Abstract
Objectives
To investigate the diagnostic feasibility of a shortened breast PET/MRI protocol in breast cancer patients.
Methods
Altogether 90 women with newly diagnosed T1tumor-staged (T1ts) and T2tumor-staged (T2ts) breast cancer were included in this retrospective study. All underwent a dedicated comprehensive breast [18F]FDG-PET/MRI. List-mode PET data were retrospectively reconstructed with 20, 15, 10, and 5 min for each patient to simulate the effect of reduced PET acquisition times. The SUVmax/mean of all malign breast lesions was measured. Furthermore, breast PET data reconstructions were analyzed regarding image quality, lesion detectability, signal-to-noise ratio (SNR), and image noise (IN). The simultaneously acquired comprehensive MRI protocol was then shortened by retrospectively removing sequences from the protocol. Differences in malignant breast lesion detectability between the original and the fast breast MRI protocol were evaluated lesion-based. The 20-min PET reconstructions and the original MRI protocol served as reference.
Results
In all PET reconstructions, 127 congruent breast lesions could be detected. Group comparison and T1ts vs. T2ts subgroup comparison revealed no significant difference of subjective image quality between 20, 15, 10, and 5 min acquisition times. SNR of qualitative image evaluation revealed no significant difference between different PET acquisition times. A slight but significant increase of IN with decreasing PET acquisition times could be detected. Lesion SUVmax group comparison between all PET acquisition times revealed no significant differences. Lesion-based evaluation revealed no significant difference in breast lesion detectability between original and fast breast MRI protocols.
Conclusions
Breast [18F]FDG-PET/MRI protocols can be shortened from 20 to below 10 min without losing essential diagnostic information.
Key Points
• A highly accurate breast cancer evaluation is possible by the shortened breast [ 18 F]FDG-PET/MRI examination protocol.
• Significant time saving at breast [ 18 F]FDG-PET/MRI protocol could increase patient satisfaction and patient throughput for breast cancer patients at PET/MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common solid tumor in women, accounting for 11.7% of all female tumors [1]. Accurate pre-therapeutic staging is of particular importance after initial diagnosis. Therefore, PET/MRI is becoming more and more accepted for local and whole-body staging similar to staging of other cancers [2,3,4,5]. This is mainly due to the excellent soft tissue contrast and the obtained multiparametric dataset that allows further tumor classification and detailed therapy planning [6, 7]. A well-known problem of hybrid examinations is long examination times [8]. The current literature reports shortened PET/MRI examinations by reducing acquisition times of PET data or the number of sequences in MRI protocols. The common clinical goal was to increase the availability of PET/MRI examinations and to increase patient satisfaction during the examination [5, 8,9,10,11,12]. Focusing on the initial staging of breast cancer patients, our current staging protocol includes a prone (multiparametric) breast [18F]FDG-PET/MRI examination followed by a supine whole-body [18F]FDG-PET/MRI exam [5]. Breast [18F]FDG-PET/MRI combines multiparametric breast MRI as the most sensitive imaging modality for breast cancer detection and tumor extent assessment with simultaneous acquired PET data and takes breast imaging to a higher diagnostic level [6, 11, 13, 14]. Because of its importance in local tumor staging and phenotyping, just skipping the breast [18F]FDG-PET/MRI examination, aiming towards faster examination protocols, is far from a solution [5]. The PET acquisition time of [18F]FDG-PET/MRI can be easily shortened to 2 min in whole-body examinations [11, 12]. If this is also possible in breast [18F]FDG-PET/MRI without losing diagnostic information, ultimately the MRI protocol is considered the time-limiting factor. Focusing on the breast MRI component, abbreviated breast MRI protocols have been suggested for breast cancer imaging in recent years [6, 13, 15,16,17,18,19]. These studies have in common that they mainly aim to feature abbreviated breast MRI as a screening method due to its high sensitivity. So far, there are no studies examining an abbreviated breast PET/MRI staging protocol for breast cancer patients. Although screening protocols do not meet the Breast Imaging-Reporting and Data System (BI-RADS) standard of a multiparametric breast MRI staging, they imply the possibility to shorten the MRI part of breast PET/MRI without losing the possibility to exclude further tumor manifestations [20].
Aiming towards faster breast [18F]FDG-PET/MRI protocols, this is the first study that systematically investigates the effect of reduced PET acquisition times on PET image quality and quantification parameters as well as a shortened, fast breast MRI protocol in a clinical setting of T1tumor-staged (T1ts) and T2tumor-staged (T2ts) breast cancer.
Material and methods
Patients
The institutional review boards of the University Duisburg-Essen and Düsseldorf, Germany (study number 17-7396-BO/6040R), approved this study, and it was performed in conformance with the Declaration of Helsinki [21]. As this was a retrospective branch of a prospective trial (register number: DRKS00005410), informed consent form was obtained at time of inclusion from all patients to cover further analysis. All patients underwent a dedicated breast [18F]FDG-PET/MRI for staging purposes.
In a retrospective evaluation, 90 females (mean age: 54 years ± 12 years; range 30–82 years) with histopathologically proven, newly diagnosed T1ts or T2ts breast cancer were selected for further analysis. The aim of the pre-selection was to include smaller tumor stages in particular, as PET and MRI detection could be more difficult here according to the smaller tumor size. Finally, 45 T1ts (up to 20 mm maximal diameter) patients and 45 T2ts (20 to 50 mm maximal diameter) patients were included [22].
Breast PET/MRI
All [18F]FDG-PET/MRI examinations were performed on an integrated 3-T PET/MRI system (Biograph mMR, Siemens Healthcare GmbH). All patients underwent a dedicated breast [18F]FDG-PET/MRI with an average delay of 68 ± 15 min after injection of bodyweight-adapted dosage of 18F-FDG (4 MBq/kg bodyweight). To ensure blood glucose levels below 150 mg/dl, blood samples were obtained prior to injection. Mean activity was 244 ± 38 MBq.
Dedicated and comprehensive breast [18F]FDG-PET/MRI examinations were performed in head-first prone position utilizing a dedicated 16-channel radiofrequency (RF) breast coil (Rapid Biomedical), developed and designed for use in integrated whole-body PET/MR imaging [23]. Simultaneously PET data and MRI data of both breasts were acquired. PET list-mode data was acquired for 20 min with one bed station. For attenuation correction (AC) of the patient tissue, a Dixon VIBE MR sequence was used (TA 19 s per bed position) [24]. MR images of the Dixon VIBE sequence were automatically segmented into four tissue classes (background air, lung, fat, and soft tissues) with pre-defined linear attenuation coefficients (LAC). The resulting AC map was completed with a bone atlas and truncation correction with the field-of-view extension method named HUGE (B0 homogenization using gradient enhancement) [25, 26]. For the RF breast coil AC, a registered CT-based AC map was implemented on the PET/MR system [23]. To evaluate the qualitative and quantitative impact of reduced PET acquisition time on PET data, the PET list-mode data of all 90 patients were reconstructed each with four different time intervals to simulate reduced PET acquisition times. Time intervals of 20, 15, 10, and 5 min were used. The 20-min PET reconstruction served as the reference standard. All PET list-mode reconstructions were performed retrospectively with e7 tools (Siemens Molecular Imaging) using the iterative ordinary Poisson ordered-subset expectation maximization algorithm (OP-OSEM) with three iterations and 21 subsets. A 4-mm full width at half-maximum Gaussian filter was applied. The resulting PET images have matrix dimensions of 344 × 344 × 127 and a resolution of 2.09 × 2.09 × 2.03 mm3 per bed position. A single Compton scatter simulation with relative scaling was applied to account for scattered events.
The originally acquired multiparametric breast MRI protocol is shown in Fig. 1 (please see Table 1 for detailed information).
Experienced breast imaging specialists at our institutions implemented a fast breast MRI protocol for this study in consensus with own experience and current literature. The protocol comprised a transversal diffusion-weighted echo-planar imaging sequence (TA 2:34 min) and three repetitions (1 × pre- and 2 × post-contrast-imaging; TA: 1:30 each excluding 30 s of pause) of a transversal 3-dimensional T1w (FLASH) sequence (TAtotal 5:00 min) of the original protocol.
Image analysis
PET data evaluation—clinical assessment
All breast PET datasets with different reconstructed time intervals for each patient (20/15/10/5 min) were analyzed regarding subjective image quality, applying a 5-point scale (see Table 2) and the number of detected breast lesions (n). Image reading was performed by a radiologist board certified in both radiology and nuclear medicine with 10 years of experience in hybrid imaging and expert in breast imaging and a radiologist with 3 years of experience in hybrid imaging. Readers were aware of breast cancer diagnosis but blinded to patient identification data. Different reconstructed images of each patient were presented separately and in random order. Any discrepancies between the two readers were resolved in a subsequent consensus reading.
PET data evaluation—objective measurements
Following quantitative PET evaluation, further quantitative imaging parameters were assessed for every PET image acquisition time of every patient in a second session: (i) standardized uptake values (SUV) SUVmean, SUVmax, SUVSD in the index lesion of the breast, and (ii) SUVmean, SUVSD of breast background enhancement in the same quadrant on the opposite breast. The PET images were analyzed starting with the 20 min/bed timeframe down to 5 min/bed timeframe to avoid bias in lesion detectability. Measurements were performed by using OsiriX (Pixmeo SARL). The index lesion of the breast was initially segmented by OsiriX using fixed threshold set to 40% of SUVmax to avoid that signal from non-malignant breast tissue was included in the evaluation. The reader checked segmentations for correctness. Additionally, a 1.5 cm volume-of-interest (VOI) was placed in the same quadrant on the opposite breast to evaluate breast background enhancement serving as the reference standard measurement. All segmentations and VOIs were copied in identical planes and positions in each reconstructed time interval for each patient. Afterwards, signal-to-noise-ratio (SNR), contrast-to-noise-ratio (CNR), and image noise (IN) for each PET time interval were calculated as described by Yan et al [27]:
MRI data evaluation—originally acquired breast MRI vs. fast breast MRI
Datasets of the originally acquired breast MRI and datasets of the fast breast MRI were analyzed regarding malignant breast lesion detectability. The PET data were not included for this evaluation. Image reading was performed by the same radiologist board certified in both radiology and nuclear medicine with 10 years of experience in hybrid imaging and expert in breast imaging and the radiologist with 3 years of experience in hybrid imaging. A reading intermission of 4 weeks towards previous PET data evaluation was performed to avoid recognition bias. Readers were aware of breast cancer diagnosis but blinded to patient identification data. A dataset of the originally acquired breast MRI and a dataset of the fast breast MRI of each patient were presented separately and in random order. Breast MRI data were analyzed for malignant lesions in accordance with the American College of Radiology MRI BI-RADS lexicon [20]. The maximum diameter of all suspicious index lesions was measured utilizing the MRI sequence that facilitated the best tumor depiction. Discrepancies between the two readers were resolved in a consensus reading.
Statistical analysis
SPSS Statistics 26 (IBM Inc.) was used for statistical analysis. Descriptive analysis was performed and data are presented as mean ± SD. A T test was used for paired group comparison. The Fisher test and Mann-Whitney U test were used for independent group comparison. p values < 0.05 were considered to be statistically significant.
Results
Subjective evaluation of PET data image quality
Altogether 90 breast cancer patients were included for data evaluation; thereof, 45 patients presented with T1ts (mean size 13.6 mm ± 3 mm, range of 8–19 mm) and 45 patients presented with T2ts (mean size 26.9 mm ± 7 mm, range of 20–50 mm) breast cancer lesions. The quality of all PET images was rated as moderate to good at all reconstruction times (see Table 3). In 2/90 (2%) patients, all PET image reconstructions were classified as non-diagnostic.
There was a slight, but not significant difference in subjective image quality between the acquisition times of 20, 15, and 10 min towards 5 min (3.4 ± 0.9 vs. 3.3 ± 0.9; see Table 3 and Fig. 2) based on an observed image quality reduction in 4/90 (4%) patients. Although three of these four patients belong to the T1ts subgroup and only one belongs to the T2ts subgroup, there was no significant difference in observed image quality reduction comparing these two tumor subgroups (Table 4). T1ts vs. T2ts breast cancer subgroup comparison of image quality revealed a minimal but not significant difference for reconstruction times of 20, 15, and 10 min (T1ts: 3.3 ± 1 vs. T2ts: 3.4 ± 0.8) towards 5 min (T1ts: 3.3 ± 1.1 vs. T2ts: 3.4 ± 0.8). In all PET reconstructions, 127 breast lesions could be detected, without any differences (Table 3).
Overall, no significant differences were found, neither comparing T1ts and T2ts breast cancer patients together at different PET reconstruction times nor comparing T1ts to T2ts patients at different PET reconstruction times.
Objective metrics of PET data image quality and SUVmax group comparison
Qualitative image evaluation using SNR revealed no significant difference between all PET acquisition times (20/15/10/5 min). The CNR decreases slightly from 43.5 to 38.9 with shortened acquisition times without being statistically significant (Table 3). Additionally IN increases slightly but significantly towards shorter PET acquisition times from 29.0 to 31.4% (p < 0.05; see Table 3 and Fig. 3). The group comparison of lesion SUVmax between all PET acquisition times (20/15/10/5 min) revealed no significant differences with a mean SUVmax value of 6.1 ± 4.5 (20 min) and 6.2 ± 4.6 (15/10/5 min).
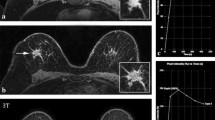
PET and MRI images of a 57-year-old breast cancer patient with an increase of image noise from 20 (A), 15 (B), 10 (C) to 5 min (D) and an associated slight subjective image quality reduction from 20/15/10 towards 5 min (image quality 3 vs. 2 according to the 5-point scale). The fast breast MRI protocol (white arrow: primary) visualized in the lower section (left: DWI, middle: ADC, right: T1-early CE-2) provides adequate diagnostic information
Lesion-based evaluation of original breast MRI vs. fast breast MRI
Altogether 136 congruent breast cancer lesions were detectable with the original breast MRI protocol and the fast breast MRI protocol, respectively. There was no significant difference in breast lesion detectability between both breast MRI protocols (see Figs. 1, 4, and 5).
The shortened diagnostic fast breast PET/MRI protocol with acquisition time (TA) of each MR sequence and simultaneous PET acquisition time (PTA). Following tracer injection (TI) and the active waiting time, dedicated breast PET/MRI in prone position was acquired. The full 20 min PET acquisition time served as the reference standard and was retrospectively shortened to 15/10/5-min intervals
Discussion
A key problem of [18F]FDG PET/MRI breast cancer staging is the time-consuming nature. With 20 min acquisition time, the dedicated breast [18F]FDG-PET/MRI is a time-consuming part of the overall examination process. Nevertheless, it is of great importance due to its local tumor staging and phenotyping abilities and should not be skipped aiming towards faster examination protocols [5, 28, 29]. Reducing the time of PET data acquisition while implementing a shortened but still diagnostic breast MRI protocol might solve the problem of long examination times.
Aiming towards faster breast [18F]FDG-PET/MRI protocols, this is the first study that systematically investigates the effect of reduced PET acquisition times on PET image quality and quantification parameters as well as the diagnostic feasibility of a fast breast MRI protocol in a clinical setting of T1ts and T2ts breast cancer.
Except for the image noise evaluation, our subjective and objective PET data evaluations show no relevant differences in image quality between each time reconstruction of PET data as well as SUXmax measurements. Image noise significantly increases slightly towards shorter acquisition times without any effect on the diagnostic value of the acquired PET images. Thus, the acquisition time of PET data can be easily shortened to 5 min without a significant loss of diagnostic abilities. This would enable a time saving of 15 min with regard to the acquisition time of the original breast [18F]FDG-PET(/MRI) protocol. Bearing in mind that this study focuses on smaller to moderate sized breast cancer, T-valued tumor size does not affect the choice of PET acquisition time. This illustrates that even smaller, metabolically active T1ts breast cancers are detectable without relevant problems by using an acquisition time of 5 min although image noise shows a slight but significant increase. Our results are in good agreement with other studies focusing on whole-body [18F]FDG-PET/MRI protocols. It was shown that whole-body [18F]FDG-PET/MRI protocols could be even shortened to about 2 min PET acquisition time [11, 30].
However, without reducing the MRI portion of the breast [18F]FDG-PET/MRI protocol by retaining good diagnostic abilities, a reduction of the PET time acquisition alone is little purposeful. Following the current literature, breast MRI protocols can be easily shortened to 3 min consisting of single T1w pre- and post-contrast-enhanced sequences for screening purposes [16, 19]. This reflects the possibility to shorten breast MRI protocols to answer the question “suspicious” or “unsuspicious.” Even if it does not meet the BI-RADS standard of a multiparametric breast MRI, it would help to exclude further tumor manifestations at women of our cohort with histologically proven breast cancer. We believe that the following sequences are necessary for a fast but still diagnostic breast MRI for individual therapy planning of patients out of our cohort: diffusion-weighted imaging, T1w-FLASH native, and two times T1w-FLASH early contrast enhanced (CE). The mentioned fast breast MRI thus results in a 7:53-min protocol including a 30-s break after the application of an MRI contrast agent. All included patients already have a histologically confirmed carcinoma. Therefore, the elimination of a T2w breast sequence in an abbreviated breast [18F]FDG-PET/MRI protocol is clearly calculated, as the main goal of this initial staging is to image the exact extent and further therapeutic planning. In most cases, breast cancer does not show a raised signal in T2w images due to its high cellularity and low water content. Consequently, T2w sequences are helpful to distinguishing benign from malignant breast lesions, which is not necessary in this cohort [31]. However, most of these lesions can also be detected on T1w images and patients of our cohort are already diagnosed with at least one breast cancer lesion. Thus, the additive gain of information by using T2w images seems negligible in this context. More important than a T2w sequence at breast cancer patients of our cohort is a DWI sequence. Breast lesions in general have altered diffusion characteristics compared to benign fibroglandular tissue, and DWI signal correlates with tumor biology. Furthermore, altered DWI signal intensity may also play a role in pre- and post-treatment management decisions by indicating therapy response [7, 32,33,34,35,36,37]. Moreover, studies show that DWI can, similar to T2w images, be used to assess lymphovascular tumor invasion as one main cause of peri-focal tumor edema [38, 39]. The high spatial resolution and assessment of vascular permeability and neoangiogenesis leads to high sensitivity of T1w-CE MRI of the breast [40]. Hereby the need of T1w-early CE sequences is essential in breast cancer screening and kinetic lesion characterization is already possible after acquiring two post-contrast sequences [15, 16, 19, 41,42,43]. Our data support the choice of sequences for a fast breast MRI by maintaining a similar malignant lesion detectability in the fast protocol. This is especially due to the fact that nearly all malignant breast lesions were well defined at early T1w-FLASH sequences. Studies reported an increased diagnostic performance after combining multiple imaging parameters [44,45,46]. Thus, an additional PET component for breast [18F]FDG-PET/MRI can increase the diagnostic performance of a breast MRI protocol. Adding a PET component offers the possibilities to achieve functional and metabolic imaging parameters at the same scanning process. This results in a better lesion characterization and improves the positive predictive value (98% vs. 77%), the specificity (100% vs. 67%), and overall diagnostic accuracy (89% vs. 78%) [46, 47]. Furthermore, the metabolic PET imaging data may give a hint of the immunohistochemical components of the breast tumor without biopsy [48,49,50,51].
Taking all previous information together and according to our data, the implemented fast breast MRI with 7:53 min is diagnostic by saving 9:58 min according to the 17:51 min that is needed for the original breast MRI protocol. This significant time saving could increase patient satisfaction and patient throughput for breast cancer patients.
Our study is not without limitations. Due to its retrospective design, patients were selected based on the availability of stored data for the additional reconstructions. Thus, the evaluation of fast breast [18F]FDG-PET/MRI applies only to our patient cohort of T1ts and T2ts high-risk breast cancer patients. Nevertheless, this is the first large and homogeneous study that evaluates a fast breast [18F]FDG-PET/MRI for this patient cohort.
Finally, breast [18F]FDG-PET/MRI protocols can be considerably shortened from 20 to approximately 8 min without losing essential diagnostic information including a 5-min PET protocol and a 7:53-min MRI protocol. This enables a considerable reduction of the breast [18F]FDG-PET/MRI protocol and consequently higher patient throughput combined with greater patient satisfaction.
Abbreviations
- AC:
-
Attenuation correction
- CNR:
-
Contrast-to-noise ratio
- EPI:
-
Echo-planar imaging
- FLASH:
-
Fast low-angle shot T1w
- IN:
-
Image noise
- LAC:
-
Linear attenuation coefficients
- PET/MRI:
-
Positron emission tomography/magnetic resonance imaging
- PPV:
-
Positive predictive value
- SNR:
-
Signal-to-noise ratio
- SUV:
-
Standardized uptake values
- T1ts :
-
T1tumor-staged
- T1ts :
-
T1tumor-staged
- T2w:
-
T2-weighted
- TA:
-
Acquisition time
- TSE:
-
Turbo spin echo
- VOI:
-
Volume-of-interest
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Bruckmann NM, Morawitz J, Fendler WP et al (2022) A role of PET/MR in breast cancer? Semin Nucl Med. https://doi.org/10.1053/j.semnuclmed.2022.01.003
Murthy V, Sonni I, Jariwala N et al (2021) The role of PSMA PET/CT and PET/MRI in the initial staging of prostate cancer. Eur Urol Focus 7:258–266
Heacock L, Weissbrot J, Raad R et al (2015) PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol 204:842–848
Kirchner J, Grueneisen J, Martin O et al (2018) Local and whole-body staging in patients with primary breast cancer: a comparison of one-step to two-step staging utilizing 18F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging 45:2328–2337
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292:520–536
Morawitz J, Kirchner J, Martin O et al (2021) Prospective correlation of prognostic immunohistochemical markers with SUV and ADC derived from dedicated hybrid breast 18F-FDG PET/MRI in women with newly diagnosed breast cancer. Clin Nuclear Med 46(3):201–205. https://doi.org/10.1097/rlu.0000000000003488
Gückel B, Gatidis S, Enck P et al (2015) Patient comfort during positron emission tomography/magnetic resonance and positron emission tomography/computed tomography examinations: subjective assessments with visual analog scales. Invest Radiol 50:726–732
Grueneisen J, Sawicki L, Schaarschmidt B et al (2016) Evaluation of a fast protocol for staging lymphoma patients with integrated PET/MRI. PLoS One 11:e0157880
Grueneisen J, Schaarschmidt B, Heubner M et al (2015) Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: a comparison to PET/CT. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2015.08.010
Lindemann ME, Stebner V, Tschischka A, Kirchner J, Umutlu L, Quick HH (2018) Towards fast whole-body PET/MR: investigation of PET image quality versus reduced PET acquisition times. PLoS One 13:e0206573
Hartung-Knemeyer V, Beiderwellen KJ, Buchbender C et al (2013) Optimizing positron emission tomography image acquisition protocols in integrated positron emission tomography/magnetic resonance imaging. Invest Radiol 48:290–294
Menezes GL, Knuttel FM, Stehouwer BL, Pijnappel RM, van den Bosch MA (2014) Magnetic resonance imaging in breast cancer: a literature review and future perspectives. World J Clin Oncol 5:61–70
Quick H (2014) Integrated PET/MR. J Magn Reson Imaging. https://doi.org/10.1002/jmri.24523
Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS (2015) Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 22:1157–1162
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB (2014) Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 32:2304–2310
Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S (2017) An abbreviated protocol for high-risk screening breast magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad Radiol 24:1132–1138
Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L (2016) An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol 13:374–380
Kuhl CK (2018) Abbreviated breast MRI for screening women with dense breast: the EA1141 trial. Br J Radiol 91:20170441
Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE (2017) BI-RADS® fifth edition: a summary of changes. Diagn Interv Imaging 98:179–190
Association WM (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) (2021) S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. Version 4.3 AWMF Registernummer: 032-045OL. https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/. Accessed 8 Sept 2022
Oehmigen M, Lindemann ME, Lanz T, Kinner S, Quick HH (2016) Integrated PET/MR breast cancer imaging: attenuation correction and implementation of a 16-channel RF coil. Med Phys 43:4808
Martinez-Möller A, Souvatzoglou M, Delso G et al (2009) Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med 50:520–526
Paulus DH, Quick HH, Geppert C et al (2015) Whole-body PET/MR imaging: quantitative evaluation of a novel model-based MR attenuation correction method including bone. J Nucl Med 56:1061–1066
Lindemann ME, Oehmigen M, Blumhagen JO, Gratz M, Quick HH (2017) MR-based truncation and attenuation correction in integrated PET/MR hybrid imaging using HUGE with continuous table motion. Med Phys 44:4559–4572
Yan J, Schaefferkoetter J, Conti M, Townsend D (2016) A method to assess image quality for low-dose PET: analysis of SNR, CNR, bias and image noise. Cancer Imaging 16:26
Umutlu L, Kirchner J, Bruckmann N-M et al (2021) Multiparametric integrated 18F-FDG PET/MRI-based radiomics for breast cancer phenotyping and tumor decoding. Cancers 13:2928
Umutlu L, Kirchner J, Bruckmann N-M et al (2022) Multiparametric 18F-FDG PET/MRI-based radiomics for prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer. Cancers 14:1727
Nevo E, Kamvosoulis P, Currie G (2022) PET/MRI, part 3: protocols and procedures. J Nucl Med Technol 50:17–24
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Guo Y, Cai YQ, Cai ZL et al (2002) Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 16:172–178
Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW (2002) In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 15:693–704
Richard R, Thomassin I, Chapellier M et al (2013) Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol 23:2420–2431
Pickles MD, Gibbs P, Lowry M, Turnbull LW (2006) Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 24:843–847
Park SH, Moon WK, Cho N et al (2012) Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol 22:18–25
Iwasa H, Kubota K, Hamada N, Nogami M, Nishioka A (2014) Early prediction of response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and gray-scale ultrasonography. Oncol Rep 31:1555–1560
Igarashi T, Furube H, Ashida H, Ojiri H (2018) Breast MRI for prediction of lymphovascular invasion in breast cancer patients with clinically negative axillary lymph nodes. Eur J Radiol 107:111–118
Auvinen P, Tammi R, Parkkinen J et al (2000) Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 156:529–536
Plecha DM, Faulhaber P (2017) PET/MRI of the breast. Eur J Radiol 94:A26–A34
Comstock CE, Gatsonis C, Newstead GM et al (2020) Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 323:746–756
Geach R, Jones LI, Harding SA et al (2021) The potential utility of abbreviated breast MRI (FAST MRI) as a tool for breast cancer screening: a systematic review and meta-analysis. Clin Radiol 76:154.e111-154.e122
Choudhery S, Chou SS, Chang K, Kalpathy-Cramer J, Lehman CD (2020) Kinetic analysis of lesions identified on a rapid abridged multiphase (RAMP) breast MRI protocol. Acad Radiol 27:672–681
Pinker K, Bogner W, Baltzer P et al (2014) Improved differentiation of benign and malignant breast tumors with multiparametric 18fluorodeoxyglucose positron emission tomography magnetic resonance imaging: a feasibility study. Clin Cancer Res 20:3540–3549
Moy L, Ponzo F, Noz ME et al (2007) Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med 48:528–537
Botsikas D, Kalovidouri A, Becker M et al (2015) Clinical utility of 18F-FDG-PET/MR for preoperative breast cancer staging. Eur Radiol 26:2297–2307
Moy L, Noz ME, Maguire GQ Jr et al (2010) Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J 16:369–376
Catalano OA, Horn GL, Signore A et al (2017) PET/MR in invasive ductal breast cancer: correlation between imaging markers and histological phenotype. Br J Cancer 116:893–902
Koo HR, Park JS, Kang KW et al (2014) 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol 24:610–618
Surov A, Meyer HJ, Wienke A (2019) Associations between PET parameters and expression of Ki-67 in breast cancer. Transl Oncol 12:375–380
Sanli Y, Kuyumcu S, Ozkan ZG et al (2012) Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med 26:345–350
Acknowledgements
Many thanks to Annika Stawitzki from the Department of Nuclear Medicine of the University Hospital Essen for the support in collecting the data.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is funded by Deutsche Forschungsgemeinschaft (DFG), the German Research Foundation (BU3075/2‑1 and KI2434/1–2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication are Kai Jannusch and Julian Kirchner.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Disclaimer
The funding foundation was not involved in trial design, patient recruitment, data collection, analysis, interpretation or presentation, writing or editing of the reports, or the decision to submit for publication. The corresponding author had full access to all data in the study and had all responsibility for the decision to submit for publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jannusch, K., Lindemann, M.E., Bruckmann, N.M. et al. Towards a fast PET/MRI protocol for breast cancer imaging: maintaining diagnostic confidence while reducing PET and MRI acquisition times. Eur Radiol 33, 6179–6188 (2023). https://doi.org/10.1007/s00330-023-09580-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09580-6