Abstract
Objectives
To quantify brain microstructural changes in recently diagnosed relapsing-remitting multiple sclerosis (RRMS) using longitudinal T1 measures, and determine their associations with clinical disability.
Methods
Seventy-nine people with recently diagnosed (< 6 months) RRMS were recruited from a single-centre cohort sub-study, and underwent baseline and 1-year brain MRI, including variable flip angle T1 mapping. Median T1 was measured in white matter lesions (WML), normal-appearing white matter (NAWM), cortical/deep grey matter (GM), thalami, basal ganglia and medial temporal regions. Prolonged T1 (≥ 2.00 s) and supramedian T1 (relative to cohort WML values) WML voxel counts were also measured. Longitudinal change was assessed with paired t-tests and compared with Bland-Altman limits of agreement from healthy control test-retest data. Regression analyses determined relationships with Expanded Disability Status Scale (EDSS) score and dichotomised EDSS outcomes (worsening or stable/improving).
Results
Sixty-two people with RRMS (mean age 37.2 ± 10.9 [standard deviation], 48 female) and 11 healthy controls (age 44 ± 11, 7 female) contributed data. Prolonged and supramedian T1 WML components increased longitudinally (176 and 463 voxels, respectively; p < .001), and were associated with EDSS score at baseline (p < .05) and follow-up (supramedian: p < .01; prolonged: p < .05). No cohort-wide median T1 changes were found; however, increasing T1 in WML, NAWM, cortical/deep GM, basal ganglia and thalami was positively associated with EDSS worsening (p < .05).
Conclusion
T1 is sensitive to brain microstructure changes in early RRMS. Prolonged WML T1 components and subtle changes in NAWM and GM structures are associated with disability.
Clinical relevance statement
MRI T1 brain mapping quantifies disability-associated white matter lesion heterogeneity and subtle microstructural damage in normal-appearing brain parenchyma in recently diagnosed RRMS, and shows promise for early objective disease characterisation and stratification.
Key Points
• Quantitative T1 mapping detects brain microstructural damage and lesion heterogeneity in recently diagnosed relapsing-remitting multiple sclerosis.
• T1 increases in lesions and normal-appearing parenchyma, indicating microstructural damage, are associated with worsening disability.
• Brain T1 measures are objective markers of disability-relevant pathology in early multiple sclerosis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disease trajectory in relapsing-remitting multiple sclerosis (RRMS) is highly variable, and difficult to predict early in the disease course. Improved objective prognostic markers, which are sensitive to early RRMS pathological features, are needed to better inform early treatment decisions from the point of diagnosis, and for future clinical trials.
Episodic immune-mediated demyelination is thought to result in axonal damage and neuronal dysfunction [1]. Identification of new or enlarging white matter lesions (WML) visible on T2-weighted or T2-FLAIR (fluid-attenuated inversion recovery) MRI is widely used as an indicator of interval inflammatory disease activity [2]. The number and volume of WML do not, however, adequately explain clinical disability in RRMS [3, 4]. Microscopic damages to grey matter (GM) and normal-appearing white matter (NAWM) are also pathological features of RRMS, [5] and heterogeneity of microstructural damage within WML that appears similar on T2-weighted MRI is observable using quantitative microstructure-sensitive MRI methods [6,7,8,9].
A subset of T2-weighted WML are visibly hypointense on T1-weighted MRI, reflecting T1 prolongation, [10] and have variously been described as ‘black hole’ or ‘T1w-hypointense’ lesions [11]. Such ‘T1w-hypointense’ lesions are thought to represent more severely damaged white matter [12] and are associated with worse clinical disability [13, 14]. Radiological evaluation of T1-weighted hypointensity is nevertheless subjective and qualitative, and will not capture intralesional T1 heterogeneity or subtle change in normal-appearing tissue.
The spin-lattice relaxation time (T1) of protons in brain parenchyma is inversely proportional to myelin density [11]. Previous imaging-histological correlation indicates that T1 quantification may provide a non-invasive marker of myelin density in multiple sclerosis (MS), [12] and is sensitive to subtle abnormalities in normal-appearing GM and NAWM in RRMS [5, 15,16,17,18,19,20]. Widespread T1 prolongation is seen in RRMS compared with healthy controls, and correlates with disability [14, 20, 21]. Quantitative mapping of T1 may also distinguish microstructural heterogeneity within WML; substantially prolonged T1 tissue volumes appear to be a better predictor of disability than visual assessment of T1-weighted hypointense WML [22]. The contribution of prolonged T1 components in WML and other brain tissues to clinical disability in early disease, and how these evolve with time remain to be explored.
The primary aims of this study were to assess whether changes in tissue microstructure integrity, reflected in T1 heterogeneity and prolongation, are quantifiable within WML and other brain regions over the first year following RRMS diagnosis, and to ascertain the relationship between T1 prolongation and clinical disability. We hypothesised that prolonged T1 components would progress with time and be associated with worsening clinical disability.
Our secondary exploratory aims were to examine intralesional T1 heterogeneity and its relationship with disability, investigate single-time point measures as predictors of clinical disability trajectory following RRMS diagnosis and establish the test-retest reliability of T1 measures.
Materials and methods
Ethics approval was obtained from the South East Scotland Research Ethics Committee 02 (REC 15/SS/0233). The study conformed to the Declaration of Helsinki 2000 (amendments in 2002 and 2004) and Good Clinical Practice ICH guidelines. All participants gave written informed consent and data were pseudo-anonymised.
Participants
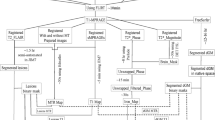
Seventy-nine patients were recruited to a single-centre sub-study of FutureMS, a prospective multi-centre longitudinal inception cohort study of people with recently diagnosed RRMS across Scotland (Fig. 1) [23, 24]. Inclusion criteria were as follows: < 6 months from diagnosis; aged ≥ 18 years; and capacity to provide informed consent. Exclusion criteria were as follows: previous use of disease-modifying therapies (DMTs); clinical trial participation prior to baseline assessment; and contraindications for MRI. FutureMS sample size calculation has been previously described; [24] Edinburgh participants were pragmatically offered extended MRI once the sub-study recruitment opened in November 2017 until March 2019. Twelve age- and sex-matched healthy controls were recruited for test-retest purposes. Exclusion criteria were contraindication for MRI and incidental MRI findings.
MRI and Expanded Disability Status Scale (EDSS) score were acquired at baseline (between November 2017 and July 2019) and 1-year follow-up. Handling of missing data and loss to follow-up are detailed elsewhere; briefly, data missing at random were imputed using predictive mean matching [23].
MRI protocol
MRI was performed on a 3-Tesla Siemens MAGNETOM Prisma clinical system at the University of Edinburgh Imaging Facility. Whole-brain T1 mapping was performed using a variable flip angle (VFA) 3D multi-echo spoiled gradient recalled echo sequence (total acquisition time: 12 min 28 s) as part of a comprehensive MRI protocol, including a 2D T2-weighted FLAIR and 3D T1-weighted MPRAGE (Table S1) [24]. Control subjects underwent an identical MRI protocol, repeated within 2 weeks, to determine test-retest agreement.
Structural MRI data processing
WML segmentation was performed on baseline 2D T2 FLAIR images using an adaptation of a previously reported thresholding method, [25] with manual correction [24]. Tissues were segmented with FreeSurfer (v6.0, http://surfer.nmr.mgh.harvard.edu/) from the T1-weighted MPRAGE [24]. These included NAWM; cortical GM (cGM); medial temporal regions (hippocampi and amygdala); basal ganglia (caudate, pallidum and putamen); thalami; and global deep GM (DGM; combined thalami, basal ganglia and medial temporal regions; Fig. 2). WML load was calculated as the percentage of occupied intracranial volume [24].
T 1 data processing
T1 parametric maps were obtained from VFA gradient echo images in MATLAB (R2018b) using previously described equations (Fig. 3; software available: https://doi.org/10.7488/ds/2965) [26, 27]. Tissue segmentations were registered to T1 maps using FSL (v6.0.1) FLIRT [28].
Median T1 within each tissue was calculated per patient (fslstats). To investigate T1 WML heterogeneity, the proportion of WML voxels within each 0.25-s interval between 0.5 and 2.00 s was calculated for each patient at baseline and follow-up (RStudio v4.0.2).
As T1 prolongation is associated with demyelination [12] and disability, [3] we also characterised WML components with relatively prolonged T1 using a thresholding approach [22]. The number of WML voxels with (1) T1 ≥ 2.00 s (here termed ‘prolonged T1’) and (2) supramedian T1 (calculated per patient relative to the baseline cohort mean of WML median T1) was thus calculated at each time point.
Statistical analysis
All statistical analyses were performed in RStudio (v4.0.2).
Test-retest healthy controls
To establish test-retest reference levels, Bland-Altman limits of agreement were calculated from healthy control data, following previously reported methodology [29]. To assess whether the difference between time points was statistically significant, sign tests were performed.
Longitudinal change in T 1 metrics
Paired two-sided t-tests were used to examine longitudinal changes in median T1 for each tissue and, as separate analyses, 1-year changes in the number of prolonged/supramedian T1 voxels and WML load. To account for WML voxels forming during the 1-year follow-up period, which could positively skew WML T1 data, a post hoc paired t-test was performed including only WML voxels already present at baseline. T1 changes in NAWM and cGM were plotted against test-retest limits for visual comparison. To control for type II error, False Discovery Rate (FDR) correction (q < 0.05) was applied for primary analyses [30].
Relationship with clinical disability
The relationship between T1 and EDSS score at baseline was investigated using ordinal logistic regression. In view of limited EDSS changes within our recently diagnosed cohort, 1-year change in EDSS score was dichotomised as either worsening EDSS, defined as ≥ 0.5 points, or stable/improving; the relationship between disability groups and both baseline and 1-year changes in T1 measures was investigated using binomial logistic regression, adjusted for covariates and interactions as appropriate (Table S2 for extended methods). FDR correction was applied separately for the three sets of analyses, which are assumed to be distinct families of hypotheses [30].
Regression analyses were repeated for prolonged and supramedian T1 WML measures. The relationship between WML prolonged T1 measures at 1-year follow-up and EDSS score at follow-up was also investigated using ordinal logistic regression.
Results
Demographics
Complete data were available from sixty-two RRMS patients (Fig. 1), and demographics (Table 1) were comparable to the wider FutureMS cohort [23]. Six participants (n = 3 worsening versus n = 3 stable/improving disability) reported a relapse in the 6 weeks prior to baseline MRI (range: 11–34 days), one of whom received oral steroid treatment (27 days before MRI). No relapses or steroid treatments were reported in the 6 weeks prior to follow-up. At follow-up, 61% (n = 38) of participants had begun treatment with DMTs, mainly dimethyl fumarate (n = 23; Table S3). There was no significant difference in DMT status between disability groups (Χ2 = 0.79, p = 0.37).
One control participant was excluded due to an unexpected incidental finding; test–retest data was therefore available from eleven controls (age 44 ± 11, 7 female).
Test-retest reliability
Test-retest agreement determined from control data is shown in Fig. S1. The difference in median T1 was not significant for all brain regions (Table S4).
Characterising T 1 in white matter lesions
Qualitative assessment showed varied proportions of WML voxels within each 0.25-s T1 range between patients, with a greater proportion in higher T1 ranges at follow-up (Fig. 4b) relative to baseline (Fig. 4a). Visual inspection showed discrete regions of relative T1 prolongation were present across WML (Fig. 5a–c), generally coinciding with T1-weighted ‘black holes’ (Fig. 5d–f).
White matter lesion T1 heterogeneity in our cohort of people with recently diagnosed relapsing-remitting multiple sclerosis (RRMS) (n = 62). Each bar represents one participant with RRMS. The colours of the stacked plot relate to the proportion of a given participant’s white matter lesion voxels within a given T1 range at (a) baseline and (b) 1-year follow-up
Spatial distribution (a–c) of prolonged T1 (i.e. ≥ 2.00 s) white matter lesion (WML) voxels (red) superimposed on all WML voxels (yellow) and whole-brain T1 maps (greyscale) in three representative relapsing-remitting multiple sclerosis study participants, with corresponding T1-weighted MPRAGE structural images (d–f)
Longitudinal change in T 1 metrics
WML median T1 decreased by a mean average of 31 ms longitudinally (t(61) = 2.98, FDR-corrected p < 0.05, 95% CI [− 52, − 10]); no other tissues showed significant longitudinal T1 changes (Table 2). Longitudinal change in NAWM and WML T1 for the majority of patient data points fell within test-retest Bland-Altman limits established in healthy controls (Fig. S2).
A longitudinal increase in WML load (mean average 0.235%, t(61) = 11.3, p < 0.001, 95% CI [0.193, 0.277]) prompted a post hoc analysis investigating only WML voxels present at baseline. This showed no significant change in median WML T1 (t(61) = 0.50, p = 0.618; Table 2).
There was a groupwise mean increase of 176 prolonged T1 WML voxels (t(61) = 5.11, p < 0.001, 95% CI [107, 246]) and an increase of 463 supramedian WML voxels (t(61) = 6.22, p < 0.001, 95% CI [314, 612]). When WML voxels which had formed during the follow-up period were excluded, changes in both measures remained significant (p < 0.001).
Relationship with clinical disability
Baseline median T1 was not associated with baseline EDSS score (all p > 0.05; Table S5) nor with EDSS worsening (all p > 0.05; Table S6) in any tissue studied. One-year changes in T1 (Table S7) within NAWM, WML, cGM, DGM, the basal ganglia and thalami (but not the medial temporal regions) were however positively associated with a greater risk of EDSS worsening (all p < 0.05; Fig. 6, and Table 3). The relationship between change in WML T1 and EDSS worsening was mediated by an interaction with age, whereby older age strengthened the association.
One-year change in T1 in our recently diagnosed relapsing-remitting multiple sclerosis cohort (n = 62). Boxplots are shown for normal-appearing white matter (NAWM), cortical grey matter (cGM), global deep grey matter (DGM), thalami, medial temporal regions, basal ganglia and white matter lesions (WML, b) including only WML voxel present at baseline. Boxplots are grouped according to dichotomised change in Expanded Disability Status Scale (EDSS) score over 1 year of < / ≥ 0.5 points. Asterisk (*) denotes significant (p < .05 with False Detection Rate correction) association between difference over 1 year in T1 and disability group after adjusting for age, baseline T1, 1-year change in lesion load, disease-modifying therapy status and any significant interaction effects (see text for details); n.s., not significant
At baseline, greater numbers of prolonged T1 and supramedian WML voxels were independently associated with higher baseline EDSS (both p < 0.05; Table 4); neither measure was predictive of EDSS worsening (Table 5). One-year changes in prolonged T1 and supramedian WML voxel counts were positively associated with EDSS worsening (p < 0.05 and p < 0.01 respectively; Table 5). Prolonged and supramedian WML T1 measures at follow-up were also positively related with follow-up EDSS score (p < 0.05 and p < 0.01 respectively; Table 4).
Discussion
Brain T1, and its relationship with clinical worsening, was quantified longitudinally in a newly diagnosed RRMS cohort. Longitudinal cohort-wide changes in median T1 were not observed for GM, NAWM or WML. There was significant T1 heterogeneity in WML; WML voxels with more marked T1 prolongation increased in number over time, and were associated with disability at baseline and 1-year follow-up. Greater T1 increases in normal-appearing brain and prolonged T1 WML components also contributed significantly to evolving disability in the year following RRMS diagnosis.
T 1 variation
Measured T1 was higher than literature values, [17, 22] which is attributable to differences in acquisition methods. Average WML T1 values were higher than NAWM, consistent with demyelination [31]. As with previous findings in RRMS, [22] wide variance in WML T1 and substantial overlap with cGM T1 values suggest heterogeneous myelin loss; [12] in our cohort, average WML T1 was lower than cGM, which may reflect the earlier disease stage and less severe WML damage. Medial temporal T1 was significantly higher than other tissues; measurements in this region are likely confounded by tissue interface effects, and therefore unreliable.
Demyelination appears to be the major determinant of T1 prolongation in white matter, [31] except within fully demyelinated WML where there is a ‘myelin floor effect’, and axonal content dominates variation in T1 [12, 32]. Increased tissue water content, [11] due to neuroinflammation or oedema, [33, 34] may also increase T1. Subtle T1 prolongation in NAWM is therefore likely to represent demyelination, whereas more markedly prolonged T1 components within WML may result from both myelin and axonal loss. Myelin and axonal content are nonetheless strongly associated, [31] and we therefore use the term microstructural damage to reflect the difficulty in establishing their individual contributions to disability in WML and normal-appearing brain.
Our multiple threshold approach in WML is similar to methods applied previously, [22, 35] and analogous to histogram-based analyses [35, 36]. Our findings indicate varying degrees of microstructural damage across individuals at diagnosis, independent of WML load, which accords with lesion variation reported in more advanced disease across MS subtypes, [35, 37,38,39] and from other microstructure-sensitive methods [6,7,8].
Longitudinal change in T 1 and prolonged WML T 1 components
We found no significant longitudinal median T1 change in grey or white matter regions over the year following diagnosis, in line with previous serial T1 measurements in early RRMS [5]. A groupwise reduction in WML T1 over time was attributable to new or enlarging WML; when only WML voxels that were present at baseline were included, no significant T1 change was found. New WML voxels with less tissue damage developing over the follow-up period therefore appear to drive the cohort-wide WML T1 decrease, rather than remyelination. WML T1 reduction over time in early RRMS has been reported previously, [19] and this potential confound should be considered for future study design.
Marked intersubject variability in the proportions of higher WML T1 components guided our focus on WML voxels with supramedian T1 and values over 2.00 s; both aim to identify more disrupted microstructure [12]. We found that the number of thresholded T1 WML voxels increased significantly over 1 year following RRMS diagnosis, indicating increasing microstructural damage within existing WML. To our knowledge, this study is the first to examine the longitudinal evolution of prolonged T1 components, although thresholding approaches using T1 [12, 22] and normalised T1-weighted images [35] have been applied cross-sectionally in MS.
T 1 and clinical disability
Although positive associations between clinical disability and T1 within NAWM [14, 21] and WML [14] have been described previously, we did not find an association between cross-sectional median T1 measures and clinical disability. Previous studies were in mixed MS subtypes at varying disease stages however, and findings varied; [40] the earlier disease stage of our more homogeneous RRMS cohort may account for these differences.
Conversely, cross-sectional prolonged T1 and supramedian WML voxel counts were associated with EDSS score at the time of diagnosis, a relationship that persisted at 1-year follow-up. The significant increase seen in these WML components over time was also associated with EDSS worsening. This is in line with the literature, where thresholded T1 has been shown to provide disability-relevant information additional to visual assessment of ‘T1-w hypointense’ WML in more advanced disease [22]. Our data suggest that prolonged T1 WML components contribute disproportionately to clinical disability in early RRMS, which may reflect both demyelination and axonal loss [12]. Thresholded WML T1 measures may therefore provide useful disease stratification in early RRMS.
Longitudinal increases in median T1 in WML tissue present at baseline in patients with worsening disability, and decreases in those with stable or improving disability, are consistent with disability-relevant microstructural damage and remyelination, respectively. An earlier study did not find any association between a derived WML load-weighted T1 measure and 1-year change in EDSS score in RRMS, [10] which may reflect differences in cohort size, disease duration and analytical approach.
In our cohort, greater 1-year increase in median T1 in NAWM, cGM and thalamus was also associated with EDSS worsening, whereas T1 was relatively stable or decreasing in participants with stable or improving EDSS, resulting in an absence of net cohort-wide T1 change in GM or NAWM. T1 therefore captures disability-relevant demyelination that is not visible on conventional MRI, and the direction of T1 change over 1 year may indicate disability trajectory in early MS.
None of our baseline measures were predictive of EDSS worsening over the following year. High individual variance in baseline brain microstructural integrity, despite a relatively homogeneous diagnosis point, and limited 1-year clinical worsening following RRMS diagnosis may be limiting factors.
Limitations
T1 quantification may be useful as a group-level patient stratifier for targeted clinical trials; the small magnitude of changes and reproducibility of measures may, however, limit application in individual patients. VFA acquisition for T1 approximation is rapid and readily implemented, but does not correct for B1 inhomogeneities, which may reduce sensitivity to biological change and introduce spatial bias, although unlikely to substantially effect longitudinal measures. WML were segmented on 3-mm 2D FLAIR slices rather than 3D FLAIR due to better contrast resolution, which may introduce partial volume effects. Although all participants were recruited within 6 months of diagnosis, the relationship with true disease onset is variable and difficult to determine reliably; [41] diagnosis was therefore chosen as a pragmatic and measurable time point. Effects of intercurrent treatment are likely to be minimal: MRI was performed a minimum of 3 weeks post-steroid treatment; DMT status was not related to disability outcome; however, treatment variation in this cohort precludes meaningful sensitivity analysis. Innate EDSS limitations are well-recognised [42]. Moreover, 1-year change in EDSS score showed significant intersubject variability (Fig. S3); overall disability worsening was minor, and confounded by improvement following the clinical episode that initiated RRMS diagnosis. Five-year follow-up of the FutureMS cohort [23] will allow more reliable evaluation of T1 measures as predictors of disease trajectory.
Conclusion
Quantitative T1 mapping is sensitive to disability-relevant microstructural changes in the year following RRMS diagnosis. T1 thresholding approaches demonstrate heterogeneity of microstructural damage in WML; WML components with prolonged T1 increase with time, and are significantly associated with clinical disability. Widespread subtle demyelination can also be detected in NAWM and GM in patients with worsening disability. Further studies in well-characterised RRMS cohorts over longer time periods will help clarify the utility of T1 mapping as a disease stratifier, and for potential application in future therapeutic trials.
Data availability
Researchers may request access to anonymised patient data from FutureMS-1 following our standard procedures. To submit an access, request please contact future-ms@ed.ac.uk. Proposals will be reviewed and approved by the FutureMS steering committee. A signed data sharing agreement will then be issued. Data will be available as soon as possible after the first publication of the results.
Abbreviations
- cGM:
-
Cortical grey matter
- DGM:
-
Deep grey matter
- DMTs:
-
Disease-modifying therapies
- EDSS:
-
Expanded Disability Status Scale
- FDR:
-
False discovery rate
- FLAIR:
-
Fluid-attenuated inversion recovery
- GM:
-
Grey matter
- MRI:
-
Magnetic resonance imaging
- NAWM:
-
Normal-appearing white matter
- RRMS:
-
Relapsing-remitting multiple sclerosis
- T 1 :
-
Spin-lattice relaxation time
- VFA:
-
Variable flip angle
- WML:
-
White matter lesions
References
Chaudhuri A (2013) Multiple sclerosis is primarily a neurodegenerative disease. J Neural Transm. https://doi.org/10.1007/s00702-013-1080-3
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. https://doi.org/10.1016/s1474-4422(17)30470-2
Barkhof F (1999) MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS). Mult Scler J. https://doi.org/10.1177/135245859900500415
Chard D, Trip SA (2017) Resolving the clinico-radiological paradox in multiple sclerosis [version 1; peer review: 3 approved]. F1000Res. https://doi.org/10.12688/f1000research.11932.1
Davies GR, Hadjiprocopis A, Altmann DR et al (2007) Normal-appearing grey and white matter T1 abnormality in early relapsing-remitting multiple sclerosis: a longitudinal study. Mult Scler J. https://doi.org/10.1177/1352458506070726
Absinta M, Sati P, Gaitán MI et al (2013) Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol. https://doi.org/10.1002/ana.23959
Harrison DM, Li X, Liu H et al (2016) Lesion heterogeneity on high-field susceptibility MRI is associated with multiple sclerosis severity. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4726
Yao B, Bagnato F, Matsuura E et al (2012) Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology. https://doi.org/10.1148/radiol.11110601
York EN, Martin SJ, Meijboom R et al (2021) MRI-derived g-ratio and lesion severity in newly diagnosed multiple sclerosis. Brain Commun. https://doi.org/10.1093/braincomms/fcab249
Thaler C, Faizy TD, Sedlacik J et al (2017) T1 recovery is predominantly found in black holes and is associated with clinical improvement in patients with multiple sclerosis. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5004
Gowland PA, Stevenson VL (2003) T1: the longitudinal relaxation time. In: Tofts PS (ed) Quantitative MRI of the brain: measuring changes caused by disease. Chichester, West Sussex; Hoboken, N.J.: Wiley, pp 111–141
Kolb H, Absinta M, Beck ES et al (2021) 7T MRI differentiates remyelinated from demyelinated multiple sclerosis lesions. Ann Neurol. https://doi.org/10.1002/ana.26194
Truyen L, van Waesberghe JH, van Walderveen MAA et al (1996) Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. https://doi.org/10.1212/wnl.47.6.1469
Parry A, Clare S, Jenkinson M, Smith S, Palace J, Matthews PM (2002) White matter and lesion T1 relaxation times increase in parallel and correlate with disability in multiple sclerosis. J Neurol. https://doi.org/10.1007/s00415-002-0837-7
Griffin CM, Chard DT, Parker GJM, Barker GJ, Thompson AJ, Miller DH (2002) The relationship between lesion and normal appearing brain tissue abnormalities in early relapsing remitting multiple sclerosis. J Neurol. https://doi.org/10.1007/Pl00007864
Niepel G, Tench CR, Morgan PS, Evangelou N, Auer DP, Constantinescu CS (2006) Deep gray matter and fatigue in MS: a T1 relaxation time study. J Neurol. https://doi.org/10.1007/s00415-006-0128-9
Steenwijk MD, Vrenken H, Jonkman LE et al (2016) High-resolution T1-relaxation time mapping displays subtle, clinically relevant, gray matter damage in long-standing multiple sclerosis. Mult Scler J. https://doi.org/10.1177/1352458515615953
Lommers E, Simon J, Reuter G et al (2019) Multiparameter MRI quantification of microstructural tissue alterations in multiple sclerosis. Neuroimage Clin. https://doi.org/10.1016/j.nicl.2019.101879
Bonnier G, Maréchal B, Fartaria MJ et al (2017) The combined quantification and interpretation of multiple quantitative magnetic resonance imaging metrics enlightens longitudinal changes compatible with brain repair in relapsing-remitting multiple sclerosis patients. Front Neurol. https://doi.org/10.3389/fneur.2017.00506
Al-Radaideh A, Athamneh I, Alabadi H, Hbahbih M (2020) Deep gray matter changes in relapsing-remitting multiple sclerosis detected by multi-parametric, high-resolution magnetic resonance imaging (MRI). Eur Radiol. https://doi.org/10.1007/s00330-020-07199-5
West J, Aalto A, Tisell A et al (2014) Normal appearing and diffusely abnormal white matter in patients with multiple sclerosis assessed with quantitative MR. PLoS One. https://doi.org/10.1371/journal.pone.0095161
Thaler C, Faizy T, Sedlacik J et al (2015) T1- thresholds in black holes increase clinical-radiological correlation in multiple sclerosis patients. PLoS One. https://doi.org/10.1371/journal.pone.0144693
Kearns PKA, Martin SJ, Chang J et al (2022) FutureMS cohort profile: a Scottish multicentre inception cohort study of relapsing-remitting multiple sclerosis. BMJ Open. https://doi.org/10.1136/bmjopen-2021-058506
Meijboom R, Wiseman SJ, York EN et al (2022) Rationale and design of the brain magnetic resonance imaging protocol for FutureMS: a longitudinal multi-centre study of newly diagnosed patients with relapsing-remitting multiple sclerosis in Scotland. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.17731.1
Zhan T, Zhan Y, Liu Z, Xiao L, Wei Z (2015) Automatic method for white matter lesion segmentation based on T1-fluid-attenuated inversion recovery images. IET Comput Vis. https://doi.org/10.1049/iet-cvi.2014.0121
Helms G, Dathe H, Dechent P (2008) Quantitative FLASH MRI at 3T using a rational approximation of the Ernst equation. Magn Reson Med. https://doi.org/10.1002/mrm.21542
York EN, Thrippleton MJ, Waldman A (2020) Magnetisation transfer saturation (MTsat) processing, [software]. University of Edinburgh. Centre for Clinical Brain Sciences. https://doi.org/10.7488/ds/2965
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. https://doi.org/10.1016/s1053-8119(02)91132-8
York EN, Meijboom R, Thrippleton MJ et al (2022) Longitudinal microstructural MRI markers of demyelination and neurodegeneration in early relapsing-remitting multiple sclerosis: Magnetisation transfer, water diffusion and g-ratio. Neuroimage Clin. https://doi.org/10.1016/j.nicl.2022.103228
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH (2004) Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. https://doi.org/10.1002/ana.20202
Fatouros PP, Marmarou A, Kraft KA, Inao S, Schwarz FP (1991) In vivo brain water determination by T1 measurements: effect of total water content, hydration fraction, and field strength. Magn Reson Med. https://doi.org/10.1002/mrm.1910170212
MacDonald HL, Bell BA, Smith MA et al (1986) Correlation of human NMR T1 values measured in vivo and brain water content. Br J Radiol. https://doi.org/10.1259/0007-1285-59-700-355
van Walderveen MAA, Kamphorst W, Scheltens P et al (1998) Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. https://doi.org/10.1212/wnl.50.5.1282
Naval-Baudin P, Pons-Escoda A, Camins À et al (2023) Deeply 3D–T1-TFE hypointense voxels are characteristic of phase-rim lesions in multiple sclerosis. Eur Radiol. https://doi.org/10.1007/s00330-023-09784-w
Griffin CM, Dehmeshki J, Chard DT et al (2002) T1 histograms of normal-appearing brain tissue are abnormal in early relapsing-remitting multiple sclerosis. Mult Scler J. https://doi.org/10.1191/1352458502ms807oa
Ormerod IEC, Miller, DH, McDonald WI (1987) The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions: a quantitative study. Brain. https://doi.org/10.1093/brain/110.6.1579
Larsson HB, Frederiksen J, Kjaer L, Henriksen O, Olesen J (1988) In vivo determination of T1 and T2 in the brain of patients with severe but stable multiple sclerosis. Magn Reson Med. https://doi.org/10.1002/mrm.1910070106
Stevenson VL, Parker GJM, Barker GJ et al (2000) Variations in T1 and T2 relaxation times of normal appearing white matter and lesions in multiple sclerosis. J Neurol Sci. https://doi.org/10.1016/s0022-510x(00)00339-7
Pontillo G, Petracca M, Monti S et al (2023) Clinical correlates of R1 relaxometry and magnetic susceptibility changes in multiple sclerosis: a multi-parameter quantitative MRI study of brain iron and myelin. Eur Radiol. https://doi.org/10.1007/s00330-022-09154-y
Kaufmann M, Kuhle J, Puhan MA et al (2018) Factors associated with time from first-symptoms to diagnosis and treatment initiation of Multiple Sclerosis in Switzerland. Mult Scler J Exp Transl Clin. https://doi.org/10.1177/2055217318814562
van Munster CEP, Uitdehaag BMJ (2017) Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. https://doi.org/10.1007/s40263-017-0412-5
Acknowledgements
With special thanks to all participants in FutureMS and the radiographers at the RIE Edinburgh. We would also like to thank other non-author contributors of the FutureMS consortium. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Collaborators: on behalf of the FutureMS consortium
Amit Akula, Sergio Baranzini, Fiona Barret, Mark Bastin, Chris Batchelor, Emily Beswick, Fraser Brown, Tracy Brunton, Javier Carod Artal, Jessie Chang, Yingdi Chen, Shuna Colville, Peter Connick, Annette Cooper, Denise Cranley, Rachel Dakin, Baljean Dhillon, Liz Elliott, James Finlayson, Peter Foley, Stella Glasmacher, Angus Grossart, Haane Haagenrud, Katarzyna Hafezi, Emily Harrison, Adil Harroud, Sara Hathorn, Tracey Hopkins, David Hunt, Aidan Hutchison, Charlotte Jardine, Kiran Jayprakash, Matt Justin, Gwen Kennedy, Lucy Kessler, Michaela Kleynhans, Juan Larraz, Katherine Love, Dawn Lyle, James MacDonald, Niall MacDougall, Jen MacFarlane, Lesley Macfarlane, Alan Maclean, Bev MacLennan, Margaret-Ann MacLeod, Nicola Macleod, Don Mahad, Sarah-Jane Martin, Conni McCarthy, Lynn McMahon, Daisy Mollison, Ian Megson, Daisy Mollison, Mary Monaghan, Lee Murphy, Katy Murray, Judith Newton, Julian Ng Kee Kwong, Jonathan O’Riordan, David Perry, Suzanne Quigley, Adam Scotson, Scott Semple, Amy Stenson, Michaela Stuart, Christine Weaver, Stuart Webb, Belinda Weller, Nicole White, Anna Williams, Stewart Wiseman, Charis Wong, Michael Wong and Rosie Woodward.
Funding
ENY was supported by a Chief Scientist Office SPRINT MND/MS Studentship (MMPP/01) and funding from the Anne Rowling Regenerative Neurology Clinic, University of Edinburgh. MJT is funded by the NHS Lothian Research and Development Office. RM and AK are funded by the UK MS Society Edinburgh Centre for MS Research grant (grant reference 133). PKAK is supported by an ECAT-Wellcome fellowship (223058/Z/21/Z). MVH is supported by the Row Fogo Charitable Trust (BRO-D.FID3668413). FutureMS, hosted by Precision Medicine Scotland Innovation Centre (PMS-IC), was funded by a grant from the Scottish Funding Council to PMS-IC and Biogen Idec Ltd. Insurance. The study E161616 FutureMS was undertaken at the Edinburgh Imaging facility RIE (Royal Infirmary of Edinburgh), University of Edinburgh. Additional funding for the University of Edinburgh 3 T MRI Research scanner in Royal Infirmary Edinburgh is provided by Wellcome (104916/Z/14/Z), Dunhill Trust (R380R/1114), Edinburgh and Lothians Health Foundation (2012/17), Muir Maxwell Research Fund, Edinburgh Imaging and University of Edinburgh. This work is supported by the UK Dementia Research Institute which receives its funding from UK DRI Ltd., funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Adam Waldman.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained (South East Scotland Research Ethics Committee 02).
Study subjects or cohorts overlap
The current article focuses on quantitative T1 data from a sub-cohort of FutureMS participants who received extended microstructural MRI, and test-retest healthy control T1 data, which have not been reported on previously.
FutureMS studies which have been previously published:
1. Glasmacher SA, Kearns PKA, Hassan Z, et al (2022) The influence of disease-modifying therapy on hidden disability burden in people with newly diagnosed relapsing-remitting multiple sclerosis. Multiple Sclerosis and Related Disorders. https://doi.org/10.1016/j.msard.2022.103837.
2. Chang Y-T, Kearns PKA, Carson A, et al (2023) Network analysis characterizes key associations between subjective fatigue and specific depressive symptoms in early relapsing-remitting multiple sclerosis. Multiple Sclerosis and Related Disorders. https://doi.org/10.1016/j.msard.2022.104429.
3. Kearns PKA, Martin SJ, Chang J, et al (2022) FutureMS cohort profile: a Scottish multicentre inception cohort study of relapsing-remitting multiple sclerosis. BMJ Open. https://doi.org/10.1136/bmjopen-2021-058506.
4. York EN, Meijboom R, Thrippleton MJ, et al (2022) Longitudinal microstructural MRI markers of demyelination and neurodegeneration in early relapsing-remitting multiple sclerosis: magnetisation transfer, water diffusion and g-ratio. Neuroimage: Clinical. https://doi.org/10.1016/j.nicl.2022.103228.
5. York EN, Martin SJ, Meijboom R, et al (2021) MRI-derived g-ratio and lesion severity in newly diagnosed multiple sclerosis. Brain Commun. https://doi.org/10.1093/braincomms/fcab249.
6. Ng Kee Kwong KC, Mollison D, Meijboom R, et al (2022) Rim lesions are demonstrated in early relapsing-remitting multiple sclerosis using 3 T-based susceptibility-weighted imaging in a multi-institutional setting. Neuroradiology. https://doi.org/10.1007/s00234-021-02768-x.
7. Chen Y, Larraz J, Wong M, et al (2022) Longitudinal retinal imaging study of newly diagnosed relapsing-remitting multiple sclerosis in Scottish population: baseline and 12 months follow-up profile of FutureMS retinal imaging cohort. BMJ Open Ophthalmology. https://doi.org/10.1136/bmjophth-2022-001024.
8. Meijboom R, York EN, Kampaite A, et al Patterns of brain atrophy in recently-diagnosed relapsing-remitting multiple sclerosis. PLoS One. 2023;18(7):e0288967. Published 2023 Jul 28. https://doi.org/10.1371/journal.pone.0288967
Methodology
-
• prospective
-
• observational
-
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harper, J.G., York, E.N., Meijboom, R. et al. Quantitative T1 brain mapping in early relapsing-remitting multiple sclerosis: longitudinal changes, lesion heterogeneity and disability. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10351-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10351-6