models change as a gradual transition from fast to asymptotic growth and is commonly used for juvenile stages of fishes, and (3) piecewise regression [(SL∼(age<BREAK) * age+(age≥BREAK) * age); R lm package], which models changes in growth as two linear functions separated by a breaking point. The break is chosen objectively as the value that produces the minimum deviance (Crawley 2007). Although less conventional than other functions (e.g., Gompertz, von Bertalanffy), the bi-phasic piecewise regression models provide more parsimonious descriptions of various metabolic, energetic, and ecological processes—including growth in fish. This is supported by a growing body of theoretical (Quince et al. 2008a) and empirical (Post and Lee 1996; Post and Parkinson 2001; Quince et al. 2008b; Samhouri et al. 2009) literature. Furthermore, piecewise regression provides a convenient method for calculating the change in growth rate (indicated by the change in slope coefficients) and the age at which growth slows (indicated by the value of the independent variable at slope flexure), thereby allowing us to determine whether the change in growth coincided with an increase in energy allocation to storage (see below: “Energy allocation”). The two linear regression models (single slope versus double slope) were compared using a nested F-test and all three models were compared using Akaike’s Information Criterion (AIC).
Feeding efficiency
To determine whether feeding rates of juvenile gag declined with size, we examined the stomach contents of 456 individuals across seven 25-mm size classes (25–200 mm SL). We examined emptiness and fullness as two metrics of feeding efficiency. For the emptiness metric, stomachs were scored with a binary response (i.e., empty versus not empty). We used logistic regression to test whether the proportions of empty stomachs increased with larger size classes of juvenile gag. For the fullness metric, stomachs were visually scored on a scale of 0 (empty) to 3 (100% full). ANOVA was used to determine whether stomach fullness decreased with increasing size classes (fixed factor) of gag. In addition, all prey items found in the stomachs were identified to lowest taxonomic level and counted.
Energy allocation
The declines in growth rates previously documented for juvenile gag occurred in the late summer months. We therefore collected juvenile gag across a broad size range (80–199 mm SL) from mid-summer through fall, encompassing the period before, during, and after reported periods of slowed growth to examine the relationship between growth and storage. The liver functions as a primary site of energy stores in fishes (Shul’man 1974; Clements and Raubenheimer 2006), which we confirmed in juvenile gag by measuring lipid concentrations (following methods of Bligh and Dyer 1959) in five tissues: (1) anterior soma (from the base of the first dorsal fin forward), (2) middle soma (from the base of the spiny dorsal posterior to the end of the soft dorsal), (3) posterior soma (from the end of the soft dorsal to the end of the caudal fin), (4) viscera, and (5) liver. Lipid concentrations in the liver were over two to three times that in the other tissues. We therefore measured the hepatosomatic index (liver mass/body mass * 1000) of juvenile gag to estimate the relative contribution of energy to storage (Fahraeus-Van Ree and Spurrell 2003; Berkeley et al. 2004; Sopinka et al. 2009). Using the index required the assumption that an increase in liver size when corrected for body size reflects an increase in energy stores for juvenile gag. Upon capture, the stomachs and livers were removed and the wet mass (g) of each gag was measured. The livers were freeze dried and weighed. Similar to our tests with growth, we wanted to examine whether the hepatosomatic index values increased for larger juvenile gag compared to smaller (younger) individuals. We therefore compared a two-slope versus a single-slope regression (Crawley 2007).
Results
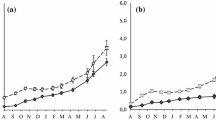
Growth of juvenile gag displayed a biphasic pattern (Fig. 3a) with a break occurring at an age of 130 days for the cohort. The rate of growth was faster for fish less than 130 days old (b 1 = 1.402, P < 0.001) and decreased by 40% for the older fish (b 2 = 0.566, P < 0.001). The piecewise regression (residual standard error = 15.2; r 2 = 0.81; AIC = 1239.79) of the length-at-age data provided significant improvement (F 2 = 14.96; P < 0.001) over the single-slope linear regression (residual standard error = 16.6; r 2 = 0.77; AIC = 1263.75). The sizes of juveniles just prior to the break between slopes (i.e., at age 130 days) were approximately 140 mm SL (mean SL126–129 days old = 139.7 mm; min SL = 127 mm; max SL = 150 mm). The Gompertz model reflected a gradual deceleration in growth with the transition occurring at an earlier age (74 days) than the piecewise regression (Fig. 3b). However, piecewise regression provided considerable improvement over the Gompertz growth function (AIC = 1244.06).
Of 456 stomachs examined, 127 (28%) were empty. Importantly, neither degree of stomach fullness (ANOVA F 1,476 = 1.82, P = 0.18) nor whether a stomach was empty (logistic regression χ2 = 0.93, P = 0.34) was related to the size of juvenile gag, indicating that feeding rates did not decline in older juveniles (Table 1). Gag fed primarily on shrimp and fish during their seagrass-associated stage (Fig. 4). Small arrow shrimp (Tozeuma carolinensis; average individual biomass = 0.037 g) dominated numerically, especially for smaller gag (∼89% of the diet of juveniles 25–149 mm SL; 58% for juveniles 150–200 mm SL). Pink shrimp (Farfantepenaeus duorarum; avg. individual biomass = 0.48 g) followed, comprising 7% of the diet of fish 25–149 mm SL, and 26% of those 150–200 mm SL. Fish—primarily pinfish (Lagodon rhomboides; avg. individual biomass = 4.76 g) and pigfish (Orthopristis chrysoptera; avg. individual biomass = 3.25 g)—gained importance in juveniles > 150 mm SL. Several shrimp (up to 11 per stomach) were typically found in any one gag stomach whereas dominant fishes were found singly.
The hepatosomatic index (i.e., energy storage) of juvenile gag increased at approximately the same size (i.e., in fish∼140 mm SL) and time (i.e., 4 week to 5 weeks prior to egress) that somatic growth rate decreased. Once again, the piecewise regression (standard residual error = 0.65; r 2 = 0.49; AIC = 87.25) better described the observed pattern (F 2 = 3.62; P = 0.037) than the linear regression with a single slope (standard residual error = 0.70; r 2 = 0.39; AIC = 90.58). Energy storage in juveniles less than 134 mm did not increase relative to body mass. In fact, the hepatosomatic index reflected a marginal decline for the smaller juveniles (b 1 = −0.025, P = 0.097) because liver mass remained stable while body mass increased. However, the hepatosomatic index increased sharply for juveniles larger than the break (b 2 = 0.016, P = 0.045; Fig. 5).
Discussion
We have shown that growth in juvenile gag is fast throughout much of their seagrass phase, but slows sharply prior to egress. These results agree with patterns previously suggested for gag, and verify that the decreased growth was not a sampling artifact affected by early emigration. Slowed growth occurred despite no detectable changes to their environment (i.e., lower water temperature), prey availability, or feeding success. In concert with slowed growth, their hepatosomatic index increased, providing strong support that energy allocation in juvenile gag shifted from growth to storage prior to egress out of seagrass habitats.
Size selective mortality of juvenile fishes often bestows an advantage to larger, faster-growing individuals, in part because smaller fishes can be more susceptible to predation (reviews by Hixon 1991; Sogard 1997). Consistent with observations from North Carolina (Ross and Moser 1995) and South Carolina (Mullaney and Gale (1996) estuaries, post settlement growth of juvenile gag in the northeastern Gulf of Mexico is remarkably fast (i.e., >1.4 mm d−1). It is certainly faster than that of other groupers that achieve similar terminal sizes (e.g., Nassau Grouper, Epinephelus striatus, 0.40 mm d−1 (Beets and Hixon 1994); Yellowedge Grouper, Epinephelus flavolimbatus, 0.43 mm d−1 (Manickchand-Heileman and Phillip 2000); Yellowmouth Grouper, Mycteroperca interstitialis, 0.64 mm d−1 (Manickchand-Heileman and Phillip 2000); Scamp, Mycteroperca phenax, 0.73 mm d−1 (Matheson et al. 1986)).Footnote 1 Fast growth may explain gag’s high survival rate during the seagrass phase (Koenig and Coleman 1998) despite high abundances of potential predators observed in the same area as the current study (e.g., paralichthyids (CD Stallings, unpublished data), carcharhinids (RD Grubbs, unpublished data)). The relatively low percentage of empty stomachs we observed indicates juvenile gag may feed at a constant rate, thus facilitating the observed growth. In addition to outpacing size-dependent predation, achieving a large size presents juvenile fishes other benefits. For example, it may provide new dietary and feeding opportunities (Persson et al. 1996). Indeed, the diet of gag shifted from numerous small prey items (e.g., hippolytid shrimps) to fewer, large prey (e.g., penaeid shrimps, fishes). This transition in diet coincides with a general increase in caloric densities of their prey (Wissing et al. 1973). The combined effects of a shift to piscivory and concomitant change in feeding strategy from actively searching to the more energetically efficient sit-and-wait strategy commonly results in a pronounced increase in size (Olson 1996; Mittelbach and Persson 1998; Post 2003). However, with juvenile gag, the shift coincided with decreased somatic growth as demonstrated by the Gompertz and piecewise regression models. Although both models provided compelling evidence for decelerating growth, our goal was not necessarily to provide coefficient estimates so much as it was to examine relative energy allocation between soma and storage. As such, piecewise regression provided a more straightforward method to examine an ecological process; it indicated that when growth slowed, both liver size and hepatosomatic index increased. Assuming the liver serves as the primary location of energy storage for juvenile gag—as indicated by our lipid measurements across several tissues—these patterns suggest energy allocation was diverted from growth to storage.
Organisms store fat to offset energetically expensive physiological requirements, such as reproduction, overwinter survival, and migration (Gosler et al. 1995). Storage allocation differs from growth, reproduction, and maintenance allocation in having no immediate metabolic demand (i.e., energy is stored at one point in time for metabolic requirements at another). Fat stores at this stage in the gag’s life likely have little to do with reproduction. These young, 100–250 mm fish will not mature until they reach 3 years to 4 years of age and are 500–600 mm in length, the implication being that considerable somatic growth will occur after egress and before reaching sexual maturity. We suggest that fat storage at this early stage in the life history is a bet-hedging strategy used in anticipation of food shortages associated with migration and/or winter, serving as insurance for survival in unpredictable environments.
Although we know little about the spatial or temporal trajectories of gag’s fall egress, one can reasonably assume that the process of finding suitable reef habitat located several to 10’s of km offshore of the seagrass beds is an energetically demanding task that may take considerable time to complete. During their offshore migration in the northeastern Gulf of Mexico, juvenile gag move from highly productive seagrass habitats to cross over relatively unproductive sand and mud habitats that provide few prey (reviews by Hemminga and Duarte 2000; Gillanders 2006) before arriving at destination reefs. Once on the reefs, they may face additional challenges (e.g., intense predation threat) that could hinder their ability to obtain food, thereby sustaining their dependency on reserves. Lindberg et al. (2006), for instance, experimentally demonstrated gag select reefs based on shelter rather than food, reinforcing the importance of predator avoidance during their first year. High predator (e.g., sharks, large groupers including adult gag) abundances are common on the reefs (C.D. Stallings, unpublished data) and may force the smaller gag to remain vigilant upon arrival, feeding at reduced rates (e.g., Stallings 2008). Their initial foraging success may be further compromised by the increased energetic demands of surviving in novel habitats, handling novel prey, and competing with the cohort of conspecifics that arrives on reefs en masse. The individual and synergistic effects of these potential restraints on feeding would require juvenile gag to rely on stored energy for survival.
Fishes in both marine and freshwater systems shift energy allocation from fast growth to storage prior to their first winter (Larson 1991; Schultz and Conover 1997; Hurst and Conover 2003; Sogard and Spencer 2004; Biro et al. 2005). This strategy allows fish to survive extended periods of food scarcity associated with winter by reaching a size at which their metabolic rates are relatively low and their physiological tolerance to cold temperatures is high (Huss et al. 2008), while also providing for sufficient energy stores. To our knowledge, evidence for this strategy has been limited to fishes overwintering at cold-temperate, high latitudes where summer growing seasons are short, winters are long and sometimes harsh, and seasonal cycles of productivity have high amplitudes. Energy allocation in young fishes at lower latitudes, where productivity is less variable, has been far less studied. In a 21-month study spanning two consecutive winters, lipid levels neither accumulated nor were depleted in immature Dusky Shiner (Notropis cummingsae) living in warm-temperate rivers (South Carolina, United States), suggesting that resources remained sufficient for growth and maintenance and that lipids were used primarily for reproduction (Schultz 1999). Although seasonal flux in productivity may be less pronounced at lower latitudes, it is not absent. Indeed, the primary prey of juvenile gag on reefs (e.g., clupeids, haemulids, carangids) become less abundant to absent during the coldest winter months (Lindberg et al. 2006). However, other factors beyond productivity dynamics could also influence allocation of energy to storage. Variations in liver lipid concentrations of neotropical fishes were correlated with changing prey concentrations caused by fluctuations in water levels of floodplain rivers (Junk 1985; Arrington et al. 2006). On coral reefs, allocation of energy to storage for a pomacentrid increased with depth (Hoey et al. 2007). Although the authors were not able to directly attribute mechanism, they suggested the pattern may have been due to depth related variation in food and activity level of the fish.
All fishes likely endure periods of food scarcity, whether related to changes in productivity associated with winter or due to other temporally-linked processes, and many species undergo ontogenetic niche shifts (Werner and Gilliam 1984). Therefore we hope this study will encourage increased research on energy allocation dynamics in warm-temperate and tropical fishes. For example, our study has produced several testable hypotheses of how gag utilize energy during their first year. Regular sampling extending from the seagrass phase through the first winter could provide information on the timing and magnitude of energy use. How energy stores are affected by climatic variation, especially relative to periods associated with reductions in food supply (e.g., winter), can be examined spatially by incorporating a range of latitudes where gag are naturally distributed (approximately 20°–35° N; e.g., Schultz and Conover 1997) and temporally across several annual cycles (e.g., Hurst and Conover 2003). Although logistically challenging, investigating the storage and utilization of energy during offshore migrations could be accomplished by intercepting gag during egress and immediately upon arrival on reefs. These efforts can of course be extended to other fishes and will broaden our understanding of fitness-related selection on species occupying lower latitudes.
Notes
Growth estimate for Epinephelus striatus (Beets and Hixon 1994) was from direct observations of YOY individuals, while the other estimates were derived from von Bertalanffy models solved for 1 year-old fish. The latter method was used due to a general lack of available data and should be interpreted with caution since models calculated from adults may provide poor estimates of juvenile growth.
References
Adams SM (1976a) The ecology of eelgrass, Zostera marina (L.), fish communities. II. Functional analysis. J Exp Mar Biol Ecol 22:293–311
Adams SM (1976b) Feeding ecology of eelgrass fish communities. Trans Am Fish Soc 105:514–519
Arrington DA, Davidson BK, Winemiller KO, Layman CA (2006) Influence of life history and seasonal hydrology on lipid storage in three neotropical fish species. J Fish Biol 68:1347–1361
Beets J, Hixon MA (1994) Distribution, persistence, and growth of groupers (Pisces: Serranidae) on artificial and natural patch reefs in the Virgin Islands. Bull Mar Sci 55:470–483
Berkeley SA, Chapman C, Sogard SM (2004) Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85:1258–1264
Biro PA, Post JR, Abrahams MV (2005) Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proc R Soc B Biol Sci 272:1443–1448
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brothers EB, McFarland WN (1981) Correlations between otolith microstructure, growth and life history transitions in newly recruited French grunts (Haemulon flavolineatum). Rapp P-V Reun Cons Int Explor Mer 178:369–374
Clements KD, Raubenheimer D (2006) Feeding and nutrition. In: Evans DH, Claiborne JB (eds) The physiology of fishes. CRC, Boca Raton, pp 47–82
Crawley MJ (2007) The R book. Wiley, West Sussex
Fahraeus-Van Ree GE, Spurrell DR (2003) Structure of and energy reserves in the liver of wild and cultured yellowtail flounder, Limanda ferruginea. Mar Biol 143:257–265
Fitzhugh GR, Koenig CC, Coleman FC, Grimes CB, Sturges W (2005) Spatial and temporal patterns in fertilization and settlement of young gag (Mycteroperca microlepis) along the west Florida shelf. Bull Mar Sci 77:377–396
Gillanders BM (2006) Seagrasses, fish, and fisheries. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Spronger, Dordrecht, pp 503–536
Glazier DS (1999) Trade-offs between reproductive and somatic (storage) investments in animals: a comparative test of the Van Noordwijk and De Jong model. Evol Ecol 13:539–555
Gosler AG, Greenwood JJD, Perrins C (1995) Predation risk and the cost of being fat. Nature 377:621–623
Hastings RW (1979) The origin and seasonality of the fish fauna on a new jetty in the northeastern Gulf of Mexico. Bull Fla State Mus Biol Sci 24:1–122
Hemminga MA, Duarte CM (2000) Fauna associated with seagrass systems. In: Seagrass ecology. Cambridge University Press, Cambridge, pp 199–247
Heppell SS, Heppell SA, Coleman FC, Koenig CC (2006) Models to compare management options for a protogynous fish. Ecol Appl 16:238–249
Hixon MA (1991) Predation as a process structuring coral reef fish communties. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 475–508
Hoey J, McCormick MI, Hoey AS (2007) Influence of depth on sex-specific energy allocation patterns in a tropical reef fish. Coral Reefs 26:603–613
Houlihan DF, Mathers EM, Foster A (1993) Biochemical correlates of growth rate in fish. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman and Hall, London, pp 45–71
Hurst TP, Conover DO (2003) Seasonal and interannual variation in the allometry of energy allocation.in juvenile striped bass. Ecology 84:3360–3369
Huss M, Bystrom P, Strand A, Eriksson LO, Persson L (2008) Influence of growth history on the accumulation of energy reserves and winter mortality in young fish. Can J Fish Aquat Sci 65:2149–2156
Junk WJ (1985) Temporary fat storage, an adaptation of some fish species to the waterlevel fluctuations and related environmental changes of the Amazon River. Amazoniana 9:315–351
Keener P, Johnson GD, Stender BW, Brothers EB, Beatty HR (1988) Ingress of postlarval gag, Mycteroperca microlepis (Pisces, Serranidae), through a South Carolina barrier island inlet. Bull Mar Sci 42:376–396
Koenig CC, Coleman FC (1998) Absolute abundance and survival of juvenile gags in sea grass beds of the Northeastern Gulf of Mexico. Trans Am Fish Soc 127:44–55
Kozlowski J, Teriokhin AT (1999) Allocation of energy between growth and reproduction: the Pontryagin Maximum Principle solution for the case of age- and season-dependent mortality. Evol Ecol Res 1:423–441
Larson RJ (1991) Seasonal cycles of reserves in relation to reproduction in Sebastes. Environ Biol Fish 30:57–70
Lindberg WJ, Frazer TK, Portier KM, Vose F, Loftin J, Murie DJ, Mason DM, Nagy B, Hart MK (2006) Density-dependent habitat selection and performance by a large mobile reef fish. Ecol Appl 16:731–746
Manickchand-Heileman SC, Phillip DAT (2000) Age and growth of the yellowedge grouper, Epinephelus flavolimbatus, and the yellowmouth grouper, Mycteroperca interstitialis, off Trinidad and Tobago. Fish Bull 98:290–298
Matheson RH, Huntsman GR, Manooch CS (1986) Age, growth, mortality, food and reproduction of the scamp, Mycteroperca phenax, collected off North Carolina and South Carolina. Bull Mar Sci 38:300–312
McManus MG, Travis J (1998) Effects of temperature and salinity on the life history of the sailfin molly (Pisces: Poeciliidae): lipid storage and reproductive allocation. Oecologia 114:317–325
Mittelbach GG, Persson L (1998) The ontogeny of piscivory and its ecological consequences. Can J Fish Aquat Sci 55:1454–1465
Mullaney MD, Gale LD (1996) Ecomorphological relationships in ontogeny: anatomy and diet in gag, Mycteroperca microlepis (Pisces: Serranidae). Copeia 167–180
Nelson WG (1979) Experimental studies of selective predation on amphipods: consequences for amphipod distribution and abundance. J Exp Mar Biol Ecol 38:225–245
Olson MH (1996) Ontogenetic niche shifts in largemouth bass: variability and consequences for first-year growth. Ecology 77:179–190
Perrin N (1992) Optimal resource allocation and the marginal value of organs. Am Nat 139:1344–1369
Persson L, Andersson J, Wahlstrom E, Eklov P (1996) Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology 77:900–911
Post DM (2003) Individual variation in the timing of ontogenetic niche shifts in largemouth bass. Ecology 84:1298–1310
Post JR, Lee JA (1996) Metabolic ontogeny of teleost fishes. Can J Fish Aquat Sci 53:910–923
Post JR, Parkinson EA (2001) Energy allocation strategy in young fish: allometry and survival. Ecology 82:1040–1051
Quince C, Abrams PA, Shuter BJ, Lester NP (2008a) Biphasic growth in fish I: theoretical foundations. J Theor Biol 254:197–206
Quince C, Shuter BJ, Abrams PA, Lester NP (2008b) Biphasic growth in fish II: empirical assessment. J Theor Biol 254:207–214
Reznick D, Yang AP (1993) The influence of fluctuating resources on life history—patterns of allocation and plasticity in female guppies. Ecology 74:2011–2019
Ricker WE (1975) Computation and interpretation of biological statistics of fish population. Fish Res Board Can Bull 191:1–382
Ross SW, Moser ML (1995) Life history of juvenile gag, Mycteroperca microlepis, in North Carolina estuaries. Bull Mar Sci 56:222–237
Samhouri JF, Vance RR, Forrester GE, Steele MA (2009) Musical chairs mortality functions: density-dependent deaths caused by competition for unguarded refuges. Oecologia 160:257–265
Schultz DL (1999) Population structure, reproduction, and lipid cycling in the dusky shiner (Notropis cummingsae) in contrasting streams. Copeia 669–683
Schultz ET, Conover DO (1997) Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia 109:516–529
Schultz ET, Conover DO (1999) The allometry of energy reserve depletion: test of a mechanism for size-dependent winter mortality. Oecologia 119:474–483
Shul’man GE (1974) Life cycles of fish: physiology and biochemistry. Wiley, New York
Shuter BJ, Post JR (1990) Climate, population viability, and the zoogeography of temperate fishes. Trans Am Fish Soc 119:314–336
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Sogard SM, Spencer ML (2004) Energy allocation in juvenile sablefish: effects of temperature, ration and body size. J Fish Biol 64:726–738
Sopinka NM, Fitzpatrick JL, Desjardins JK, Stiver KA, Marsh-Rollo SE, Balshine S (2009) Liver size reveals social status in the African cichlid Neolamprologus pulcher. J Fish Biol 75:1–16
Stallings CD (2008) Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 89:2090–2095
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size structured populations. Annu Rev Ecol Syst 15:393–425
Wissing TE, Darnell RM, Ibrahim MA, Berner L (1973) Caloric values of marine animals from the Gulf of Mexico. Contrib Mar Sci 17:1–7
Acknowledgments
M. Chasar, C. Grimes, S. Handy, A. Kiefert, J. Kimmel, S. Owens, J. Romine, L. Spataro, S. Stewart, and T. Sumner provided technical support. Thanks also to R. Grubbs, D. Johnson, and J. Samhouri for discussion concerning various aspects of the manuscript. This research was funded in part by the National Marine Fisheries Service MARFIN (NA90AA-H-MF748), Florida Sea Grant (FSG-RILR-B32), and the Florida Department of Environmental Protection (DNR C7223). Markiewicz was supported in part by the Norman Meinkoth Field Biology Award from Swarthmore College. Stallings was supported in part by the U.S. National Oceanic and Atmospheric Association (Northern Gulf of Mexico Cooperative Institute 191001-363558-01).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stallings, C.D., Coleman, F.C., Koenig, C.C. et al. Energy allocation in juveniles of a warm-temperate reef fish. Environ Biol Fish 88, 389–398 (2010). https://doi.org/10.1007/s10641-010-9655-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9655-4