Abstract
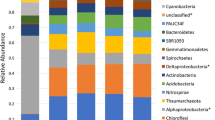
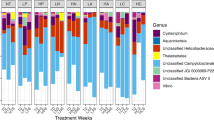
Diseases of marine organisms, including sponges on coral reefs, are being reported with increasing frequency worldwide. Aplysina Red Band Syndrome (ARBS) occurs across the Caribbean basin, predominantly affecting Aplysina cauliformis, one of the most common reef sponges in this region. Existing ARBS lesions and their effects on the sponge holobiont have been documented, yet little is known about the biochemical and microbial changes associated with the onset of infection. Due to the transmissible nature of ARBS, infection can be induced and monitored through sponge-to-sponge direct contact. Nine-day contact experiments with healthy-diseased and healthy-healthy sponge pairings were conducted in the Bahamas in January and July to compare individual sponges sampled initially and at one of three successive time points. Temporal changes in bacterial assemblages and photosymbiont abundance (via concentrations of chlorophyll a), and concentrations of total protein, heat shock protein 70, and major secondary metabolites that may correspond with disease onset were characterized. All healthy sponges in contact with diseased sponges developed ARBS by day 9 in January and by day 6 in July, suggesting that observed changes in the holobiont corresponded with the development of ARBS. The concentrations of several major secondary metabolites, as well as heat shock protein 70 and chlorophyll a, changed significantly in samples of visibly healthy tissue from initially healthy sponges that became diseased. In contrast, the composition of the associated bacterial community changed in all attached sponges over time. These results suggest that infection with ARBS elicits rapid responses by the sponge holobiont, providing a model system in which to investigate immune responses in an early metazoan.



Similar content being viewed by others
References
Aguilar-Camacho JM, McCormack, GP (2017) Molecular responses of sponges to climate change. In: Carballo JL, Bell JJ (eds) Climate change, ocean acidification and sponges Springer, Cham, Switzerland, pp 79–104
Angermeier H, Kamke J, Abdelmohsen UR, Krohne G, Pawlik JR, Lindquist NL, Hentschel U (2011) The pathology of sponge orange band disease affecting the Caribbean barrel sponge Xestospongia muta. FEMS Microbiol Ecol 75:218–230
Angermeier H, Glöckner V, Pawlik JR, Lindquist NL, Hentschel U (2012) Sponge white patch disease affecting the Caribbean sponge Amphimedon compressa. Dis Aquat Org 99:95–102
Bayer K, Schmitt S, Hentschel U (2008) Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba. Environ Microbiol 10:2942–2955
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheshire AC, Wilkinson CR (1991) Modelling the photosynthetic production by sponges on Davies Reef, Great Barrier Reef. Mar Biol 109:13–18
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinformatics 10:171. https://doi.org/10.1186/1471-2105-10-171
De Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110
Deignan LK, Pawlik JR, Erwin PM (2018) Agelas wasting syndrome alters prokaryotic symbiont communities of the Caribbean brown tube sponge, Agelas tubulata. Microb Ecol 76:459–466
Diaz CM, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Easson CG, Thacker RW (2014) Phylogenetic signal in the community structure of host-specific microbiomes of tropical marine sponges. Front Microbiol 5:532
Easson CG, Slattery M, Momm HG, Olson JB, Thacker RW, Gochfeld DJ (2013) Exploring individual- to population-level impacts of disease on coral reef sponges: using spatial analysis to assess the fate, dynamics, and transmission of Aplysina red band syndrome (ARBS). PLoS ONE. 8: e79976
Easson CG, Slattery M, Baker DM, Gochfeld DJ (2014) Complex ecological associations: competition and facilitation in a sponge-algal interaction. Mar Ecol Prog Ser 507:153–167
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucl Acid Res 19:7843–7853
Erwin PM, Pita L, López-Legentil S, Turon X (2012) Stability of sponge-associated bacteria over large seasonal shifts in temperature and irradiance. Appl Environ Microbiol 78:7358–7368
Erwin PM, Thacker RW (2007) Phylogenetic analyses of marine sponges within the order Verongida: a comparison of morphological and molecular data. Invert Biol 126:220–234
Fan L, Liu M, Simister R, Webster NS, Thomas T (2013) Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J 7:991–1002
Flatt PM, Gautschi JT, Thacker RW, Musafija-Girt M, Crews P, Gerwick WH (2005) Identification of the cellular site of polychlorinated peptide biosynthesis in the marine sponge Dysidea (Lamellodysidea) herbacea and symbiotic cyanobacterium Oscillatoria spongeliae by CARD-FISH analysis. Mar Biol 147:761–774
Freeman CJ, Easson CG, Matterson KO, Thacker RW, Baker DM, Paul VJ (2020) Microbial symbionts and ecological divergence of Caribbean sponges: a new perspective on an ancient association. ISME J 14:1571–1583
Freeman CJ, Thacker RW (2011) Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceanogr 56:1577–1586
Freeman CJ, Thacker RW, Baker DM, Fogel ML (2013) Quality or quantity: is nutrient transfer driven more by symbiont identity and productivity than by symbiont abundance? ISME J 7:1116–1125
Gil-Agudelo DL, Myers C, Smith GW, Kim K (2006) Changes in the microbial communities associated with Gorgonia ventalina during aspergillosis infection. Dis Aquat Org 69:89–94
Gochfeld DJ, Easson CG, Freeman CJ, Thacker RW, Olson JB (2012a) Disease and nutrient enrichment as potential stressors on the Caribbean sponge Aplysina cauliformis and its bacterial symbionts. Mar Ecol Prog Ser 456:101–111
Gochfeld DJ, Kamel HN, Olson JB, Thacker RW (2012b) Trade-offs in defensive metabolite production but not ecological function in healthy and diseased sponges. J Chem Ecol 38:451–462
Gochfeld DJ, Diaz MC, Renegar DA, Olson JB (2019) Histological and ultrastructural features of Aplysina cauliformis affected by Aplysina red band syndrome. Invert Biol. 138: e12247
Godefroy N, Le Goff E, Martinand-Mari C, Belkhir K, Vacelet J, Baghdiguian S (2019) Sponge digestive system diversity and evolution: filter feeding to carnivory. Cell Tissue Res 377:341–351
Hentschel U, Piel J, Degnan SM, Taylor MW (2012) Genomic insights into the marine sponge microbiome. Nature Rev Microbiol 10:641–654
Hervé M (2019) RVAideMemoire: Testing and Plotting Procedures for Biostatistics R package version 09–73. https://CRANR-projectorg/package=RVAideMemoire
Hewson I, Button JB, Gudenkauf BM, Miner B, Newton AL, Gaydos JK, Wynne J, Groves CL, Hendler G, Murray M, Fradkin S, Breitbart M, Fahsbender E, Lafferty KD, Kilpatrick AM, Miner CM, Raimondi P, Lahner L, Friedman CS, Daniels S, Haulena M, Marliave J, Burge CA, Eisenlord ME, Harvell CD (2014) Densovirus associated with sea-star wasting disease and mass mortality. Proc Natl Acad Sci 111:17278–17283
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical ecosystem engineers. Ecol 78:1946–1957
Kandler NM, Wahab MAA, Noonan SHC, Bell JJ, Davy SK, Webster NS, Luter HM (2018) In situ responses of the sponge microbiome to ocean acidification. FEMS Microbiol Ecol 94:2018,fiy205 [doi: https://doi.org/10.1093/femsec/fiy205]
Kelly SR, Garo E, Jensen PR, Fenical W, Pawlik JR (2005) Effects of Caribbean sponge secondary metabolites on bacterial surface colonization. Aquatic Microb Ecol 40:191–203
Keren R, Lavy A, Mayzel B, Ilan M (2015) Culturable associated bacteria of the sponge Theonella swinhoei show tolerance to high arsenic concentrations. Front Microbiol 6:154
Keren R, Mayzel B, Lavy A, Polishchuk I, Levy D, Fakra SC, Pokroy B, Ilan M (2017) Sponge-associated bacteria mineralize arsenic and barium on intracellular vesicles. Nat Comm 8:14393
Lafferty KD, Hofmann EE (2016) Marine disease impacts, diagnosis, forecasting, management and policy. Phil Trans Roy Soc b: Biol Sci 371:1689.
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, pp 115–175
Lesser MP, Fiore C, Slattery M, Zaneveld J (2016) Climate change stressors destabilize the microbiome of the Caribbean barrel sponge, Xestospongia muta. J Exp Mar Biol Ecol 475:11–18
López-Legentil S, Song B, McMurray SE, Pawlik JR (2008) Bleaching and stress in coral reef ecosystems: hsp70 expression by the giant barrel sponge Xestospongia muta. Mol Ecol 17:1840–1849
Luter HM, Webster NS (2017) Sponge disease and climate change. In: Carballo JL, Bell JJ (eds) Climate change, ocean acidification and sponges. Springer, Cham, Switzerland, pp 411–428
Luter HM, Whalan S, Webster NS (2010) Prevalence of tissue necrosis and brown spot lesions in a common marine sponge. Mar Freshwater Res 61:484–489
Mehbub MF, Lei J, Franco C, Zhang W (2014) Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar Drugs 12:4539–4577
Meyer JL, Castellanos-Gell J, Aeby GS, Häse CC, Ushijima B, Paul VJ (2019) Microbial community shifts associated with the ongoing Stony Coral Tissue Loss Disease outbreak on the Florida reef tract. Front Microbiol 10:2244.
Mydlarz LD, Jones LE, Harvell CD (2006) Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Ann Rev Ecol Evol System 37:251–288
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018) vegan: Community Ecology Package R package version 25–2. https://CRANR-projectorg/package=vegan
Olson JB, Gochfeld DJ, Slattery M (2006) Aplysina red band syndrome: a new threat to Caribbean sponges. Dis Aquat Org 71:163–168
Olson JB, Thacker RW, Gochfeld DJ (2014) Molecular community profiling reveals impacts of time, space, and disease status on the bacterial community associated with the Caribbean sponge Aplysina cauliformis. FEMS Microbiol Ecol 87:268–279
Pantos O, Bythell JC (2006) Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16S rRNA techniques. Dis Aquat Org 69:79–88
Pantos O, Cooney RP, Le Tissier MDA, Barer MR, O’Donnell AG, Bythell JC (2003) The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis. Environ Microbiol 5:370–382
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York
Paul VJ, Freeman CJ, Agarwal V (2019) Chemical ecology of marine sponges: new opportunities through “-omics.” Int Comp Biol 59:765–776
Pawlik JR, Chanas B, Toonen RJ, Fenical W (1995) Defenses of Caribbean sponges against predatory reef fish. I. Chemical Deterrency Mar Ecol Prog Ser 127:183–194
Pawlik JR, Steindler L, Henkel TP, Beer S, Ilan M (2007) Chemical warfare on coral reefs: sponge metabolites differentially affect coral symbiosis in situ. Limnol Oceanogr 52:907–911
Pita L, Erwin PM, Turon X, López-Legentil S (2013) Till death do us part: Stable sponge-bacteria associations under thermal and food shortage stresses. PLoS ONE. https://doi.org/10.1371/journal.pone.0080307
Pita L, Hoeppner MP, Ribes M, Hentschel U (2018) Differential expression of immune receptors in two marine sponges upon exposure to microbial-associated molecular patterns. Sci Rep 8:16081.
Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R (2016) Unprecedented disease-related coral mortality in southeastern Florida. Sci Rep 6:31374.
Puyana M, Fenical W, Pawlik JR (2003) Are there activated chemical defenses in sponges of the genus Aplysina from the Caribbean? Mar Ecol Prog Ser 246:127–135
Puyana M, Pawlik J, Blum J, Fenical W (2015) Metabolite variability in Caribbean sponges of the genus Aplysina. Rev Bras Farmacogn 25:592–599
Ramsby BD, Hoogenboom MO, Whalan S, Webster NS (2018) Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol Ecol 27:2124–2137
Ribes M, Calvo E, Movilla J, Logares R, Coma R, Pelejero C (2016) Restructuring of the sponge microbiome favors tolerance to ocean acidification. Envr Micro Rep 8:536–544
Rix L, Ribes M, Coma R, Jahn MT, de Goeij JM, van Oevelen D, Escrig S, Meibom A, Hentschel U (2020) Heterotrophy in the earliest gut: a single-cell view of heterotrophic carbon and nitrogen assimilation in sponge-microbe symbioses. ISME J 14:2554–2567
Sacristán-Soriano O, Banaigs B, Casamayor EO, Becerro MA (2011) Exploring the links between natural products and bacterial assemblages in the sponge Aplysina aerophoba. Appl Environ Microbiol 77:862–870
Santos-Gandelman JF, Giambiagi-deMarval M, Muricy G, Barkay T, Laport MS (2014) Mercury and methylmercury detoxification potential by sponge-associated bacteria. Antonie Van Leeuwenhoek 106:585–590
Sarkis S, Boettcher A, Ueda N, Hohn C (2005) A simple transport procedure for juvenile calico scallops, Argopecten gibbus (Linnaeus, 1758). J Shellfish Res 24:377–380
Schmidt EW, Obraztsova AY, Davidson SK, Faulkner DJ, Haygood MG (2000) Identification of the antifungal peptide-containing symbiont of the marine sponge Theonella swinhoei as a novel δ-proteobacterium, “Candidatus Entotheonella palauensis”. Mar Biol 136:969–977
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schütte UME, Abdo Z, Bent SJ, Shyu C, Williams CJ, Pierson JD, Forney LJ (2008) Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Appl Microbiol Biotechnol 80:365–380
Shore A, Caldwell JM (2019) Modes of coral disease transmission: how do diseases spread between individuals and among populations? Mar Biol 166:45.
Smith EP (2020) Ending reliance on statistical significance will improve environmental inference and communication. Estuaries Coasts 43:1–6
Stabili L, Cardone F, Alifano P, Tredici SM, Piraino S, Corriero G, Gaino E (2012) Epidemic mortality of the sponge Ircinia variabilis (Schmidt, 1962) associated to proliferation of a Vibrio bacterium. Invert Microbiol 64:802–813
Stockton S (2016) Variability in antibacterial chemical defenses in Caribbean sponges of the genus Aplysina. Honors Thesis, University of Mississippi
Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, DeSalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M (2009) Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J 3:512–521
Sweet M, Bulling M, Cerrano C (2015) A novel sponge disease caused by a consortium of microorganisms. Coral Reefs 34:871–883
Taylor MW, Radax R, Steger D, Wagner M (2007) Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347
Team RDC (2019) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011 URL: https://www. R-project Org
Teeyapant R, Proksch P (1993) Biotransformation of brominated compounds in the marine sponge Verongia aerophoba- evidence for an induced chemical defense? Naturwissenschaften 80:369–370
Thomas T, Moitinho-Silva L, Lurgi M, Björk JR, Easson C, Astudillo-García C, Olson JB, Erwin PM, López-Legentil S, Luter H, Chaves-Fonnegra A, Costa R, Schupp PJ, Steindler L, Erpenbeck D, Gilbert J, Knight R, Ackermann G, Lopez JV, Taylor MW, Thacker RW, Montoya JM, Hentschel U, Webster NS (2016) Diversity, structure and convergent evolution of the global sponge microbiome. Nature Comm 7:11870. https://doi.org/10.1038/ncomms11870
Thoms C, Ebel R, Proksch P (2006) Activated chemical defense in Aplysina sponges revisited. J Chem Ecol 32:97–123
Unson MD, Holland ND, Faulkner DJ (1994) A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar Biol 119:1–11
Van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ, Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA (2012) Global diversity of sponges (Porifera). PLoS ONE. 7: e35105
Vargas S, Leiva L, Wörheide G (2020) Short-term exposure to high-temperature water causes a shift in the microbiome of the common aquarium sponge Lendenfeldia chondrodes. Microb Ecol. https://doi.org/10.1007/s00248-020-01556-z
Walton CJ, Hayes NK, Gilliam DS (2018) Impacts of a regional, multi-year, multi-species coral disease outbreak in southeast Florida. Front Mar Sci. 8: 323
Ward JR, Lafferty KD (2004) The elusive baselines of marine disease: are diseases in ocean ecosystems increasing? PLoS Biol. https://doi.org/10.1371/journal.pbio.0020120
Webster NS (2007) Sponge disease: a global threat? Environ Microbiol 9:1363–1375
Webster NS, Xavier JR, Freckelton M, Motti CA, Cobb R (2008) Shifts in microbial and chemical patterns within the marine sponge Aplysina aerophoba during a disease outbreak. Envr Microbiol 10:3366–3376
Wilkinson CR (1983) Net primary productivity in coral reef sponges. Science 219:410–412
Wilson MC, Mori T, Rückert C, Uria AR, Helf MJ, Takada K, Gernert C, Steffens UAE, Heycke N, Schmitt S, Rinke C, Helfrich EJN, Brachmann AO, Gurgui C, Wakimoto T, Kracht M, Crüsemann M, Hentschel U, Abe I, Matsunaga S, Kalinowski J, Takeyama H, Piel J (2014) An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 506:58–62
Wulff J (2012) Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344
Zea S, Henkel TF, Pawlik JR (2014) The Sponge Guide: a picture guide to Caribbean sponges. 3rd ed. Available online at www.spongeguide.org
Acknowledgements
We thank M Ansley, S Criddle, S Lee, D Lee, C Williams, and Dr. JL Stevens for their help with the laboratory analyses, and Drs. E Hunkin and M Slattery for their help in the field. Dr. E Mueller and the Perry Institute of Marine Science provided logistical support. Samples were collected under Bahamas Department of Marine Resources Scientific Research Permit MAF/FIS/1,12,46A,79. Funding was provided by National Science Foundation grants OCE-1214303 to DJG and JBO, OCE-0727833 to JBO and OCE-0727996 to DJG and a National Institute of Undersea Science and Technology grant to DJG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Carly Kenkel
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Olson, J.B., Easson, C.G. & Gochfeld, D.J. Temporal changes in the sponge holobiont during the course of infection with Aplysina Red Band Syndrome. Coral Reefs 40, 1211–1226 (2021). https://doi.org/10.1007/s00338-021-02126-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-021-02126-6