Abstract
Objectives
To compare the repeatability and interrelation of various late gadolinium enhancement (LGE) assessment techniques for monitoring fibrotic changes in myocarditis follow-up.
Materials and methods
LGE extent change between baseline and 3-month cardiovascular magnetic resonance (CMR) was compared in patients with acute myocarditis using the full width at half maximum (FWHM), gray-scale thresholds at 5 and 6 standard deviations (SD5 and SD6), visual assessment with threshold (VAT) and full manual (FM) techniques. In addition, visual presence score (VPS), visual transmurality score (VTS), and a simplified visual change score (VCS) were assessed. Intraclass-correlation (ICC) was used to evaluate repeatability, and methods were compared using Spearman’s correlation.
Results
Forty-seven patients (38 male, median age: 27 [IQR: 21; 38] years) were included. LGE extent change differed among quantitative techniques (p < 0.01), with variability in the proportion of patients showing LGE change during follow-up (FWHM: 62%, SD5: 74%, SD6: 66%, VAT: 43%, FM: 60%, VPS: 53%, VTS: 77%, VCS: 89%). Repeatability was highest with FWHM (ICC: 0.97) and lowest with SD5 (ICC: 0.89). Semiquantitative scoring had slightly lower values (VPS ICC: 0.81; VTS ICC: 0.71). VCS repeatability was excellent (ICC: 0.93). VPS and VTS correlated with quantitative techniques, while VCS was positively associated with VPS, VTS, VAT, and FM, but not with FWHM, SD5, and SD6.
Conclusion
FWHM offers the least observer-dependent LGE follow-up after myocarditis. VPS, VTS, and VCS are practical alternatives, showing reliable correlations with quantitative methods. Classification of patients exhibiting either stable or changing LGE relies on the assessment technique.
Clinical relevance statement
This study shows that LGE monitoring in myocarditis is technique-dependent; the FWHM method yields the most consistent fibrotic tracking results, with scoring-based techniques as reliable alternatives.
Key Points
-
Recognition of fibrotic changes during myocarditis follow-up is significantly influenced by the choice of the quantification technique employed.
-
The FWHM technique ensures highly repeatable tracking of myocarditis-related LGE changes.
-
Segment-based visual scoring and the simplified visual change score offer practical, reproducible alternatives in resource-limited settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myocarditis presents with diverse clinical manifestations. While most patients recover fully, a subset faces severe complications, such as heart failure and cardiovascular death [1]. Identifying individuals at risk of unfavorable outcomes is crucial for tailoring effective therapeutic interventions.
In recent years, diagnostic approaches for myocarditis have evolved significantly. While an endomyocardial biopsy is preferred for cases involving shock or severe arrhythmias, cardiovascular magnetic resonance imaging (CMR) has become the primary diagnostic tool for clinically stable patients with suspected acute myocarditis, recommended as a first-line evaluation for cardiomyopathies (Class I, Level B recommendation) [2]. CMR, especially with late gadolinium enhancement (LGE), offers a robust method to confirm myocyte injury during the acute phase and reliably assess fibrotic changes within affected myocardial regions over the course of inflammation [3, 4]. The persistence of the LGE postacute phase correlates with adverse outcomes, while its absence at follow-up indicates full recovery [5, 6]. Despite the importance of follow-up CMR examinations for disease monitoring and prognostication, current guidelines lack consensus on the optimal LGE assessment technique after the acute phase of myocarditis, representing an active area of investigation [7, 8].
Recently, there has been a growing interest in applying quantitative methods to LGE in myocarditis, akin to their routine use for other cardiomyopathies. These methods often involve gray-scale thresholds combined with semiautomated or fully manual techniques, providing a detailed yet labor-intensive approach to LGE quantification [9, 10]. Simpler, semiquantitative methods, utilizing segment-based scoring systems, remain widely used for LGE assessment [11, 12]. Experienced clinicians may even visually discern significant changes in LGE patterns and extent throughout the entire left ventricular (LV) myocardium, potentially bypassing the need for intricate quantitative analyses. This approach can benefit from clinical intuition and the integration of contextual information into the evaluation process. Limited data exist on directly comparing quantitative and semiquantitative methods for measuring LGE extent in acute myocarditis at a single time point, while their performance in LGE follow-up assessment remains mostly unknown [13].
Thus, our objective was to evaluate the performance and interchangeability of various techniques for assessing LGE changes postacute myocarditis. Our study hypothesized that semiquantitative assessment techniques are as reproducible as quantitative methods in evaluating LGE changes during myocarditis, and they are interchangeable. To address this hypothesis, we introduced a simplified visual assessment method, in addition to segment-based methods, for a direct comparison of LGE changes across the entire LV myocardium, referred to as the ‘visual change score.’
Materials and methods
Study population
The study had institutional review board approval, followed the Declaration of Helsinki principles. Patients were retrospectively selected from a myocarditis registry at our institution, with retroactive written informed consent according to institution regulations. Consecutive patients were included, who underwent baseline CMR within 7 days of the initial clinical episode and follow-up CMR at 90 (± 15) days, with a study period between January 2016 and December 2019. All participants were referred to our center with a first episode of suspected acute myocarditis. Patients underwent a diagnostic work-up in line with the latest guidelines of the European Society of Cardiology, including a 12-lead electrocardiogram at presentation, and met the updated Lake Louise Criteria for a CMR-based diagnosis of acute myocarditis [7, 8]. Inclusion criteria required positive LGE on baseline CMR. Coronary artery disease was ruled out in all participants either through invasive coronary angiography, CT coronary angiography, or based on a low pretest probability (< 30 years of age). Medical records were scrutinized for underlying comorbidities that could potentially impact LV myocardium, leading to the exclusion of patients with a history of prior myocarditis, congenital heart disease, cardiac surgery, reduced LV ejection fraction, known cardiomyopathies, and systemic conditions with potential myocardial involvement, such as collagen disorders, eosinophilic disorders, sepsis, tuberculosis, or malignancies undergoing chemotherapy. The study population is outlined in Fig. 1.
CMR protocol
The studies utilized magnetic resonance scanners, either a 1.5 Tesla (Achieva, Philips Medical Systems) or a 3.0 Tesla (Skyra, Siemens), equipped with dedicated phased array coils for cardiac examinations. All acquisitions were breath-held in end-expiration. Cine-balanced steady-state free precession images assessed cardiac function and geometry in standard orientations. Native T1 and T2 Mapping, along with T2-weighted images of the LV with and without fat suppression, were obtained in short axis. LGE images were acquired 10 minutes postadministration of 0.2 mmoL/kg gadobutrol (Gadovist, Bayer Schering Pharma), following standard orientations. The optimal inversion time for LGE was determined individually using an inversion time scout sequence, ranging between 190 and 270 ms. For 1.5 Tesla CMR studies, a three-dimensional (3D) LGE sequence was employed (field of view 350 × 350 mm2, matrix dimensions of 256 × 256, repetition time/echo time of 3.6/1.8 ms, a flip angle of 15°, in-plane resolution of 1.5 × 1.5 mm2, and a slice thickness of 8 mm). At 3.0 Tesla, a two-dimensional (2D) Phase-Sensitive Inversion-Recovery sequence was used (field of view 330 × 330 mm2, matrix dimensions of 192 × 256, repetition time/echo time of 663/2 ms, a flip angle of 20°, in-plane resolution of 1.3 × 1.3 mm2, and a slice thickness of 8 mm). The CMR diagnosis of myocarditis was established based on regional or global myocardial edema, defined by increased native T2-signal or T2-mapping values, along with evidence of myocardial injury indicated by elevated native T1-mapping values, increased extracellular volume, or positive LGE.
LGE data analysis
LGE involvement in the LV was assessed using commercial postprocessing software (Intellispace Portal, Version 10, Philips Healthcare). The quantitative evaluation involved manually delineating LV endo- and epicardial boundaries on short-axis LGE images. Subsequently, various clinically available LGE quantification techniques were applied. These included semiautomated methods, where regions of interest (ROIs) were initially placed in both non-enhanced areas and the seemingly normal remote myocardium. In the next step, the hyper-enhanced myocardial regions were segmented in several ways: automatically using the full width at half maximum (FWHM) method or relative to gray-scale thresholds set at predefined values, 5 and 6 standard deviations above the mean signal intensity for the remote myocardial tissue (referred to as the 5 SD and 6 SD techniques), or by manually adjusting the threshold by the reader to achieve the best match (referred to as visual assessment with a user-defined threshold, VAT). Lastly, a full manual (FM) quantification method was employed, where the reader manually delineated the extent of LGE on each LV slice. The extent of LGE was calculated as a percentage relative to the entire LV myocardial volume. Change in LGE extent during follow-up was defined as a ≥ 1% difference in LGE volume between baseline and follow-up relative to the entire LV volume. This cut-off was based on the previous prognostic study of Aquaro et al, who used the same threshold, and a significant LGE change from 6.2 [IQR: 2; 10] % to 4.1 [IQR: 2; 8] % was found over 6 months follow-up [6].
For semiquantitative LGE assessment, the 17-segment model of the American Heart Association was applied to the entire LV myocardium [14]. LGE presence and transmurality were coded (0 = no LGE, 1 = < 25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%), and visual presence score (VPS) and visual transmurality score (VTS) were calculated, each multiplied by the number of affected myocardial segments, resulting in VPS (0–17) and VTS (0–68). Change in LGE extent was defined as the change of VPS or VTS score during follow-up.
For a simplified assessment of LGE change, baseline and follow-up short-axis LGE images were displayed side by side. Readers estimated the percentage LGE difference of the entire LV myocardium, consolidated into a five-point Likert scale: 0 = no change, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%, termed the ‘visual change score’ (VCS). Positive values denote LGE progression, while negative values indicate regression.
Baseline and follow-up CMR studies were independently evaluated by three observers (M.K., M.P., J.M.S.) with over 5 years of CMR experience and Level 3 certification from the European Association of Cardiovascular Imaging or equivalent authority. Consensus readings were used to estimate the correlation between each technique. To test interobserver reliability, one observer (M.K.) repeated measurements with a 6-month interval.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR). Categorical variables are expressed as counts and percentages. The Wilcoxon signed-rank test compared patient characteristics between baseline and follow-up for continuous variables, and the McNemar test compared categorical data. Friedman’s test assessed the extent of LGE among different quantitative methods. Spearman’s correlation evaluated the relationship between LGE assessment techniques, with correlation coefficients (ρ) indicating a very strong (≥ 0.70), strong (0.40 to < 0.70), moderate (0.30 to < 0.40), weak (0.20 to < 0.30), and no or negligible (0.01 to < 0.20) relationship [15, 16]. Intra- and interobserver repeatability were assessed using intraclass correlation (ICC), categorized as poor (< 0.5), moderate (0.5 to < 0.75), good (0.75 to < 0.9), or excellent (0.9–1.0) agreement [17]. A 2-sided p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 23).
Results
Baseline characteristics
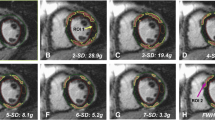
The study included 47 patients meeting specific criteria (38 male, median age 27 [IQR: 21; 38] years). Baseline patient characteristics are detailed in Table 1, and diagnostic parameters are summarized in Table 2. Figure 2 illustrates an example of LGE follow-up assessment using various techniques.
Example of LGE assessment using various quantitative techniques and visual scoring. Cardiovascular magnetic resonance images at baseline and 3-month follow-up of a 35-year-old male with acute myocarditis. Upper panels show LGE images in short-axis orientation, while lower panels present bulls-eye views with quantitative and visual scores. The color scale represents the LGE extent. Changes in LGE extent (percentage, %) from baseline to follow-up: FWHM 6% to 3%, SD5 16% to 8%, SD6 13% to 6%, VAT 6% to 4%, FM 8% to 3%. VPS decreased from 8 to 5, and VTS reduced from 16 to 8, corresponding a VCS of -3, indicating a 51–75% reduction in LGE
LGE findings between baseline and at follow-up
All patients exhibited nonischemic (subepicardial) LGE in at least one myocardial segment. The extent of LGE at baseline and follow-up, assessed with different techniques, is summarized in Fig. 3 and Table 3. Significant variation in LGE extent change was noted among quantitative techniques (p < 0.01), with SD5 showing the largest change (−2.4% [IQR: −7.2; −0.1%]) and VAT the smallest (−0.3% [IQR: −2.1; 0.3%]). Absolute change in LGE extent across techniques exceeded the predefined threshold of a significant change (≥ 1% of LV), leading to the disparate categorization of patients at follow-up, as demonstrated also in Fig. 4. Consequently, there was a significant disparity among LGE assessment techniques in categorizing patients with progressive, unchanged, or regressive LGE at follow-up. The highest proportion of patients with LGE change was identified with SD5 (60%), while the lowest was with VAT (34%), as summarized in Table 3.
LGE extent at baseline and follow-up CMR with various quantitative techniques. The chart shows LGE extent with various quantitative methods. Columns depict baseline and follow-up LGE percentages. Horizontal lines denote max and min values. Colored boxes highlight data between the first and third quartiles, with lines indicating medians
Absolute difference between LGE extent change among various quantitative techniques. Plots show LGE changes using different quantitative techniques, measuring LGE as a percentage (%) of left ventricular volume. Significant variability in LGE change, surpassing the ≥ 1% LV threshold, is evident. The largest difference is between SD5 and VAT techniques. Upper and lower lines represent max and min points, colored boxes enclose the first and third quartiles, with lines indicating medians
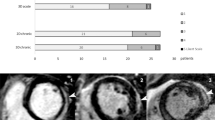
Semiquantitative grading showed LGE regression in 53% of the patients with VPS and 77% with VTS during follow-up. Visual assessment with VCS demonstrated higher sensitivity for detecting LGE extent change, with positive results in 89% of patients. Importantly, neither semiquantitative scoring nor visual assessment using VCS showed LGE progression between baseline and follow-up CMR. Figure 5 offers a comparative view of VCS with traditional assessment techniques regarding proportional LGE changes during follow-up.
Relative changes in LGE extent during follow-up, illustrated for both quantitative and semiquantitative techniques in comparison to visual change score. Plots show LGE change from baseline to follow-up CMR, adjusted for visual change score. Data from quantitative and semiquantitative techniques was converted into VCS categories, as 1–25%, 26–50%, 51–75%, and 76–100% change. Positive values denote LGE progression, negative values indicate LGE regression. Upper and lower lines depict max and min points, while colored boxes indicate first and third quartiles, with lines showing medians
Repeatability of LGE assessment
Quantitative postprocessing methods showed excellent interobserver repeatability for LGE assessment at baseline and follow-up, with the highest ICC values observed for FHWM (up to 0.98 [IQR: 0.96; 0.99]), as summarized in Table 4. ICCs at follow-up CMR were slightly reduced for SD5 and SD6 (0.79 [IQR: 0.65; 0.87] and 0.78 [IQR: 0.64; 0.87], respectively), indicating potentially lower reliability for methods involving gray-scale thresholds in quantifying smaller amounts of LGE. The repeatability of assessing changes in LGE extent was excellent for most quantitative techniques, with FWHM having the highest ICC (0.97 [IQR: 0.94; 0.98]), and good for SD5 (0.89 [IQR: 0.81; 0.93]).
Interobserver repeatability of semiquantitative scoring was good for VPS at baseline (0.80 [IQR: 0.59; 0.89]), VTS at baseline (0.77 [IQR: 0.52; 0.89]) and follow-up (0.89 [IQR: 0.56; 0.90]), but moderate for VPS at follow-up (0.66 [IQR: 0.39; 0.81]). VPS change showed good interobserver repeatability (0.81 [IQR: 0.63; 0.90]), which was moderate for VTS change (0.71 [IQR: 0.53; 0.83]).
Notably, VCS demonstrated excellent interobserver repeatability for assessing changes between baseline and follow-up (0.93 [IQR: 0.89; 0.96]). Inter- and intraobserver repeatability of various assessment techniques are summarized in Fig. 6.
Association between different LGE assessment techniques
We found a positive correlation between visual scoring using VPS and VTS and all quantitative techniques, with a very strong association between FM and VTS (ρ = 0.73, p < 0.01), a moderate interrelationship between FWHM and VPS (ρ = 0.31, p = 0.03), and strong agreement between all other methods, as summarized in Table 5. Furthermore, VPS and VTS showed a strong association with VCS (ρ = 0.68 and ρ = 0.60, respectively, p < 0.01 both). We also observed a moderate positive association of VCS with VAT and FM (ρ = 0.35, p = 0.02 and ρ = 0.38, p = 0.01, respectively), but not with other quantitative techniques.
Discussion
We demonstrated excellent repeatability with commonly used postprocessing software for quantitative tracking of LGE in acute myocarditis, with the least observer dependence observed with the FWHM technique. Semiquantitative scoring using VPS and VTS emerged as a reliable alternative for assessing LGE changes in myocarditis, showing a strong association with most quantitative methods, albeit with slightly lower overall repeatability compared to quantitative approaches. Our simplified method for a direct comparison of LGE throughout the entire LV myocardium, referred to as the visual change score, proved highly capable of detecting LGE alterations over time with excellent repeatability. Importantly, the categorization of patients’ LGE status at follow-up CMR (progression/regression/no change) showed substantial variation across LGE assessment techniques.
A recent meta-analysis of 11 studies revealed that extensive LGE at the index CMR doubles the risk of future cardiovascular events following acute myocarditis. However, comparing LGE burden across studies is deemed challenging due to varied definitions, such as LGE affecting more than 2 myocardial segments, 10% of the LV mass, or 17 grams, depending on the assessment technique [18]. This highlights the need for comprehensive studies. Gräni et al conducted an extensive study on 670 patients, demonstrating that the FWHM technique had the highest repeatability for baseline LGE assessment in myocarditis [13], consistent with our findings. Almost all quantitative techniques in our study showed excellent repeatability, with SD5 and SD6 remaining in the good range for the follow-up. Given the LGE decrease at the follow-up CMR in most patients in our cohort, it can be speculated that these threshold-based techniques may exhibit decreased interobserver reliability with smaller amounts of LGE. CMR is also increasingly used for ongoing disease monitoring. Prior longitudinal studies revealed that LGE decreases in up to a quarter of patients six months after the acute phase of myocarditis, indicating that LGE does not necessarily imply definite fibrosis [19]. In a recent study by Aquaro et al involving 187 patients with repeated CMR after acute myocarditis over a median clinical follow-up of seven years, an increase in LGE extent at the 6-month follow-up was associated with future cardiovascular events (HR 2.60, 95% CI 1.05–6.90). Importantly, even the persistence of LGE on follow-up CMR was an independent risk factor when concomitant resolution of myocardial edema was observed (HR 4.50, 95% CI 1.30–14.50) [6]. This underscores the importance of precise evaluation of LGE changes over time in myocarditis patients. However, quantitative analysis is often time-consuming, requiring segmentation of complete LV endo- and epicardial myocardium contours. Additionally, the use of various postprocessing methods at different time points during imaging follow-up may introduce errors, raising questions about the interchangeability of these techniques for longitudinal studies. Our findings highlight excellent reliability in assessing LGE changes in myocarditis when using the same quantification technique for the follow-up, with the FWHM showing the highest repeatability. Notably, the absolute difference in LGE change among various postprocessing techniques in our study exceeded the predefined threshold of ≥ 1% of the LV mass [6], indicating their lack of interchangeability for follow-up studies. Consequently, the proportion of patients classified with LGE progression, regression, or no change varied markedly depending on the postprocessing technique used, suggesting that the prognostic information derived from quantitative LGE analysis may strongly depend on the chosen technique. Semiquantitative LGE scoring with VPS and VTS is a reliable alternative to quantitative techniques, with slightly lower repeatability in our study. Nevertheless, there is a positive correlation between the performance of VPS and VTS and most quantitative methods in tracking LGE changes during follow-up. We also tested a simplified visual assessment method, referred as ‘visual change score’, for monitoring global LGE changes in the entire LV myocardium during follow-up. VCS was highly reproducible in our study for the direct comparison of LGE between baseline and follow-up CMR. Moreover, it exhibited a strong correlation with VPS and VTS, and a moderate correlation with FM LGE quantification and semiautomated LGE assessment using the VAT technique. Simplified visual analysis has been supported by evidence in cardiac imaging [20,21,22]. Even prior large-scale trials, such as the CE-MARC trial, have employed a visual approach to estimate wall motion abnormalities, ischemia testing, coronary stenosis, and infarct scar size [23]. This simplified, less time-consuming method may be particularly valuable in small centers where sophisticated and often more expensive postprocessing techniques are not available. However, the prognostic potential of VCS remains unknown, and further studies with clinical follow-up and repeated CMR are necessary, especially since we couldn’t establish a correlation of VCS with FHWM, SD5, and SD6 techniques, which have been the preferred techniques in prior prognostic studies using CMR in myocarditis follow-up [6, 10].
Our study has limitations. While our study is limited by a smaller sample size and a comparatively young median patient age, the use of CMR in diagnosing acute myocarditis, coupled with stringent exclusion criteria, has allowed for a more accurately defined cohort of acute myocarditis cases. This approach offers a clearer distinction than larger studies that depend only on clinical criteria [24]. We used a previously established 1% threshold for change in LGE volume [6], which may yield lower specificity in larger LGE volumes. An increased threshold for LGE extent change would also probably smoothen the difference between the quantification methods in regards of categorizing patients with changing or stable LGE. This was not further tested in our study, and the sensitivity and specificity of this threshold for true biological changes remain underexplored. Variations in hardware and software could still influence technique performance. Our sample size did not allow for comparisons between 1.5 T and 3 T scanners or various LGE sequences, but our previous research suggested similar diagnostic quality for 2D and 3D LGE techniques [25]. The lack of clinical follow-up limits our ability to determine the prognostic value of certain techniques, including VCS, which was not the primary focus of this study. Larger studies with clinical follow-up are needed to address these limitations.
In conclusion, our study highlights non-interchangeability among methodologies for tracking myocarditis fibrotic changes. Among common postprocessing techniques, FWHM shows the highest degree of repeatability of LGE assessment during follow-up in this patient population. Visual scoring with VPS and VTS is a reliable alternative, though with slightly lower repeatability. We introduced a simplified visual change score for direct LGE change comparison, revealing strong correlations with semiquantitative techniques. This approach is valuable for smaller centers with limited resources. Additional studies with clinical follow-up are necessary to determine its prognostic potential.
Abbreviations
- CMR:
-
Cardiovascular magnetic resonance
- FHWM:
-
Full width at half maximum
- FM:
-
Full manual
- ICC:
-
Intraclass correlation
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- SD5:
-
Gray-scale thresholds set at 5 standard deviations above remote myocardium
- SD6:
-
Gray-scale thresholds set at 6 standard deviations above remote myocardium
- T:
-
Tesla
- VAT:
-
Visual assessment with threshold
- VCS:
-
Visual change score
- VPS:
-
Visual presence score
- VTS:
-
Visual transmurality score
References
Eichhorn C, Greulich S, Bucciarelli-Ducci C, Sznitman R, Kwong RY, Gräni C (2022) Multiparametric cardiovascular magnetic resonance approach in diagnosing, monitoring, and prognostication of myocarditis. JACC Cardiovasc Imaging 15:1325–1338
Arbelo E, Protonotarios A, Gimeno JR et al (2023) 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J 44:3503–3626
Yang F, Wang J, Li W et al (2020) The prognostic value of late gadolinium enhancement in myocarditis and clinically suspected myocarditis: systematic review and meta-analysis. Eur Radiol 30:2616–2626
Leiner T, Bogaert J, Friedrich MG et al (2020) SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J Cardiovasc Magn Reson 22:76
Basso C (2022) Myocarditis. N Engl J Med 387:1488–1500
Aquaro GD, Ghebru Habtemicael Y, Camastra G et al (2019) Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol 74:2439–2448
Ferreira VM, Schulz-Menger J, Holmvang G et al (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72:3158–3176
Caforio ALP, Pankuweit S, Arbustini E et al (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648
Fischer K, Marggraf M, Stark AW et al (2020) Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS One 15:e0227134
Gräni C, Eichhorn C, Bière L et al (2017) Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 70:1964–1976
Károlyi M, Kolossváry M, Weber L et al (2023) Association between ECG parameters and late gadolinium enhancement along the course of myocarditis. Int J Cardiovasc Imaging 39:1169–1178
Grün S, Schumm J, Greulich S et al (2012) Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol 59:1604–1615
Gräni C, Eichhorn C, Bière L et al (2019) Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J Cardiovasc Magn Reson 21:14
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–42
Dancey CP, Reidy J (2004) Statistics Without Maths for Psychology: using SPSS for Windows. Prentince-Hall, London, England
Leclezio L, Jansen A, Whittemore V, de Vries P (2015) Pilot validation of the tuberous sclerosis-associated neuropsychiatric disorders (TAND) checklist. Pediatric Neurol 52:16–24
Han X (2020) On statistical measures for data quality evaluation. J Geogr Inf Syst 12:178–187
Georgiopoulos G, Figliozzi S, Sanguineti F et al (2021) Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis: a systematic review and meta-analysis. Circ Cardiovasc Imaging 14:e011492
Mahrholdt H, Wagner A, Deluigi CC et al (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590
Li Y, Li C, Jin H, Huang W (2016) Magnetic resonance imaging in interventional therapy of patients with acute myocardial infarction prior to and after treatment. Exp Ther Med 12:1755–1759
Gudmundsson P, Rydberg E, Winter R, Willenheimer R (2005) Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol 101:209–212
Sirol M, Gzara H, Gayat E et al (2016) Comparison between visual grading and planimetric quantification of microvascular obstruction extent assessment in reperfused acute myocardial infarction. Eur Radiol 26:2166–2175
Greenwood JP, Maredia N, Younger JF et al (2012) Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 379:453–460
Kytö V, Sipilä J, Rautava P (2013) The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart 99:1681–1684
Polacin M, Kapos I, Gastl M et al (2021) Comparison of 3D and 2D late gadolinium enhancement magnetic resonance imaging in patients with acute and chronic myocarditis. Int J Cardiovasc Imaging 37:305–313
Weber L, Sokolska JM, Nadarevic T et al (2022) Impact of myocardial injuryon regional left ventricular function in the course of acute myocarditis withpreserved ejection fraction: insights from segmental feature tracking strainanalysis using cine cardiac MRI. Int J Cardiovasc Imaging 38:1851–1861
Acknowledgements
Márton Kolossváry was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the ÚNKP-23-3-5-OE-71 new national excellence program of the Ministry of Culture and Innovation from the source of the National Research, Development and Innovation Fund of Hungary. Project no. PD 147269 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the PD_23 funding scheme. Justyna M. Sokolska was a beneficiary of the Polish National Agency for Academic Exchange (NAWA) in ‘The Iwanowska Programme’ at the Department of Cardiology, MRI Cardiac Unit, University Hospital Zurich, Switzerland.
Funding
The authors state that this work has not received any funding. Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Robert Manka.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Hatem Alkadhi is a Deputy Editor of European Radiology. He has not taken part in the review or selection process of this article.
Statistics and biometry
Mihály Károlyi and Márton Kolossváry have significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Study participants have been a part of the following studies, without overlap in the study results: Weber et al [26] and Károlyi et al [11].
Methodology
-
Retrospective
-
Observational
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Károlyi, M., Polacin, M., Kolossváry, M. et al. Comparative analysis of late gadolinium enhancement assessment techniques for monitoring fibrotic changes in myocarditis follow-up. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10756-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10756-x