Abstract
Objectives
A published tumour regression grade (TRG) score for squamous anal carcinoma treated with definitive chemoradiotherapy based on T2-weighted MRI yields a high proportion of indeterminate responses (TRG-3). We investigate whether the addition of diffusion-weighted imaging (DWI) improves tumour response assessment in the early post treatment period.
Materials and methods
This retrospective observational study included squamous anal carcinoma patients undergoing MRI before and within 3 months of completing chemoradiotherapy from 2009 to 2020. Four independent radiologists (1–20 years’ experience) scored MRI studies using a 5-point TRG system (1 = complete response; 5 = no response) based on T2-weighted sequences alone, and then after a 12-week washout period, using a 5-point DWI-TRG system based on T2-weighted and DWI. Scoring confidence was recorded on a 5-point scale (1 = low; 5 = high) for each reading and compared using the Wilcoxon test. Indeterminate scores (TRG-3) from each reading session were compared using the McNemar test. Interobserver agreement was assessed using kappa statistics.
Results
Eighty-five patients were included (mean age, 59 years ± 12 [SD]; 55 women). T2-weighted TRG-3 scores from all readers combined halved from 24% (82/340) to 12% (41/340) with DWI (p < 0.001). TRG-3 scores changed most frequently (41%, 34/82) to DWI-TRG-2 (excellent response). Complete tumour response was recorded clinically in 77/85 patients (91%). Scoring confidence increased using DWI (p < 0.001), with scores of 4 or 5 in 84% (287/340). Interobserver agreement remained fair to moderate (kappa range, 0.28–0.58).
Conclusion
DWI complements T2-weighted MRI by reducing the number of indeterminate tumour responses (TRG-3). DWI increases radiologist’s scoring confidence.
Clinical relevance statement
Diffusion-weighted imaging improves T2-weighted tumour response assessment in squamous anal cancer, halving the number of indeterminate responses in the early post treatment period, and increases radiologists’ confidence.
Key Points
-
Tumour response based on T2-weighted MRI is often indeterminate in squamous anal carcinoma.
-
Diffusion-weighted imaging alongside T2-weighted MRI halved indeterminate tumour regression grade scores assigned by four radiologists from 24 to 12%.
-
Scoring confidence of expert and non-expert radiologists increased with the inclusion of diffusion-weighted imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous carcinoma of the anal canal is on the rise worldwide, with an annual incidence of 0.5–2 in 100,000 [1]. Definitive radiotherapy with concomitant mitomycin C and 5-fluorouracil (or capecitabine) is the therapy of choice for localised disease, with good outcomes [2, 3]. Timely identification of locoregional treatment failure, occurring in a minority of cases (11–14%) [4, 5], allows these patients to be considered for salvage surgery, which in turn leads to local pelvic control in approximately 60% of cases and to a 5-year survival rate of 30–60% [2]. Early detection of salvageable local disease relapse during imaging response assessment and surveillance is key.
MRI is recommended for locoregional staging and response assessment [2, 3, 6, 7], and has a growing role in radiotherapy planning [8]. High-resolution T2-weighted sequences are typically obtained in planes parallel and perpendicular to the anal canal. MRI tumour response assessment based on T2-weighted sequences can be challenging in the early post treatment period, due to the overlapping features between therapy-induced inflammation (hyperintense tissue oedema mixed with hypointense fibrosis, anatomical distortion) and residual tumour, typically intermediate in signal [9]. A 5-point MRI tumour regression grade (TRG) system based on T2-weighted sequences has been utilised to classify squamous anal carcinoma tumour response to chemoradiotherapy [10]. In a prospective single-centre cohort, the number of indeterminate TRG scores (TRG-3) was considerable in the early post treatment period, corresponding to 58% of the total at 3 months and to 26% of the total at 6 months post chemoradiotherapy, emphasising the problem posed by treatment-related inflammation.
Diffusion-weighted imaging (DWI) is used routinely to aid the assessment of a variety of abdominal malignancies [11, 12]. Specifically, in rectal cancer treated with neoadjuvant chemoradiotherapy, DWI increases diagnostic accuracy in the evaluation of complete response [13] and detection of small-volume residual tumour before endoscopy [14]. We hypothesised that DWI could benefit early tumour response evaluation in squamous anal carcinoma and improve the diagnostic confidence of non-expert radiologists. Thus, the primary aim of this study was to determine whether DWI improves tumour response assessment by reducing indeterminate responses (TRG-3) in the early post treatment period. Secondary aims were to assess the impact of DWI on subjective TRG scoring confidence and interobserver agreement amongst expert and non-expert radiologists.
Materials and methods
Patients
Institutional board waiver of informed consent was obtained for this retrospective study of consecutive MRI data obtained as part of the standard care pathway. Patients with biopsy-proven squamous cell carcinoma of the anal canal undergoing treatment with definitive chemoradiotherapy between February 2009 and May 2020 were identified from the Picture Archiving and Communication System (PACS) and electronic patient record (EPR) of a tertiary care institution (Guy’s and St Thomas’ NHS Foundation Trust). Inclusion criteria were baseline and post treatment MRI (within 3 months of treatment completion) available from PACS; TNM 8th ed. T2 stage; or greater tumours [15], equivalent to tumour diameter >2 cm. Exclusion criteria were absence of DWI on baseline or post treatment MRI; DWI of insufficient diagnostic quality; no visible tumour on baseline MRI; and prior tumour surgical excision. The patient flowchart is shown in Fig. 1.
Treatment and clinical follow-up
Radiotherapy was delivered to a mean dose of 50.86 Gy (range 50.4–54 Gy) using a linear accelerator (Elekta or Varian) applying a 3D conformal or intensity-modulated technique. Concomitant chemotherapy consisted of mitomycin C (12 mg/m2 on day 1) with either 5-fluorouracil (1000 mg/m2/day, continuous venous infusion, on days 1–4 and 29–32) or capecitabine orally (825 mg/m2 twice a day on radiation days).
Following completion of chemoradiotherapy, patients were clinically assessed at 8–10 weeks, then every 3 months for the first 2 years, and every 6 months afterwards as per standard institutional practice. Endoscopic evaluation ± MRI/CT was undertaken if canal ± locoregional/distant recurrence was suspected clinically.
MRI acquisition
Patients were scanned supine on one of five 1.5- or 3.0-Tesla MRI scanners (Magnetom Avanto, Aera or Skyra, Siemens Healthineers) using an 18-channel pelvic phased array coil. The examination protocol included axial and sagittal T2-weighted turbo spin-echo (TSE) sequences covering the whole pelvis, and high-resolution small-field-of-view T2-weighted TSE sequences perpendicular and parallel to the anal canal. DWI consisted of a single-shot spin-echo echo planar imaging (EPI) axial diffusion-weighted sequence encompassing the pelvis with three b-values (0, 100, 800 s/mm2). Apparent diffusion coefficient (ADC0-800) maps and calculated high b-value images (b = 1400 or 1600 s/mm2) were created automatically at the time of acquisition. Patients did not undergo any additional preparation prior to the examination. Typical acquisition parameters are summarised in Supplemental Table 1.
Image analysis
Baseline and post treatment MRI were evaluated sequentially by four independent observers, blinded to clinical outcome: two senior radiology residents (K.M., A.A.), with 1 year’s experience in oncologic pelvic MRI but no specific experience in staging squamous anal carcinoma (referred to as ‘non-expert observers’) and two subspecialist radiologists (D.P., V.G.) with over 10 years’ experience (‘expert observers’).
Anonymised scans were presented in a randomised order. Post treatment MRIs were assessed next to baseline MRIs on dual monitors (Sectra IDS7 workstations, Sectra AB). The first reading session was based on multiplanar T2-weighted sequences alone. Tumour response was evaluated according to a previously published 5-point tumour regression grade (TRG) score [10], outlined in Table 1. In addition to TRG, the following primary tumour characteristics were recorded: size (maximum diameter in any plane); location (lower canal, mid canal, upper canal/anorectum); invasion of adjacent structures (prostate, vagina, ischioanal fossa). The second reading session, separated from the first by a 12-week wash out period, included T2-weighted and DWI assessed in conjunction. Multiplanar T2 sequences, acquired/calculated b-value images and corresponding ADC maps were displayed simultaneously. DWI image quality was scored subjectively as inadequate, adequate or good by each observer, documenting the nature of image degradation as free text, when present. Tumour response was re-scored according to a modified DWI-TRG system, outlined in Table 1. Post treatment DWI images were regarded positive for residual disease when diffusion restriction (signal hyperintensity on high b-value images matched by hypointensity on ADC map) remained present at the site of the tumour, excluding linear diffusion restriction spatially matched to anorectal mucosa. A single-slice, free-hand region of interest was drawn around the tumour on ADC maps, with reference to the corresponding DWI and T2-weighted images, and the mean tumour ADC value recorded for baseline MRI. During both reading sessions, observers scored their subjective confidence in assessing tumour response on a scale of 1 (low) to 5 (high).
Statistical analysis
Statistical analyses were performed by a senior statistician (P.B.) using Stata (v15.1; StataCorp LP). Normally distributed variables were expressed as mean ± standard deviation. Categorical variables were expressed as absolute numbers and their percentages. The McNemar test was used to compare the number of indeterminate TRG vs. other TRG scores between reading sessions. Interobserver agreement was assessed using the kappa statistics (kappa < 0.21 = poor agreement; 0.21–0.40 = fair; 0.41–0.60 = moderate; 0.61–0.80 = good; > 0.80 = excellent). Kappa values and their standard errors were used to perform a z-test to compare the level of agreement between reading sessions. The Wilcoxon matched-pairs test was used to compare observer confidence scores. Analyses were performed for each observer separately, and for all observers combined. A p value <.05 was taken to represent statistical significance.
Results
Patients and clinical response
Baseline patient characteristics are summarised in Table 2. The final cohort consisted of 85 patients, ranging in age between 34 and 86 years (mean, 59 years ± 12 [SD]; 55 women). Mean tumour size was 5.1 ± 2.1 cm. A large proportion of patients had locally advanced disease at baseline (53%, 45/85), defined as T3 stage or greater, and/or tumours located in the upper canal/anorectum (52%, 44/85). Mean clinical follow-up duration was 32 ± 18 months. Clinical disease recurrence was recorded in 36% (31/85) of patients: local, in 18% (15); nodal, in 9% (8); metastatic, in 14% (12). Clinical complete tumour response was recorded in 91% (77/85) of patients at 8–10 weeks from the end of treatment. There was partial response in 6% (5/85). Progressive disease was recorded in 4% (3/85). Local recurrence was documented in 8% (7/85) of patients: 5% (4), corresponding to late recurrence, beyond 12 months from the end of treatment; 4% (3), corresponding to microscopic subclinical recurrence at 6–9 months from the end of treatment. Four patients underwent salvage surgery by means of abdominoperineal excision of the rectum.
MRI and tumour regression grade
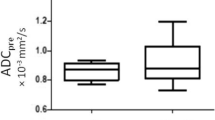
DWI image quality was scored as inadequate in 3 cases, which were excluded; adequate in 26% (22/85); and good in 74% (63/85). The most common problems affecting DWI quality were low signal-to-noise ratio and susceptibility artefacts, particularly from air/gas at the anal margin and in the rectal lumen. Calculated b-value (b1400 or b1600 s/mm2) images were available in 70/85 (82%) cases. Baseline tumour conspicuity on b800 images and ADC maps was high in all included cases, with mean tumour ADC values of 0.910 ± 0.182 × 10−3 mm2/s.
TRG scores from the four radiologists are shown in Fig. 2. With the inclusion of DWI, the number of indeterminate TRG-3 scores decreased significantly for three of the four radiologists examined individually (difference range, 11–19%; p range, < 0.001–0.04), and for all radiologists combined (difference, 12%; p < 0.001) (Table 3). For all observers combined, the number of TRG-3 cases halved from 24% (82/340) of the total based on T2-weighted MRI alone to 12% (41/340) based on T2-weighted plus DWI. Indeterminate TRG-3 scores changed most frequently to DWI-TRG-2 (41%, 34/82), corresponding to excellent response (Figs. 3 and 4); 9% (7/82) changed to DWI-TRG-4, corresponding to minimal response (Fig. 5). The remaining 50% of TRG-3 (41/82) corresponded to indeterminate DWI-TRG-3 scores (Fig. 6).
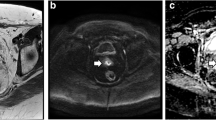
Images in a 57-year-old man with squamous anal carcinoma. T2-weighted MRI (right column), high b-value DWI (middle column) and DWI ADC map (left column). At baseline MRI, tumour staged as T4 invades the pelvic sidewall and prostate (upper row). After treatment (lower row), a region of indeterminate intermediate T2 signal in the lower rectal wall (TRG-3) does not correspond to restricted diffusion (arrows); linear diffusion restriction is spatially matched to anorectal mucosa (DWI-TRG-2)
Images in a 53-year-old woman with squamous anal carcinoma. T2-weighted MRI (right column), high b-value DWI (middle column) and DWI ADC map (left column). At baseline MRI, tumour of the lower canal staged as T2 (upper row). Persistence of intermediate T2 signal (TRG-3) at the site of tumour (arrows) does not correspond to restricted diffusion, in keeping with excellent response (DWI-TRG-2)
Images in a 41-year-old woman with squamous anal carcinoma. T2-weighted MRI (right column), high b-value DWI (middle column) and DWI ADC map (left column). At baseline MRI, tumour staged as T4 invades the vagina posteriorly and left pelvic sidewall anteriorly (upper row). After treatment (lower row), the presence of both linear and nodular diffusion restriction within a cavity left by tumour shrinkage, not spatially matched to anorectal mucosa (arrows), was deemed indeterminate (TRG-3 and DWI-TRG-3) by most observers. Complete response was recorded clinically
Images in a 64-year-old woman with squamous anal carcinoma. T2-weighted MRI (right column), high b-value DWI (middle column) and DWI ADC map (left column). At baseline MRI, tumour of the lower canal staged as T3 (upper row). After treatment (lower row), a small nodule of restricted diffusion in the 7 o’clock position (arrow) lies within a broader area of indeterminate intermediate T2 signal (TRG-3), confirming residual disease (DWI-TRG-4). Incomplete response was recorded clinically
Subjective TRG scoring confidence
Observers’ confidence in assessing response increased with the addition of DWI. Scores were higher for each of the four observers (p < 0.001), and for all observers combined (Supplemental Table 2). For all observers combined, 84% (287/340) of confidence scores were 4 or 5 for T2-weighted plus DWI, compared to 55% (188/340) for T2-weighted MRI alone.
Interobserver agreement
Interobserver agreement was between fair and moderate (Supplemental Table 3), with kappa values ranging between 0.28 and 0.58. The highest agreement was achieved by non-expert observers assessing response on T2-weighted plus DWI. No significant differences in interobserver agreement were found between the two response assessment methods (p = 0.16–0.40).
Correspondence between MRI TRG and clinical tumour response
Correspondence between MRI TRG scores and clinical tumour response is summarised in Table 4. Patients with complete clinical response (n = 77) were assigned TRG scores of 1 or 2 in 88% (270/308) of cases using T2-weighted plus DWI, versus 73% (226/308) based on T2-weighted MRI alone. Patients with partial clinical response (n = 5) were assigned scores of 3 or above in 75% (15/20) and 65% (13/20) of cases respectively. Eleven out of 12 TRG scores in 3 patients with progressive disease were ‘4’ or ‘5’. In 7 patients with documented local recurrence after an initial complete response, 64% (18/28) TRG-2 and 93% (26/28) DWI-TRG-2 scores were recorded, in line with complete responders without subsequent recurrence.
Discussion
Assessing tumour response in the initial period following chemoradiotherapy can be challenging in patients with squamous anal carcinoma. An MRI tumour regression grade (TRG) system based on multiplanar T2-weighted sequences alone has been proposed to standardise assessment, yielding over 50% of indeterminate TRG-3 responses at 3 months from the end of treatment [10]. In our study, we found that DWI as a complement to T2-weighted sequences improved early MRI response assessment (1–3 months post treatment) by halving the total number of indeterminate responses from 24 to 12%. Qualitative evaluation of DWI signal changes, specifically the resolution of tumour hyperintensity on high b-value images, increased the subjective TRG scoring confidence of both expert and non-expert observers. Indeterminate TRG-3 scores changed most frequently (41%) to DWI-TRG-2, corresponding to excellent response. TRG-3 scores changed to DWI-TRG-4 in 9% of cases, indicating minimal response/residual disease, potentially allowing earlier consideration of salvage surgery, associated with favourable 5-year survival rates as high as 64% [16].
To our knowledge, external validation of the previously proposed TRG system for squamous anal carcinoma has yet to be undertaken. Our findings highlight the potential for DWI to provide early reassurance on the presence of a favourable response to definitive chemoradiotherapy, and to lower the number of patients referred for examination under anaesthesia and biopsy, given the majority were downgraded to DWI-TRG-2.
The value of DWI has already been demonstrated in rectal adenocarcinoma, where active surveillance may be considered for complete responders following neoadjuvant chemoradiotherapy [17]. DWI has higher sensitivity in restaging versus T2-weighted MRI alone (84% vs. 50%) [18]. As observed in our study, DWI improves performance by differentiating post-radiation fibrosis from viable tumour [13, 19, 20]. In rectal adenocarcinoma, active surveillance with DWI is now an alternative to surgery following neoadjuvant therapy with complete response [14].
Digital rectal examination has traditionally been the mainstay for determining complete local response in squamous anal carcinoma, and there is ongoing debate as to the benefit of imaging versus clinical evaluation. Treatment-related oedema and/or fibrosis can be difficult to distinguish from persistent active disease clinically. Treatment-related effects may even complicate the interpretation of post treatment biopsies. Proximal anorectal squamous carcinomas and locally advanced tumours represent a further challenge for clinical assessment, as their extent may not be fully appreciable by rectal examination, even under general anaesthesia [3]. It remains accepted that it may take up to 6 months for complete tumour resolution to occur. In the ACT II trial, the optimum time to assess complete response was reported as 26 weeks based on digital rectal examination and abdominopelvic CT [21]. Our findings suggest that T2-weighted MRI plus DWI may allow for earlier evaluation.
In a previous study by Kochhar et al [10], a high number of indeterminate TRG-3 scores were found in the early post treatment period (3 months post chemoradiotherapy) based on T2-weighted sequences alone, corresponding to 58% of the total. In our study, this proportion was lower, corresponding to 24% of the total based on T2-weighted sequences alone. Such difference highlights a variability in local practice, even between large-volume centres, and emphasises the need for consensus radiological guidelines for tumour response assessment.
Interobserver agreement amongst the four observers was only fair to moderate, with kappa values ranging between 0.28 and 0.58. No significant improvement in agreement was found by combining T2-weighted sequences with DWI. Importantly, non-expert observers did not show less agreement that might suggest a difficulty interpreting DWI. On the contrary, they reached the highest agreement assessing response on T2-weighted plus DWI.
A 5-point TRG system may be redundant for squamous carcinoma treated with chemoradiotherapy: only 12 patients were classified as having a TRG score of 1 or 5, as currently defined. In the study by Kochhar et al [10], no patients were scored as TRG-5 and only 2/74 patients were scored as TRG-1. A modified 3-point TRG incorporating DWI may be worth assessing in future prospective studies, as proposed for rectal cancer [22].
The value of DWI in SCCA has been investigated to date in a small number of studies, assessing its role in tumour volumetry and staging [23], and its predictive and prognostic value [24, 25]. Non-specialist or non-expert radiologists should familiarise themselves with the common interpretation pitfalls associated with DWI [26,27,28]. In line with previous publications, the most common problems affecting DWI quality in our study were low signal-to-noise ratio and susceptibility artefacts from air/gas at the anal margin and in the rectal lumen. All observers in our study acknowledged the value of calculated high b-value images in terms of T2 shine-through reduction [29].
We acknowledge several limitations. First, given the retrospective nature of our study, selection bias might have affected our results. Complex and advanced cases are referred to our tertiary surgical oncology centre. Likely because of this, a high proportion (53%) of locally advanced tumours was included in our sample. Second, due to the definitive nature of chemoradiotherapy and the high number of complete responders, it was not possible to correlate imaging with tumour histopathology after treatment in the majority of cases. Third, interpretation of post treatment MRI was particularly challenging, due to the presence of marked anatomical distortion, tumour cavities and fistulous tracts. Fourth, minor variations in the imaging acquisition across multiple 1.5- and 3.0-Tesla scanners could not be avoided. There was also some variability in the timing of post treatment MRI, which ranged between 1 and 3 months. Fifth, unlike Kochhar et al previously, we performed response MRI at a single early time point (1–3 months), instead of two time points (3 and 6 months). It is difficult to speculate how many of the TRG-3 would have resolved to TRG-2 on T2-weighted sequences alone at 6 months. Finally, our response assessment methods did not consider regional nodal response, an important prognostic factor for squamous anal carcinoma patients [3]. Combined local and regional nodal response assessment using both FDG PET-CT and MRI (including DWI) 3 months after chemoradiotherapy with curative intent was found to be the strongest predictor of patient outcome by Adusumilli et al in a single-centre series of 75 patients [30].
It must be stressed that the predictive and prognostic value of MRI response assessment against clinical reference standards remains to be proven from large prospective series in squamous anal carcinomas [9, 10]. Any conclusion regarding the predictive or prognostic value of DWI over T2-weighted sequences is beyond the scope of this study. We found no obvious correlation between MRI TRG and the onset of local recurrence after initial response.
In summary, the inclusion of DWI alongside T2-weighted MRI increases diagnostic confidence and improves early tumour response assessment in squamous anal carcinoma, by reducing the number of indeterminate responses following chemoradiotherapy.
Change history
28 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00330-023-10115-2
Abbreviations
- CR:
-
Complete response
- DWI:
-
Diffusion-weighted imaging
- LRec:
-
Local recurrence
- PD:
-
Progressive disease
- PR:
-
Partial response
- TRG:
-
Tumour regression grade
References
Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A (2017) International trends in anal cancer incidence rates. Int J Epidemiol 46:924-938
Glynne-Jones R, Nilsson PJ, Aschele C et al (2014) Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol 111:330-339
Rao S, Guren MG, Khan K et al (2021) Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 32:1087-1100
de Meric de Bellefon M, Lemanski C, Castan F et al (2020) Long-term follow-up experience in anal canal cancer treated with intensity-modulated radiation therapy: clinical outcomes, patterns of relapse and predictors of failure. Radiother Oncol 144:141-147
Shakir R, Adams R, Cooper R et al (2020) Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 106:329-339
Gourtsoyianni S, Goh V (2014) MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging 39:2-17
NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma (2023) Available via https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf. Accessed 6 Mar 2023
Bird D, Henry AM, Sebag-Montefiore D, Buckley DL, Al-Qaisieh B, Speight R (2019) A systematic review of the clinical implementation of pelvic magnetic resonance imaging-only planning for external beam radiation therapy. Int J Radiat Oncol Biol Phys 105:479-492
Goh V, Gollub FK, Liaw J et al (2010) Magnetic resonance imaging assessment of squamous cell carcinoma of the anal canal before and after chemoradiation: can MRI predict for eventual clinical outcome? Int J Radiat Oncol Biol Phys 78:715-721
Kochhar R, Renehan AG, Mullan D, Chakrabarty B, Saunders MP, Carrington BM (2017) The assessment of local response using magnetic resonance imaging at 3- and 6-month post chemoradiotherapy in patients with anal cancer. Eur Radiol 27:607-617
Wagner M, Ronot M, Doblas S et al (2016) Assessment of the residual tumour of colorectal liver metastases after chemotherapy: diffusion-weighted MR magnetic resonance imaging in the peripheral and entire tumour. Eur Radiol 26:206-215
Bharwani N, Miquel ME, Powles T et al (2014) Diffusion-weighted and multiphase contrast-enhanced MRI as surrogate markers of response to neoadjuvant sunitinib in metastatic renal cell carcinoma. Br J Cancer 110:616-624
Kim SH, Lee JM, Hong SH et al (2009) Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 253:116-125
Gollub MJ, Das JP, Bates DDB et al (2021) Rectal cancer with complete endoscopic response after neoadjuvant therapy: what is the meaning of a positive MRI? Eur Radiol 31:4731-4738
Amin MB, Edge SB, American Joint Committee on Cancer (2016) AJCC Cancer Staging Manual, 8th Ed. Springer International Publishing ISBN 9783319406176
Harris DA, Williamson J, Davies M, Evans MD, Drew P, Beynon J (2013) Outcome of salvage surgery for anal squamous cell carcinoma. Colorectal Dis 15:968–973
van der Valk MJM, Hilling DE, Bastiaannet E et al (2018) Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391:2537-2545
van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S (2013) Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 269:101-112
Maas M, Lambregts DM, Nelemans PJ et al (2015) Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol 22:3873-3880
Sassen S, de Booij M, Sosef M et al (2013) Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol 23:3440-3449
Glynne-Jones R, Sebag-Montefiore D, Meadows HM et al (2017) Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): a post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol 18:347-356
Lee MA, Cho SH, Seo AN et al (2017) Modified 3-point MRI-based tumor regression grade incorporating DWI for locally advanced rectal cancer. AJR Am J Roentgenol 209:1247-1255
Prezzi D, Mandegaran R, Gourtsoyianni S et al (2018) The impact of MRI sequence on tumour staging and gross tumour volume delineation in squamous cell carcinoma of the anal canal. Eur Radiol 28:1512-1519
Muirhead R, Bulte D, Cooke R et al (2020) A prospective study of diffusion-weighted magnetic resonance imaging as an early prognostic biomarker in chemoradiotherapy in squamous cell carcinomas of the anus. Clin Oncol 32:874-883
Owczarczyk K, Prezzi D, Cascino M et al (2019) MRI heterogeneity analysis for prediction of recurrence and disease free survival in anal cancer. Radiother Oncol 134:119-126
van Griethuysen JJM, Bus EM, Hauptmann M et al (2018) Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - effect of applying a micro-enema to improve image quality. Eur J Radiol 99:131-137
Min LA, Vacher YJL, Dewit L et al (2020) Gross tumour volume delineation in anal cancer on T2-weighted and diffusion-weighted MRI - reproducibility between radiologists and radiation oncologists and impact of reader experience level and DWI image quality. Radiother Oncol 150:81-88
Lambregts DMJ, van Heeswijk MM, Delli Pizzi A et al (2017) Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching. Eur Radiol 27:4445-4454
Bates DDB, Golia Pernicka JS, Fuqua JL et al (2020) Diagnostic accuracy of b800 and b1500 DWI-MRI of the pelvis to detect residual rectal adenocarcinoma: a multi-reader study. Abdom Radiol (NY) 45:293-300
Adusumilli, P., Elsayed, N., Theophanous, S. et al. Combined PET-CT and MRI for response evaluation in patients with squamous cell anal carcinoma treated with curative-intent chemoradiotherapy. Eur Radiol 32, 5086–5096 (2022). https://doi.org/10.1007/s00330-022-08648-z
Acknowledgements
The authors acknowledge funding support from the Wellcome/Engineering and Physical Sciences Research Council Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z); Cancer Research UK National Cancer Imaging Translational Accelerator Award (C4278/A27066); UK Research & Innovation London Medical Imaging and Artificial Intelligence Centre; and National Institute for Health Research Biomedical Research Centre at Guy’s & St Thomas’ Hospitals and King’s College London.
Funding
The authors state that this work has not received any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Davide Prezzi.
Conflict of interest
Prof Vicky Goh has received research funding for a PhD student from Siemens Healthineers, unrelated to this study. The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Mr Paul Bassett kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects (n=40) have been previously reported in ‘Owczarczyk K, Prezzi D, Cascino M et al (2019) MRI heterogeneity analysis for prediction of recurrence and disease free survival in anal cancer. Radiother Oncol 134:119-126’. The study’s aims differed entirely from those of this manuscript and there is no overlap of results or conclusions.
Methodology
-
retrospective
-
observational
-
performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Reference 30 was corrected from “Lambregts DM, Rao SX, Sassen S, Martens MH et al (2014) MRI and difusion-weighted MRI volumetry for identifcation of complete tumor responders after preoperative chemoradiotherapy in patients with rectal cancer: a bi-institutional validation study. Ann Surg 262:1034-1039” to: “Adusumilli, P., Elsayed, N., Theophanous, S. et al. Combined PET-CT and MRI for response evaluation in patients with squamous cell anal carcinoma treated with curative-intent chemoradiotherapy. Eur Radiol 32, 5086–5096 (2022). https://doi.org/10.1007/s00330-022-08648-z”
Supplementary information
ESM 1
(PDF 160 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prezzi, D., Muthuswamy, K., Amlani, A. et al. Diffusion-weighted imaging complements T2-weighted MRI for tumour response assessment in squamous anal carcinoma. Eur Radiol 33, 7575–7584 (2023). https://doi.org/10.1007/s00330-023-09942-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09942-0