Abstract
Objective
To evaluate the image quality of an ultra-low contrast medium and radiation dose CT pulmonary angiography (CTPA) protocol for the diagnosis of acute pulmonary embolism using a clinical photon-counting detector (PCD) CT system and compare its performance to a dual-energy-(DE)-CTPA protocol on a conventional energy-integrating detector (EID) CT system.
Methods
Sixty-four patients either underwent CTPA with the novel scan protocol on the PCD-CT scanner (32 patients, 25 mL, CTDIvol 2.5 mGy·cm) or conventional DE-CTPA on a third-generation dual-source EID-CT (32 patients, 50 mL, CTDIvol 5.1 mGy·cm). Pulmonary artery CT attenuation, signal-to-noise ratio, and contrast-to-noise-ratio were assessed as objective criteria of image quality, while subjective ratings of four radiologists were compared at 60 keV using virtual monoenergetic imaging and polychromatic standard reconstructions. Interrater reliability was determined by means of the intraclass correlation coefficient (ICC). Effective dose was compared between patient cohorts.
Results
Subjective image quality was deemed superior by all four reviewers for 60-keV PCD scans (excellent or good ratings in 93.8% of PCD vs. 84.4% of 60 keV EID scans, ICC = 0.72). No examinations on either system were considered “non-diagnostic.” Objective image quality parameters were significantly higher in the EID group (mostly p < 0.001), both in the polychromatic reconstructions and at 60 keV. The ED (1.4 vs. 3.3 mSv) was significantly lower in the PCD cohort (p < 0.001).
Conclusions
PCD-CTPA allows for considerable reduction of contrast medium and radiation dose in the diagnosis of acute pulmonary embolism, while maintaining good to excellent image quality compared to conventional EID-CTPA.
Clinical relevance statement
Clinical PCD-CT allows for spectral assessment of pulmonary vasculature with high scan speed, which is beneficial in patients with suspected pulmonary embolism, frequently presenting with dyspnea. Simultaneously PCD-CT enables substantial reduction of contrast medium and radiation dose.
Key Points
• The clinical photon-counting detector CT scanner used in this study allows for high-pitch multi-energy acquisitions.
• Photon-counting computed tomography allows for considerable reduction of contrast medium and radiation dose in the diagnosis of acute pulmonary embolism.
• Subjective image quality was rated best for 60-keV photon-counting scans.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a frequent and potentially lethal condition. Timely diagnosis and therapy is crucial for optimizing the clinical outcome. In the first decade of the twenty-first century, computed tomography pulmonary angiography (CTPA) has become the reference standard in the diagnostic workup for suspected acute PE due to high sensitivity, ubiquitous availability, and short acquisition time [1,2,3,4]. In recent years, dual-energy (DE) CT has proven to be of incremental value in the diagnosis of PE by offering additional spectral information and reducing the required radiation dose [5, 6]. Even further functional information is gained via various post-processing applications that exploit the fact that elements with high atomic numbers like iodine (n = 53) possess different absorption characteristics at varying energy levels [7, 8]. In the imaging of PE, two post-processing applications are of particular significance: visualization of the pulmonary perfusion in color-coded iodine-distribution-maps [9] and virtual monoenergetic imaging (VMI), which means that images are reconstructed at a virtual monoenergetic level (keV). Especially monoenergetic low-keV images have been shown to improve image quality in low-contrast settings [10]. In the past, various technical approaches for DE-CT imaging have been developed, where as technical commonality all of them are based on an energy-integrating detector (EID) [11].
With the introduction of the first clinical photon-counting detector (PCD) CT, a novel multi-energy-CT approach has recently become available [12]. In directly converting PCD-CT, the specific energy level of every individual incoming photon is measured [13]. Among the most important advantages of the PCD technology are the suppression of electronic noise below predefined thresholds, higher spatial resolution with preserved dose efficiency, and full spectral information even in high-pitch scans. Notably, the impact of these theoretical advantages on iodine contrast and radiation dose requirements in patients with pulmonary embolism has not been evaluated to date.
Therefore, the aim of this study was to assess the image quality of a high-pitch PCD-CTPA scan protocol with ultra-low contrast medium and radiation dose for the diagnosis of acute pulmonary embolism and compare its performance to an established DE-CTPA protocol on a third-generation dual-source CT scanner.
Material and methods
Patient population and study design
The local institutional review board approved this study and waived the need for written informed individual consent. In this retrospective investigation, a total of 64 patients who underwent CTPA between October 2021 and March 2022 for diagnostic workup of suspected acute pulmonary embolism were included. Of those, 32 individuals received their examinations on a clinical PCD-CT scanner (Naeotom Alpha, Siemens Healthcare GmbH), whereas the other 32 CTPAs were performed on an EID-based dual-source DE-CT system (Somatom Force, Siemens Healthcare GmbH). For every patient, age, gender, height, and weight, as well as lateral and anterior–posterior chest diameter were documented. In addition, body mass indices and effective thoracic diameter were calculated. Detailed information regarding the study population are summarized in Table 1.
CT scan protocols
PCD-CT scans were performed on a first-generation clinical PCD-CT scanner that employs dual-source beam geometry and is equipped with two cadmium-telluride photon-counting detectors (QuantaMax, Siemens Healthcare GmbH) offering a 50-cm field-of-view (FOV; primary PCD array). EID scans were performed on a third-generation 192-slice dual-source CT scanner equipped with latest EID technology (Stellar, Siemens Healthcare GmbH) offering a 35.3-cm FOV in dual-energy mode.
In the patient group examined on the PCD-CT system, acquisition parameters were determined to achieve approximately half the radiation dose of our well-established dual-energy CTPA acquisition protocol. Tube potential was 120 kV using the “QuantumPlus” scan mode in a high-pitch setting (pitch of 2.0) with an image quality setting of “BQ 50.” In the EID group, tube potential was set to 90 kV/Sn 150 kV with a reference tube current–time product of 60/46 mAs which represents our dual-energy acquisition protocol in daily routine. The helical pitch factor was set to 0.55 for DE image acquisition. Automated tube-current modulation (CARE Dose 4D, Siemens Healthcare GmbH) was applied for all examinations in both patient groups.
Before each scan, a predefined amount of iodinated contrast medium was applied (Imeron® 350, Bracco) via an antecubital vein using an automated injector. In the EID group, 50 mL of contrast medium was administered with a flow rate of 4 mL/s, corresponding to an iodine delivery rate of 1400 mg/s [14, 15]. Based on recent studies discussing a potential for contrast medium dose savings in PCD-CT by up to 50% while still obtaining diagnostic attenuation [13, 16], we aimed for a significant reduction in terms of halving the dose of our standard dual-energy acquisition protocol. In doing so, we approached the lower limit of 25 mL step by step, starting from our standard dose (supplementary figure). Due to shorter acquisition time and a smaller bolus of contrast medium, we adapted flow rate and trigger threshold of our injection protocol to ensure a sufficient bolus length. As a result in the PCD group, 25 mL was injected with an iodine delivery rate of 875 mg/s as an “ultra-low contrast medium approach.” Detailed parameters of the applied CT scan protocols are provided in Table 2.
For study purposes, two stacks of axial images (3-mm slice thickness) were reconstructed for each examination: (1) polychromatic low-energy threshold 120-kV images referred to as “T3D” by the vendor for PCD-CT or blended series mimicking a single-energy 120-kV scan for EID-CT and (2) monoenergetic image series at 60 keV for both detector technologies. All images were reconstructed using specific iterative image reconstruction algorithms (ADMIRE Br40/Qr40 for EID-CT; QIR Bv36 for PCD-CT) at a strength level of 3. Detailed reconstruction parameters are provided in Table 2.
Evaluation of objective image quality
For analyzing and comparing the objective image quality of CTPAs, the mean CT attenuation in Hounsfield units was assessed for the pulmonary vasculature (pulmonary trunk, left upper lobe artery, right lower lobe artery), the descending aorta, and the periscapular musculature (left teres major muscle) by placing standardized circular regions of interest (ROIs) in 3-mm axial images at corresponding sites both in 60 keV and polychromatic 120 kV images. The periscapular musculature was chosen as reference tissue as it shows homogenous attenuation without relevant contrast enhancement in the arterial phase [17]. If a pulmonary embolism was present, the ROI was placed proximal to the embolus. The ROIs were drawn by one reader (PP, 2 years of clinical experience in CT imaging). In accordance to previous publications by other research groups, the contrast-to-noise ratio (CNR) and the signal-to-noise ratio (SNR) were calculated for each CTPA in the 60 keV and the polychromatic images using the following formulas [17, 18]:
Evaluation of subjective image quality
Four radiologists (R1-4) with different expertise in the field of cardiovascular CT imaging (reader 1, P.P., 2 years; reader 2, P.G., 5 years; reader 3, H.H., 7 years; reader 4, B.P., 12 years) assessed the subjective image quality of every 60 keV and polychromatic CTPA using a 4-point rating scale. Readers were blinded regarding both scanner type and energy level. Rating scores were determined as follows: (1) excellent overall image quality, no motion artifacts, excellent CTPA contrast, excellent diagnostic confidence down to peripheral branches, no relevant subjective image noise; (2) good overall image quality, minor motion artifacts, good CTPA contrast, good diagnostic confidence down to subsegmental level, minor motion artifacts, minor subjective image noise; (3) moderate to poor overall image quality, severe motion artifacts, just barely acceptable CTPA contrast, practicable evaluation to at least segmental level, high subjective image noise, still of diagnostic quality; (4) non-diagnostic image quality.
Radiation exposure
Both the volume computed tomography dose index (CTDIvol) (mGy) and the dose-length product (DLP) (mGy·cm) were recorded from the dose report automatically generated by the CT scanner. For estimation of the effective radiation dose (ED), a conversion factor of 0.018 mSv/mGy·cm was applied. For PCD scans, size-specific dose estimates (SSDEs) were recorded as calculated by the scanner, whereas for EID scans, SSDEs were calculated as described previously [19].
Statistical analysis
Dedicated statistical software (SPSS Statistics for windows, version 25, IBM) was used for all statistical analyses. p values < 0.05 were considered statistically significant. To compare continuous variables (presented as means ± standard deviations), the Mann–Whitney U test was used. Subjective image ratings are presented as absolute numbers and relative frequencies. For comparison of scale ratings, the Mann–Whitney U test was used as described previously [20]. Intraclass correlation coefficients (ICCs) were calculated in order to evaluate inter-reader reliability. Moreover, the percentage of CTPA scans (at T3D/120 kV and at 60 keV) rated with a score of 1 (= excellent image quality) or 2 (= good image quality) was calculated for each reader.
Results
Patient population
Of the 64 patients included, 38 were women (20 in the PCD group vs.18 in the EID group) and 26 were men (12 in the PCD group vs. 14 in the EID group). The mean age was 65.2 (± 17.1) years in the PCD group and 71.0 (± 14.0) years in the EID group. The mean BMI was 27.6 (± 6.0) kg/m2 in the PCD cohort vs. 26.1 (± 3.3) kg/m2 in the EID group. The incidence of PE was n = 10 (31.3% of cases) in the PCD group vs. n = 7 (21.9% of cases) in the EID group. Characteristics of the respective patient collectives are summarized in Table 1.
Objective image quality
CT attenuation values within relevant vascular structures were substantially higher in the EID group than in the PCD group, both in the polychromatic images and in the 60-keV images: the pulmonary trunk (316.1 ± 111.3 [PCD, 60 keV] vs. 586.3 ± 162.1 [EID, 60 keV], p < 0.001; 239.4 ± 78.3 [PCD, T3D] vs. 430.2 ± 117.7 [EID, blended], p < 0.001), the right lower lobe artery (336.2 ± 120.0 [PCD, 60 keV] vs. 556.5 ± 154.9 [EID, 60 keV], p < 0.001; 256.4 ± 84.0 [PCD, T3D] vs. 424.9 ± 117.6 [EID, blended], p < 0.001), and the left upper lobe artery (331.1 ± 120.7 [PCD, 60 keV] vs. 534.1 ± 148.1 [EID, 60 keV], p < 0.001; 250.1 ± 87.4 [PCD, T3D] vs. 410.2 ± 25.7 [EID, blended], p < 0.001). Also, CT attenuation values for the descending aorta were significantly higher in the EID group than in the PCD group (179.4 ± 72.6 [PCD, 60 keV] vs. 304.2 ± 122.1 [EID, 60 keV], p < 0.001; 141.3 ± 52.1 [PCD, T3D] vs. 230.3 ± 86.4 [EID, blended], p < 0.001). There was no significant difference in CT attenuation values of periscapular musculature between PCD and EID scans with 57.9 ± 8.9 vs. 56.0 ± 6.5 for 60 keV images (p = 0.277) and 52.2 ± 6.5 vs. 54.4 ± 6.0 for T3D/blended images (p = 0.428).
Both SNR and CNR were significantly higher for EID scans in 60 keV, as well as in the blended image series for all evaluated vessel sections, e.g., in case of the pulmonary trunk 30.6 ± 8.6 (SNR)/27.7 ± 8.6 (CNR) in the 60 keV EID scans vs. 17.6 ± 4.8 (SNR)/14.2 ± 4.8 (CNR) in the 60 keV PCD scans (all p < 0.001). A detailed comparison of all ROI measurements and SNR/CNR calculations is provided in Table 3.
Subjective image quality
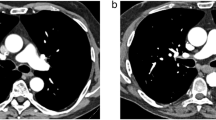
All CTPAs in both groups were of diagnostic image quality. For 60 keV images, the CTPA image quality was considered excellent or good in 31/32/32/30 cases (96.9%/100%/100%/93.8%; R1/R2/R3/R4) in the PCD group and in 31/30/32/30 cases (96.9%/93.8%/100%/93.8%) in the EID group. An image example is given in Fig. 1, demonstrating CTPA with bilateral pulmonary embolism with PCD and EID at 60 keV. For T3D/blended images, the CTPA image quality was rated as excellent or good in 30/32/28/29 cases (93.8%/100%/87.5%/90.6%) in the PCD group and in 31/32/32/30 cases (96.9%/100%/100%/93.8%) in the EID group. For each group, the overall percentage of CTPAs rated with a subjective image quality score of “1” or “2,” indicating excellent or good image quality, was calculated with the following results: in the PCD group, 93.8% of 60 keV CTPAs and 90.6% of T3D CTPAs were rated with a subjective image quality score of “1” or “2,” while in the EID group 84.4% of 60 keV CTPAs and 90.6% of blended (120 kV) CTPAs were rated with a subjective image quality score of “1” or “2.” Subjective evaluation of image quality is summarized in Table 4.
PCD-CT (a, b) and EID-CT (c, d) demonstrating bilateral pulmonary embolism. Dual-energy EID-CT exhibits significant pulsation artifacts (c; arrow) and blurring of thrombus material (c; arrowheads) compared with high-pitch 60-keV PCD-CT. Despite application of a low-dose protocol, PCD-CT delivers excellent intraluminal contrast, paired with high image sharpness and delineation of the thrombus material (a, b; arrows). PCD-CT, photon-counting detector CT; EID-CT, energy-integrating detector CT
According to Koo et al, the intraclass correlation coefficient suggested moderate reliability for ratings of PCD T3D (ICC 0.71), for ratings of PCD scans at 60 keV (ICC 0.72), and for ratings of blended EID scans at 120 kV (ICC 0.67). In contrast, ratings of EID VMI at 60 keV demonstrated poor reliability with an ICC of 0.47 [21].
Radiation dose
The mean ED (1.4 ± 0.5 mSv [PCD] vs. 3.3 ± 0.8 mSv [EID]), as well as SSDE (3.1 ± 0.8 mGy [PCD] vs. 5.7 ± 2.2 mGy [EID]) and DLP (80.0 ± 25.3 mGy·cm [PCD] vs. 181.7 ± 101.8 mGy·cm [EID]) were significantly lower in the PCD group (all p < 0.001). Detailed results of all evaluated radiation dose parameters are summarized in Table 5.
Discussion
In this study, we assessed objective and subjective image quality criteria for an ultra-low contrast medium and radiation dose CT pulmonary angiography scan protocol performed on a first-generation clinical photon-counting detector CT. Our results indicate that despite drastic reductions in both contrast medium and radiation dose administered, the resulting image quality is suitable for the diagnostic workup of patients with suspected acute pulmonary embolism.
According to the ALARA (“as low as reasonably achievable”) principle, there is strong clinical interest to keep both contrast agent dose and radiation dose as low as possible. PCD technology offers considerable advantages over standard EID scanners in this respect. First clinical experiences with PCD in cardiovascular imaging have recently been published investigating CT angiography scans of the aorta and the coronary vessels [22,23,24]. In addition, several studies have been conducted concerning (ultra)-low contrast medium CTPA protocols using conventional EID technology. For example, Rajiah et al evaluated a 30-mL protocol on a 128-slice dual-source EID scanner with promising results [25]. Brendlin et al suggested a contrast-enhanced ultra-low radiation dose high-pitch CT protocol with reduced scan range resulting in an effective radiation dose of 0.7 ± 0.3 mSv [25, 26], while Lu et al evaluated a high-pitch CTPA with iterative reconstruction at 80 kVp and as little as 20 mL of contrast medium on a dual-source EID-CT scanner [18]. Notably, a disadvantage of conventional EID-based single-energy examinations is the inability to perform monoenergetic extrapolarization to enhance the iodine contrast in case of suboptimal vascular opacification. Moreover, spectral image information for the calculation of iodine distribution maps, which show incremental advantages in the diagnosis of pulmonary artery embolism, is lacking [5]. The EID-based DE technology has experienced a considerable hype in the past 10 years, mostly due to decisive advantages over conventional single-energy acquisition [5, 6, 10, 27]. However, whether this acquisition technology is associated with an increased radiation dose remains in question [28,29,30]. Other restrictions, such as a limited spectral FOV in conventional detector dual-source approaches [11], which can be critical in obese patients, must also be considered (Table 2). In contrast to conventional EID scanners, PCD scanners offer the benefit to combine all the requirements for an ideal CT examination: Full FOV spectral data acquisition (50 cm), low contrast medium, and low radiation dose, as well as the full spectrum of spectral image post-processing applications (Fig. 2).
To the authors’ best knowledge, this is the first study investigating a PCD for CTPA. We were able to show that the acquisition of CTPAs on a PCD-CT scanner with a total of 25 mL of iodinated contrast medium is feasible while maintaining excellent to good image quality in nearly 100% of examinations. The option of significantly reducing the volume of contrast medium (in our case by half compared to our in-house standard EID protocol) is especially advantageous in patients with kidney preconditions, since the risk of contrast-medium induced nephropathy increases with the applied volume of contrast medium [31]. A lowered iodine delivery rate can be compensated in parts by the increased image contrast due to virtual low-kV imaging [32,33,34]. Furthermore, our results indicate that a reduction of effective radiation dose by 51.2% (1.4 mSv vs. 3.3 mSv in the EID group) can be realized without a loss of subjective image quality or diagnostic confidence (Fig. 3). Notably, assessed objective image quality parameters were superior in the EID group. According to our experience, the decrease in contrast-to-noise-ratio between detector systems is acceptable in any case, especially when taking higher resolution, less motion artifacts, and the achieved severe dose savings achieved with the PCD system into account. With a mean of 80.0 mGy·cm, DLP in the PCD group was significantly lower in comparison to the usual radiation exposure for CTPA in Europe (138 mGy·cm) and the USA (420 mGy·cm), as recently evaluated by Bos et al [35].
Subjective image quality was considered best by all readers for 60 keV PCD examinations with excellent or good ratings in 93.8% of PCD-CTPAs. The main reason for higher subjective image ratings in the PCD group was lower susceptibility to motion and especially breathing artifacts. In other words, images derived from conventional EID-based dual-energy CT often appear blurry (Fig. 1). Technically, this is mainly due to the fact that the helical pitch factor for dual-energy acquisitions on conventional EID scanners is limited by the vendors (in case of the EID system used in this study default setting of 0.55), whereas the PCD-equipped scanner allows for multi-energy data acquisition with higher pitch factors (2.0 in our study setting) associated with significantly shorter scan times. This issue is especially advantageous for patients who have difficulty following breath-holding commands due to dyspnea, which would be a quite expected symptom in patients presenting with pulmonary embolism [1]. No CTPA examination was deemed “non-diagnostic” on either scanner, irrespective of the acquisition or reconstruction settings.
Our study has several limitations: First, we did not evaluate the diagnostic accuracy of the ultra-low contrast medium PCD-CTPA protocol. However, the similar frequency of detected pulmonary embolisms (10 vs. 7 out of 32 CTPAs in the PCD/EID group) and diagnostic image quality ratings in all examinations suggest that PCD-CTPA most likely is not inferior to the EID group. Second, the iodine delivery rates differed between study groups. Moreover, the reconstruction kernels between detector systems differed slightly. Third, with less pulsation artifacts and the shorter contrast medium bolus in the PCD group, image impression of PCD-CTPAs varied from EID-CTPAs; thus, readers may have become accustomed to either scanners’ characteristics despite being blinded for their subjective image quality reading. Last, at the time of image acquisition and data evaluation, no dedicated software was available for calculating iodine distribution maps from PCD-CTPA data. Therefore, image quality and diagnostic value of suchlike maps could not be evaluated for PCD-CTPA in the current study, warranting further investigations in that regard.
Conclusion
High-pitch photon-counting CT pulmonary angiography allows for significant reduction of contrast medium and radiation dose in the diagnosis of acute pulmonary embolism, while maintaining good to excellent image quality in comparison with dual-energy pulmonary angiography on a conventional CT scanner with energy-integrating detector technology.
Abbreviations
- CNR:
-
Contrast-to-noise ratio
- CTDI:
-
Computed tomography dose index
- CTPA:
-
Computed tomography pulmonary angiography
- DE:
-
Dual-energy
- DLP:
-
Dose-length product
- ED:
-
Effective dose
- EID:
-
Energy-integrating detector
- FOV:
-
Field of view
- ICC:
-
Intraclass correlation coefficient
- PCD:
-
Photon-counting detector
- PE:
-
Pulmonary embolism
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- SSDE:
-
Size-specific dose estimates
- VMI:
-
Virtual monoenergetic imaging
References
Konstantinides SV, Meyer G, Becattini C et al (2020) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41:543–603
Tapson VF (2008) Acute pulmonary embolism. N Engl J Med 358:1037–1052
Stein PD, Fowler SE, Goodman LR et al (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354:2317–2327
Ota M, Nakamura M, Yamada N et al (2002) Prognostic significance of early diagnosis in acute pulmonary thromboembolism with circulatory failure. Heart Vessels 17:7–11
Weidman EK, Plodkowski AJ, Halpenny DF et al (2018) Dual-energy CT angiography for detection of pulmonary emboli: incremental benefit of iodine maps. Radiology 289:546–553
Petritsch B, Kosmala A, Gassenmaier T et al (2017) Diagnosis of pulmonary artery embolism: comparison of single-source CT and 3rd generation dual-source CT using a dual-energy protocol regarding image quality and radiation dose. Rofo 189:527–536
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology 276:637–653
Johnson TR (2012) Dual-energy CT: general principles. AJR Am J Roentgenol 199:S3-8
Dissaux B, Le Floch PY, Robin P et al (2020) Pulmonary perfusion by iodine subtraction maps CT angiography in acute pulmonary embolism: comparison with pulmonary perfusion SPECT (PASEP trial). Eur Radiol 30:4857–4864
Apfaltrer P, Sudarski S, Schneider D et al (2014) Value of monoenergetic low-kV dual energy CT datasets for improved image quality of CT pulmonary angiography. Eur J Radiol 83:322–328
Siegel MJ, Kaza RK, Bolus DN et al (2016) White paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, part 1: technology and terminology. J Comput Assist Tomogr 40:841–845
Rajendran K, Petersilka M, Henning A et al (2022) First clinical photon-counting detector CT system: technical evaluation. Radiology 303:130–138
Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D (2018) Photon-counting CT: technical principles and clinical prospects. Radiology 289:293–312
Kosmala A, Gruschwitz P, Veldhoen S et al (2020) Dual-energy CT angiography in suspected pulmonary embolism: influence of injection protocols on image quality and perfused blood volume. Int J Cardiovasc Imaging 36:2051–2059
Petritsch B, Pannenbecker P, Weng AM et al (2020) Comparison of dual- and single-source dual-energy CT for diagnosis of acute pulmonary artery embolism. Rofo. https://doi.org/10.1055/a-1245-0035
Emrich T, O’Doherty J, Schoepf UJ et al (2022) Reduced iodinated contrast media administration in coronary CT angiography on a clinical photon-counting detector CT system: a phantom study using a dynamic circulation model. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000911
Sabel BO, Buric K, Karara N et al (2016) High-pitch CT pulmonary angiography in third generation dual-source CT: image quality in an unselected patient population. PLoS One 11:e0146949
Lu GM, Luo S, Meinel FG et al (2014) High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol 24:3260–3268
Boone JM SK, Cody DD et al (2011) Size-specific dose estimates in pediatric and adult body CT examinations: report no. 204Am Assoc Phys Medicine, College Park
Sullivan GM, Artino AR Jr (2013) Analyzing and interpreting data from likert-type scales. J Grad Med Educ 5:541–542
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15:155–163
Euler A, Higashigaito K, Mergen V et al (2022) High-pitch photon-counting detector computed tomography angiography of the aorta: intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose. Invest Radiol 57:115–121
Mergen V, Sartoretti T, Klotz E et al (2022) Extracellular volume quantification with cardiac late enhancement scanning using dual-source photon-counting detector CT. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000851
Si-Mohamed SA, Boccalini S, Lacombe H et al (2022) Coronary CT angiography with photon-counting CT: first-in-human results. Radiology 303:303–313
Rajiah P, Ciancibello L, Novak R, Sposato J, Landeras L, Gilkeson R (2019) Ultra-low dose contrast CT pulmonary angiography in oncology patients using a high-pitch helical dual-source technology. Diagn Interv Radiol 25:195–203
Brendlin AS, Winkelmann MT, Peisen F et al (2021) Diagnostic performance of a contrast-enhanced ultra-low-dose high-pitch CT protocol with reduced scan range for detection of pulmonary embolisms. Diagnostics (Basel) 11(7):1251
Petritsch B, Pannenbecker P, Weng AM et al (2021) Split-filter dual-energy CT pulmonary angiography for the diagnosis of acute pulmonary embolism: a study on image quality and radiation dose. Quant Imaging Med Surg 11:1817–1827
Schenzle JC, Sommer WH, Neumaier K et al (2010) Dual energy CT of the chest: how about the dose? Invest Radiol 45:347–353
Sauter AP, Shapira N, Kopp FK et al (2020) CTPA with a conventional CT at 100 kVp vs. a spectral-detector CT at 120 kVp: comparison of radiation exposure, diagnostic performance and image quality. Eur J Radiol Open 7:100234
Tabari A, Gee MS, Singh R et al (2020) Reducing radiation dose and contrast medium volume with application of dual-energy CT in children and young adults. AJR Am J Roentgenol 214:1199–1205
Faucon AL, Bobrie G, Clement O (2019) Nephrotoxicity of iodinated contrast media: from pathophysiology to prevention strategies. Eur J Radiol 116:231–241
Wannasopha Y, Leesmidt K, Srisuwan T, Euathrongchit J, Tantraworasin A (2022) Value of low-keV virtual monoenergetic plus dual-energy computed tomographic imaging for detection of acute pulmonary embolism. PLoS One 17:e0277060
Dane B, Patel H, O’Donnell T et al (2018) Image quality on dual-energy CTPA virtual monoenergetic images: quantitative and qualitative assessment. Acad Radiol 25:1075–1086
Meier A, Wurnig M, Desbiolles L, Leschka S, Frauenfelder T, Alkadhi H (2015) Advanced virtual monoenergetic images: improving the contrast of dual-energy CT pulmonary angiography. Clin Radiol 70:1244–1251
Bos D, Yu S, Luong J et al (2022) Diagnostic reference levels and median doses for common clinical indications of CT: findings from an international registry. Eur Radiol 32:1971–1982
Funding
Open Access funding enabled and organized by Projekt DEAL. P.G. (Z-02CSP/18) and J.P.G. (Z-3BC/02) were financially supported by the Interdisciplinary Center of Clinical Research Würzburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bernhard Petritsch.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Institutional research grant from Siemens Healthcare GmbH, not related to this specific project. Jan-Peter Grunz is a research consultant for Siemens Healthineers. Bernhard Petritsch and Thorsten A. Bley receive speaker honorar from Siemens Healthineers. The other authors of this manuscript declare no further relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Andreas M. Weng) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Study permission was obtained from the Institutional Review Board.
Study subjects or cohorts overlap
None of the study subjects or cohorts have been previously reported.
Methodology
• Retrospective
• Diagnostic study
• Performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pannenbecker, P., Huflage, H., Grunz, JP. et al. Photon-counting CT for diagnosis of acute pulmonary embolism: potential for contrast medium and radiation dose reduction. Eur Radiol 33, 7830–7839 (2023). https://doi.org/10.1007/s00330-023-09777-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09777-9