Abstract
Ob jectives
The European Society of Radiology identified 10 common indications for computed tomography (CT) as part of the European Study on Clinical Diagnostic Reference Levels (DRLs, EUCLID), to help standardize radiation doses. The objective of this study is to generate DRLs and median doses for these indications using data from the UCSF CT International Dose Registry.
Methods
Standardized data on 3.7 million CTs in adults were collected between 2016 and 2019 from 161 institutions across seven countries (United States of America (US), Switzerland, Netherlands, Germany, UK, Israel, Japan). DRLs (75th percentile) and median doses for volumetric CT-dose index (CTDIvol) and dose-length product (DLP) were assessed for each EUCLID category (chronic sinusitis, stroke, cervical spine trauma, coronary calcium scoring, lung cancer, pulmonary embolism, coronary CT angiography, hepatocellular carcinoma (HCC), colic/abdominal pain, appendicitis), and US radiation doses were compared with European.
Results
The number of CT scans within EUCLID categories ranged from 8,933 (HCC) to over 1.2 million (stroke). There was greater variation in dose between categories than within categories (p < .001), and doses were significantly different between categories within anatomic areas. DRLs and median doses were assessed for all categories. DRLs were higher in the US for 9 of the 10 indications (except chronic sinusitis) than in Europe but with a significantly higher sample size in the US.
Conclusions
DRLs for CTDIvol and DLP for EUCLID clinical indications from diverse organizations were established and can contribute to dose optimization. These values were usually significantly higher in the US than in Europe.
Key Points
• Registry data were used to create benchmarks for 10 common indications for CT identified by the European Society of Radiology.
• Observed US radiation doses were higher than European for 9 of 10 indications (except chronic sinusitis).
• The presented diagnostic reference levels and median doses highlight potentially unnecessary variation in radiation dose.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Radiation doses for computed tomography (CT) are highly variable across patients, institutions, and countries [1,2,3,4,5]. Because of the rising use of CT [6,7,8], the carcinogenic risk of ionizing radiation [9,10,11,12,13,14], and the relatively high radiation doses associated with CT, greater standardization is needed across institutions and countries for CT dose [4, 15]. Variation in radiation doses at CT is primarily attributable to how the scans are conducted and the setting of technical parameters, rather than patient, institutional, or machine characteristics, all of which have a smaller contribution to variation in observed doses [4]. Thus, dose optimization can be achieved across most machine types through optimization of technical parameters. The establishment of diagnostic reference levels (DRLs) was first implemented by the International Commission on Radiological Protection (ICRP) in 1996 to aid in optimization of medical radiation exposures [16]. The DRLs were identified as doses that should not be exceeded on a patient of routine size unless there was a particular need to do so. Some organizations require the documentation of medical necessity if these values are exceeded. The European Council Directive 2013/59/EURATOM requires the establishment, regular review, and use of DRLs for member states [15]. The US National Council on Radiation Protection and Measurements (NCRP) implemented an additional concept of achievable doses (ADs) in 1999 reflecting the median. These doses were identified as doses that should help improve further dose optimization and encourage faculties to achieve lower doses than the DRL [17, 18].
DRLs and achievable doses are commonly used in clinical practice to guide radiology facilities and practitioners in efforts to optimize CT doses. Most commonly, the DRL is set at the 75th percentile of the dose distribution and the achievable dose at the 50th percentile for a geographical area [2, 17,18,19]. These are often created broadly within anatomic areas, and yet even within anatomical regions, different clinical indications have different image quality requirements.
The European study on clinical diagnostic reference levels for X-ray medical imaging (EUCLID), funded by the European Commission and led by the European Society of Radiology, has established clinical indication-based DRLs for ten common clinical indications to provide better guidance to radiologists and technologists. In this large-scale survey, dose data of 4299 adult patients with standard size were collected across 14 European countries via an online survey [20, 21]. Since the EUCLID categories provide a useful approach for setting dose targets and thresholds, we would expect to see greater variation between the EUCLID categories than within the EUCLID categories because of the different image quality requirements of different CT indications. In this study, we used data from the University of California San Francisco (UCSF) CT International Dose Registry to describe DRLs and median doses for EUCLID clinical indications and compare radiation doses between facilities of the United States of America (US) and Europe (EU).
Materials and methods
UCSF CT International Dose Registry
Details regarding the UCSF CT International Dose Registry have been previously described [4]. In short, the registry includes imaging data from 161 imaging facilities and hospitals associated with 27 healthcare institutions from seven countries (United States of America (US), Switzerland, Netherlands, Germany, UK, Israel, and Japan) that use Radimetrics dose management software (RadimetricsTM Enterprise Platform, Bayer AG, Leverkusen, Germany). Radimetrics was selected as the only one of several available dose management software because it was already being used by several hospitals internationally at the time the dose registry was initiated. Due to the need for secure data transfer, only one dose management software was used as data source. All customers of Radimetrics were invited to participate in the registry, and institutions who elected to participate and were able to meet the logistical requirements such as establishing data connections and receiving institutional approval to share data are included. At each imaging facility, imaging data are assembled on a local server, stripped of patient identifying information and then transferred to the registry [4]. The UCSF Committee on Human Research and the institutional review boards of the collaborating institutions approved the study, waived informed consent, or relied on the UCSF approval.
Study population
Data are included for diagnostic CT examinations performed in adult patients ages 18 years and older between 1 January 2016 and 31 December 2019. All patient sizes were included. Exams for research, surgical or interventional procedures, combined with positron emission tomography (i.e., PET-CT), or single photon emission tomography (i.e., SPECT-CT), and done for radiation oncology guidance were excluded.
Indication for CT examination
The indication for each CT examination was determined by applying natural language processing techniques to the study description and protocol name included in the Digital Imaging and Communications in Medicine (DICOM) metadata. This included searching for explicit terms and classifying exams using the most specific and highest dose category. In the EUCLID study, ten common clinical indications for undergoing CT were identified [21]. These EUCLID clinical indications or categories include (1) sinus to asses for sinusitis and polyps; (2) stroke, which we aligned with a routine brain examination, and imaging of the brain to assess for hemorrhage; (3) cervical spine for trauma; (4) coronary calcium scoring; (5) lung cancer, which we aligned with routine chest examinations, as well as cancer diagnosis and staging, and not screening; (6) pulmonary embolism; (7) coronary angiography; (8) hepatocellular carcinoma (HCC), suspected or evaluation of known; (9) colic/abdominal pain, which we aligned with imaging for suspected kidney stones; and (10) abdominal pain, suspected appendicitis, which we aligned with routine abdomen. EUCLID category of “stroke” most closely aligns with a routine head CT performed to exclude hemorrhage, so brain perfusion scans and cerebrovascular CT angiograms were excluded.
Dose metrics
Results are provided for complete CT examinations including all scans performed as part of the examination (e.g., a complete examination would include all imaging acquisitions and might include one scan with and one without intravenous contrast). Bolus scans were identified and the dose was included in the total exam dose, but these scans were not considered when determining examination phase. Results are reported for volumetric CT-dose index (CTDIvol) reflecting the average radiation exposure per section and the dose-length product (DLP) reflecting the total radiation output for the examination. Patient diameter was defined as the average of the water equivalent diameter from each CT acquisition over the entire imaging range and used to adjust radiation doses by size [4, 22].
Statistical analysis
For each clinical indication, the median dose was defined as the 50th percentile in dose distribution and the DRL was defined as the 75th percentile in dose distribution. The 95% confidence intervals (CI) of the DRLs and median doses were calculated by bootstrapping using random sampling with replacement [23].
Analysis of variance was used to determine if there was greater variation between categories, as compared to within categories. The distribution of DRLs and median doses were assessed for the United States (US) and Europe (EU) after adjusting for patient size and age. For the international comparison, only US and European CT exams were investigated further because the registry includes sufficient sample sizes for each EUCLID category; sample sizes for facilities in Japan and Israel were insufficient. The adjusted doses were estimated via log-linear regression estimating the dose for patients with average age and size. Bootstrapping within each country was used to determine if the DRLs and median doses varied by country when stratified by anatomic area. The relative DRLs and relative median doses (and 95% CI) between the US and Europe were calculated. Analysis was done using R version 3.6.3.
Results
A total of 3,718,217 CT scans performed between January 1, 2016, and December 31, 2019, across the 10 EUCLID clinical indications are included (Table 1). The number of CT scans for each indication ranged from 8,933 for hepatocellular carcinoma to over 1.2 million for stroke. The examinations were performed on 383 CT scanners across 74 machine models from the four largest CT manufacturers. Most of the 161 participating facilities contributed to most of the clinical indications with some notable exceptions, e.g., coronary calcium scoring was recorded at only 73 facilities, coronary angiography at 66 facilities, and HCC at 19 facilities. Overall 84 % of all CT examinations were performed at the US facilities (n = 141) and 8 % at the European facilities (n = 11).
DRLs and median doses
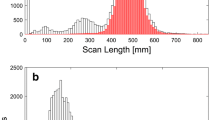
There was greater variation for CTDIvol and DLP between categories, as compared to within categories (p < .001) (Table 2 and Fig. 1). Within each anatomic area, the DRLs and median doses were significantly different across the EUCLID categories (Table 2, Fig. 1, p < .05). For example, in the chest the median doses for CTDIvol increased from 5.7 mGy for coronary calcium scoring to 8.0 mGy for lung cancer, to 10.4 mGy for pulmonary embolism, to 13.3 mGy for coronary CT angiography, reflecting more than a 2-fold difference among the four chest categories (Table 2, Fig. 1a). The corresponding DRLs for CTDIvol varied more than 3-fold ranging from 7.0 mGy for coronary calcium scoring to 25.4 mGy for coronary CT angiography. Even larger differences across these chest categories were observed for the DRLs and median values of DLP (Table 2 and Fig. 1b). For chest imaging, a 9-fold difference in the DRLs for DLP was observed, ranging from 106 mGy cm [95% CI 106, 107] for coronary calcium scoring to 935 mGy cm [95% CI 920, 949] for coronary CT angiography. The CI were narrow due to large sample size. Within each anatomic area, the differences between the EUCLID categories for median doses and DRLs were significantly different (all p-values < .05). The corresponding values adjusted for patient size changed relatively little (supplement 1).
Comparison between US and Europe
For most (9 of the 10) EUCLID categories, after adjusting for patient size and age, the DRLs and median doses were significantly higher in the US (all p < .05, Tables 3 and 4, Fig. 2a and b). The largest differences were seen for chest CT. Using CTDIvol, pulmonary embolism had an approximately 3-fold higher dose in the US (relative median dose 2.96 [95% CI 2.92, 3.00], relative DRL 2.73 [95% CI 2.70, 2.76]), and coronary calcium scoring had an approximately 4-fold higher dose in the US (relative median dose 3.92 [95% CI 3.85, 4.00], relative DRL 4.25 [95% CI 4.15, 4.38]). Lung cancer had an approximately 2-fold higher dose in the US (relative median dose 2.51 [95% CI 2.50, 2.52], relative DRL 2.22 [95% CI 2.20, 2.24]). Coronary CT angiography had a 2-fold higher dose in the US. In the US for other categories, the doses were modestly higher, i.e., approximately 22 to 77% higher (range in the relative median dose 1.31 to 1.67 and relative DRLs 1.22 to 1.77). US doses were comparable or lower only for chronic sinusitis (relative median dose 1.06 [95% CI 1.05, 1.06], relative DRL 0.72 [95% CI 0.71, 0.72]). Similar results were seen for DLP (Table 4 and Fig. 2b). For example, pulmonary embolism had an approximately 3-fold higher dose in the US (relative median dose 3.05 [95% CI 2.99, 3.10], relative DRL 2.89 [95% CI 2.84, 2.94]).
Distribution of dose metrics (a CTDIvol, b DLP) for each EUCLID category with comparison between the United States (US) and Europe (EU), showing median doses (50th percentile, black open circle for US and gray open box for EU), and diagnostic reference levels (DRLs) (75th percentile, black circle for US and gray box for EU). Doses were adjusted for patient size and age. a Comparison of DRLs and median doses for CTDIvol (in mGy) between US and Europe for each EUCLID indication. b Comparison of DRLs and median doses for DLP (in mGy cm) between US and Europe for each EUCLID indication.
The use of multiphase scanning was similar for some indications between the US and Europe. For example, 99% of examinations performed for hepatocellular carcinoma use multiphase scanning in both the US and Europe. However, for other indications there were substantial differences between US and Europe in multiphase scanning: coronary CT angiography (66% vs 46%), colic/abdominal pain (6% vs 29%), and stroke (11% vs 18%).
Comparison with published literature
Most publications describing benchmark doses focus on the DRLs (75th percentile). The DRLs for DLP of the EUCLID indications of stroke, cervical spine trauma, coronary calcium scoring, coronary CT angiography, and lung cancer were within the range of values reported in the literature (Fig. 3b, supplement 2). The reported values are based on the literature review from the EUCID study of the European Society of Radiology and additional database research in PubMed [24]. For several EUCLID indications, including chronic sinusitis, pulmonary embolism, and the abdominal imaging categories (colic/abdominal pain, appendicitis, and HCC), the observed DRLs for DLP were higher than reported in the literature. For CTDIvol the observed values were within published values for most EUCLID indications (Fig. 3a, supplement 2). Only DRLs for chronic sinusitis, pulmonary embolism, and colic/abdominal pain were higher.
Comparison between published diagnostic reference levels (DRLs) (a CTDIvol and b DLP) and observed DRLs in this report for each EUCLID indication. The maximum published value is shown with a triangle, the minimum published value with a circle (each in gray). The observed values are shown in black circles (EUCLID). a Comparison between published and observed DRLs for CTDIvol of each EUCLID indication. b Comparison between published and observed DRLs for DLP of each EUCLID indication.
Adjusted for patient age and size, US DRLs exceeded published DRLs for 6 of 10 indications (chronic sinusitis, cervical spine trauma, pulmonary embolism, all abdominal indications) in terms of DLP and twice in terms of CTDIvol (chronic sinusitis and colic/abdominal pain). European DRLs exceeded published DRLs for DLP only for sinusitis and colic/abdominal pain, and DRLs for CTDIvol exceeded only for sinusitis. For 4 of 10 indications for DLP (coronary calcium scoring, lung cancer, pulmonary embolism, HCC) and 6 of 10 indications for CTDIvol (cervical spine trauma, all chest indications, HCC), European doses were lower than published DRLs.
Compared with recently published results from the large-scale, survey-based EUCLID study on standard-sized patients, most DRLs from the UCSF CT International Dose Registry, which includes all patient sizes, were higher than DRLs from the EUCLID study, except for stroke and lung cancer [21].
Discussion
The DRLs and median doses for EUCLID indications included in this report can be used as a starting point for dose optimization. Because the observed large differences across the EUCLID categories greatly exceeded the dose differences within categories, our results support the use of the EUCLID clinical indication-specific CT protocols, rather than combining all indications within anatomic areas.
The advantage of these data is their large size, inclusion of all examinations as opposed to sampled examination, inclusion of a large number of different machine makes and models, and representation of data from 161 imaging facilities who participate in the UCSF CT International Dose Registry. These data primarily reflect US practice, and all were assembled from facilities that invested in dose management software, which should also be mentioned as limitations of the study. Because fewer European countries are represented, it is impossible to know if these reflect European practice more broadly or just the practice of a small number of imaging facilities in the small number of included countries.
The European Union has asked that member nations locally set DRLs for CT [15]. This is based on the presumption that doses vary by machine manufacturer and model and therefore must be set in a way that takes into consideration the type of equipment. We have shown previously that within our registry, machine make and model are relatively small contributors to dose variability, whereas how machines are used and the choice of technical parameters are the largest contributors of dose variability [4]. Thus, these benchmarks should be relevant for most imaging centers.
The large differences in doses between the US and Europe persisted after accounting for patient size and age. Inter-country variability results from the way the machines are used, the choice of technical parameters, and the decisions that clinical staff make to alter dose levels and hence contribute to dose variation [4]. The reasons for these differences are likely multifactorial including greater awareness of the need to optimize dose in Europe, which is reflected by the creation of EUCLID categories in the first place. Differences in the usage of multiphase scanning, e.g., coronary CT angiography, could reflect different practice cultures between Europe and the US. Reducing the number of acquired series is the easiest strategy to reduce radiation dose.
We present both DRLs and median doses for ten common clinical indications for CT, which represent the current status quo of the used radiation doses from the participating facilities. ICRP Publication 135 describes that DRLs are not intended to be used as a trigger or alarm level for individual patients and that DRLs are not limits. They can be used as benchmarks for further investigations when a representative sample of examinations exceed the local, national, or regional DRL value. Median doses are an additional tool for dose optimization activities. However, if local practices result in radiation doses far below median dose values, adequate image quality should be ensured [17].
Previously published DRLs for clinical indications were mainly from survey-based methods or from selective submission to established registries. These are likely not as accurate as doses based on a comprehensive assessment such as included in this report. Larger surveys are necessary to account for variations based on size and variations that may result from inclusion of a single or small number of scanners [25]. The recently published EUCLID study is also based on a large-scale survey that collected at least 20 CT examinations of standard-sized patients from each hospital for each EUCLID indication [21]. The differences in the DRLs of the UCSF CT International Dose Registry and this study may be mainly attributable to the inclusion of all patients and all CT scans in the registry rather than only selected patients.
The primary limitation in these data is that assignment to the EUCLID categories may be inaccurate. We assessed the CT doses for the EUCLID clinical indications using data stored within the DICOM data and if these data were non-specific (e.g., the indication was coded only as a routine CT), we would have not been able to correctly assign the CT to the appropriate category. The observation that all of the facilities did not contribute to all EUCLID categories likely reflects our inability to assign some CT scans to the appropriate category, rather than reflecting that the facility did not performing certain types of scans. However, this likely would have added random noise to our estimates and thus diminished differences. Hence, differences between categories may be even greater than reported. Other limitations include the relatively small size of the European studies, the use of only one dose-monitoring software as data source, and that we did not include a measure of imaging quality, as no agreed upon measure exists.
In conclusion, dose metrics from large multi-center studies can help create representative DRLs. These DRLs can be used for dose optimization and institutional evaluation to examine whether institutions routinely exceed these benchmarks. This analysis supports that the clinical indication-specific categories provide more nuanced and accurate reflections of dose requirements that categories based on anatomic area alone. The provided DRLs and median doses for the EUCLID indications can be used as a tool for defining these benchmarks. These benchmarks exceed previously published DRLs for some indications, perhaps providing evidence that practice can be improved, and a greater standardization is needed. Similarly, the higher values for US institutions also might suggest the opportunity for additional dose reduction in the US.
Change history
22 February 2022
In this article the following funding information was added: Open Access funding enabled and organized by Projekt DEAL.
Abbreviations
- AD:
-
Achievable dose
- CI:
-
Confidence interval
- DRL:
-
Diagnostic reference level
- EUCLID:
-
European Study on Clinical Diagnostic Reference Levels for X-ray Medical Imaging
References
Smith-Bindman R, Lipson J, Marcus R et al (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169:2078–2086
European Commission (2014) Diagnostic reference levels in thirty-six European countries, Radiation Protection N° 180. Publications Office of the European Union, Luxembourg
Parakh A, Euler A, Szucs-Farkas Z, Schindera ST (2017) Transatlantic comparison of CT radiation doses in the era of radiation dose-tracking software. AJR Am J Roentgenol 209:1302–1307
Smith-Bindman R, Wang Y, Chu P et al (2019) International variation in radiation dose for computed tomography examinations: prospective cohort study. BMJ k364:4931
Lukasiewicz A, Bhargavan-Chatfield M, Coombs L et al (2014) Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology 271:445–451
Medicare Payment Advisory Commission (2019) A data book: healthcare spending and the Medicare program. Medicare Payment Advisory Commission, Washington, DC
Smith-Bindman R, Kwan ML, Marlow EC et al (2019) Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA 322:843–856
IMV Medical Information Division (2019) IMV 2019 CT Market Outlook Report. IMV, Des Plaines, IL
International Agency for Research on Cancer (2012) Radiation - IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, International Agency for Research on Cancer
National Research Council (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. The National Academies Press, Washington, DC
Richardson DB, Cardis E, Daniels RD et al (2015) Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 351:h5359
Cardis E, Vrijheid M, Blettner M et al (2005) Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 331:77
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Preston DL, Ron E, Tokuoka S et al (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
European Commission (2014) Council Directive 2013/59/Euratom of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation, and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Off J Eur Union 13:1–73
Harding K, Thomson WH (1997) Radiological protection and safety in medicine - ICRP 73. Eur J Nucl Med 24:1207–1209
Vano E, Miller DL, Martin CJ et al (2017) ICRP Publication 135: diagnostic reference levels in medical imaging. Ann ICRP 46:1–144
Field S, Arthur RJ, Coakley AJ et al (1999) Guidelines on patient dose to promote the optimisation of protection for diagnostic medical exposures, Report of an Advisory Group on Ionising Radiation
Rosenstein M (2008) Diagnostic reference levels for medical exposure of patients: ICRP guidance and related ICRU quantities. Health Phys 95:528–534
Frija G, Damilakis J, Jaschke W et al. WP 2: Identification of procedures and clinical indications & review of existing clinical DRLs. The final list of clinical indications for CT and interventional radiology for which DRLs will be established. ESR EuroSafe Imaging. Available via http://www.eurosafeimaging.org/wp/wp-content/uploads/2017/09/EUCLID_clinical-indications_final.pdf. Accessed 14 Nov 2019
Tsapaki V, Damilakis J, Paulo G et al (2021) CT diagnostic reference levels based on clinical indications: results of a large-scale European survey. Eur Radiol. https://doi.org/10.1007/s00330-020-07652-5
McCollough C, Bakalyar DM, Bostani M et al (2014) Use of water equivalent diameter for calculating patient size and size-specific dose estimates (SSDE) in CT: the report of AAPM Task Group 220. AAPM report, 6–23
Efron B, Tibshirani RJ (1994) An introduction to the bootstrap. CRC Press
Damilakis J, Frija G, Hierath M et al (2018) European study on clinical diagnostic reference levels for x-ray medical imaging, Deliverable 2.1: report and review on existing clinical DRLs. Available via http://www.eurosafeimaging.org/wp/wp-content/uploads/2017/09/D2.1_Report-and-review-on-existing-clinical-DRLs_final_published-on-website.pdf. Accessed 13 Nov 2020
Stamm G (2011) Collective radiation dose from MDCT: critical review of surveys studies. In: Tack D, Kalra M, Gevenois P (eds) Radiation dose from multidetector CT. Medical radiology. Springer, Berlin, pp 209-229. https://doi.org/10.1007/174_2011_433
L’Agence fédérale de Contrôle nucléaire (AFCN) (2018) Niveaux de référence diagnostiques en radiologie. Bruxelles
Wachabauer D, Röthlin F (2017) Aktualisierung der diagnostischen Referenzwerte für Österreich. Wien
Bundesamt für Strahlenschutz (2016) Bekanntmachung der aktualisierten diagnostischen Referenzwerte für diagnostische und interventionelle Röntgenanwendungen. Bundesamt für Strahlenschutz, Salzgitter, Germany
Aberle C, Ryckx N, Treier R, Schindera S (2020) Update of national diagnostic reference levels for adult CT in Switzerland and assessment of radiation dose reduction since 2010. Eur Radiol 30:1690–1700
Schegerer AA, Nagel HD, Stamm G, Adam G, Brix G (2017) Current CT practice in Germany: results and implications of a nationwide survey. Eur J Radiol 90:114–128
van der Molen AJ, Schilham A, Stoop P, Prokop M, Geleijns J (2013) A national survey on radiation dose in CT in The Netherlands. Insights Imaging 4:383–390
Radiation and Nuclear Safety Authority (2013) Reference levels for patient radiation exposure in computed tomography examinations of adults. Radiation and Nuclear Safety Authority, Helsinki, Finnland
Foley SJ, McEntee MF, Rainford LA (2012) Establishment of CT diagnostic reference levels in Ireland. Br J Radiol 85:1390–1397
Kanal KM, Butler PF, Sengupta D, Bhargavan-Chatfield M, Coombs LP, Morin RL (2017) U.S. Diagnostic reference levels and achievable doses for 10 adult CT examinations. Radiology 284:120–133
Public Health England (2014) Doses from computed tomography (CT) examinations in the UK – 2011 review. Public Health England
Wallace A, Hayton A, Thomas P, Beveridge T (2015) The 2011 – 2013 National Diagnostic Reference Level - service report. Australian Radiation Protection and Nuclear Safety Agency, Yallambie
Japan Network for Research and Information on Medical Exposures (2015) Diagnostic reference levels based on latest surveys in Japan - Japan DRLs 2015. Japan Network for Research and Information on Medical Exposures
Salama DH, Vassileva J, Mahdaly G et al (2017) Establishing national diagnostic reference levels (DRLs) for computed tomography in Egypt. Phys Med 39:16–24
Palorini F, Origgi D, Granata C, Matranga D, Salerno S (2014) Adult exposures from MDCT including multiphase studies: first Italian nationwide survey. Eur Radiol 24:469–483
Holroyd JR, Edyvean S (2018) Doses from cervical spine computed tomography (CT) examinations in the UK. Br J Radiol 91:20170834
Treier R, Aroua A, Verdun FR, Samara E, Stuessi A, Trueb PR (2010) Patient doses in CT examinations in Switzerland: implementation of national diagnostic reference levels. Radiat Prot Dosimetry 142:244–254
Sundhedsstyrelsen Statens Institut for Strålebeskyttelse (2015) CT Referencedoser. Sundhedsstyrelsen Statens Institut for Strålebeskyttelse, Copenhagen
Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP (2017) A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 11:268–273
Mafalanka F, Etard C, Rehel JL et al (2015) Establishment of diagnostic reference levels in cardiac CT in France: a need for patient dose optimisation. Radiat Prot Dosimetry 164:116–119
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Fukushima Y, Tsushima Y, Takei H, Taketomi-Takahashi A, Otake H, Endo K (2012) Diagnostic reference level of computed tomography (CT) in Japan. Radiat Prot Dosimetry 151:51–57
Funding
Open Access funding enabled and organized by Projekt DEAL. The registry and analysis was supported by the US National Institutes of Health (R01- CA181191) and the Patient-Centered Outcomes Research Institute (CD-1304-7043, DI-2018C1-11375). Funders had no role in study design, collection, analysis, interpretation, and reporting of data; or decision to publish. The views in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee, or other funders.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Rebecca Smith-Bindman.
Conflict of Interest
R.S-B is a founder of Alara Imaging, Inc., a company focused on collecting and reporting radiation dose information associated with CT as part of quality measurement.
D.B. was supported as a Clinician Scientist within the University Medicine Essen Academy (UMEA) program, funded by the German Research Foundation (DFG; grant FU356/12-1) and the Faculty of Medicine, University of Duisburg-Essen.
J.E.: Activities related to the present article: No relevant relationships. Activities not related to the present article: Consultant for W. L. Gore & Associates; Speaker’s fee from Ionetix; Institution has grants/grants pending from National Institutes of Health, International Atomic Energy Agency, Canon Medical Systems, GE Healthcare, Roche Medical Systems, and W. L. Gore and Associates.
R.G. is a Key Opinion Leader, Siemens Healthineers, Advanced Therapies (Interventional Imaging).
All other authors have nothing to disclose.
Statistics and Biometry
One of the authors has significant statistical expertise.
Informed Consent
Written informed consent was waived by the Institutional Review Board.
Ethical Approval
The institutional review boards at the University of California San Francisco (and the collaborating institutions) approved the study or relied on the university’s approval.
Methodology
• prospective
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
D. Bos began this work while a visiting scientist with the Department of Epidemiology and Biostatistics at the University of California San Francisco, San Francisco, CA, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bos, D., Yu, S., Luong, J. et al. Diagnostic reference levels and median doses for common clinical indications of CT: findings from an international registry. Eur Radiol 32, 1971–1982 (2022). https://doi.org/10.1007/s00330-021-08266-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08266-1