Abstract
Introduction
Radiographs of the hand and teeth are frequently used for medical age assessment, as skeletal and dental maturation correlates with chronological age. These methods have been criticized for their lack of precision, and magnetic resonance imaging (MRI) of the knee has been proposed as a more accurate method. The aim of this systematic review is to explore the scientific and statistical evidence for medical age estimation based on skeletal maturation as assessed by MRI of the knee.
Materials and methods
A systematic review was conducted that included studies published before April 2021 on living individuals between 8 and 30 years old, with presumptively healthy knees for whom the ossification stages had been evaluated using MRI. The correlation between “mature knee” and chronological age and the risk of misclassifying a child as an adult and vice versa was calculated.
Results
We found a considerable heterogeneity in the published studies —in terms of study population, MRI protocols, and grading systems used. There is a wide variation in the correlation between maturation stage and chronological age.
Conclusion
Data from published literature is deemed too heterogenous to support the use of MRI of the knee for chronological age determination. Further, it is not possible to assess the sensitivity, specificity, negative predictive value, or positive predictive value for the ability of MRI to determine whether a person is over or under 18 years old.
Key Points
• There is an insufficient scientific basis for the use of magnetic resonance imaging of the knee in age determination by skeleton.
• It is not possible to assess the predictive value of MRI of the knee to determine whether a person is over or under 18 years of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining a person’s age is important for many legal processes, e.g., regarding child labor, sexual assault, prostitution, and sometimes for elite athletes, but is particularly relevant in the asylum procedure. In most European countries, the cut-off age of minority is 18 years of age [1, 2]. Individuals below the age of 18 are entitled to have their legal rights as minors respected in accordance with national and international laws and regulations [3]. Medical tests to assess a person’s psychological and physiological development level have been used as methods for chronological age assessment, particularly in young unaccompanied asylum seekers when their date of birth is unknown, or when they lack documents to confirm their age [4]. There is a correlation between skeletal and dental development and chronological age; hence, methods for the assessment of skeletal maturation based on radiographs of the hand and maturation of the teeth are frequently used to assign a chronological age to an individual. However, those methods have been criticized for their lack of precision [5, 6].
Magnetic resonance imaging (MRI) has been proposed as a more advanced imaging technique for the evaluation of skeletal maturation. MRI is radiation free and can be applied to several bones, e.g., the clavicle and the hand. MRI of the knee is a method which in the recent decade has been proposed to potentially provide a more accurate method for chronological age assessment than traditional radiographic methods [7,8,9].
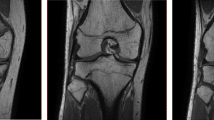
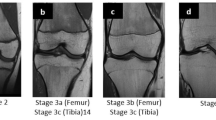
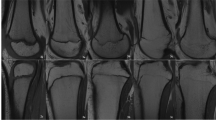
Grading of skeletal maturation of the knee is based on the appearances of the physeal line, or physis, which is the site of growth in a long bone. The physis is a fine structure consisting of mesenchymal cells in different maturation stages. The physis becomes thinner and thinner throughout the process of skeletal maturation, before it disappears and endochondral ossification ceases. There are six different grading systems for skeletal maturation of the knee assessed by MRI: (1) Schmeling and Kellinghaus, (2) Vieth, (3) Dedouit, (4) Dedouit, Kellinghaus, and Schmeling, modified version, (5) Jopp and (6) Schmeling. All grading systems classify the maturation into exclusive stages based on the characterization and delineation of the thin physeal line (Tables 1 and 2).
The aim of this systematic review is to explore the scientific evidence for medical age estimation based on skeletal maturation as assessed by MRI of the knee. We also wanted to explore the likelihood for a minor to be misclassified as an adult, or vice versa, for an adult to be misclassified as a minor, when MRI of the knee is used for chronological age estimation in a forensic setting.
Materials and methods
Protocol and registration
This systematic review was conducted at the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU), an assignment by the Ministry of Health and Social Affairs in Sweden, and published in Swedish in October 2021 [23]. SBU uses a peer-reviewed protocol for systematic reviews. The systematic review process follows the general concepts covered by Preferred Reporting Items for Systematic Reviews and Meta-analyses, PRISMA [24].
Eligibility criteria
A study was considered eligible if it reported data for living study participants between the ages of 8 and 30 years with no pathological problems of the knee or ankle (population) for whom the ossification stages of the knee (distal femur) had been evaluated using MRI (index test). The chronological age was known through records (reference test), and the diagnostic accuracy (outcome) was reported as sensitivity/specificity or by a correlation of age and ossification stage. Only cross-sectional studies and longitudinal studies written in English, German, or any Scandinavian language were included.
Literature search
A systematic literature search was conducted by an information specialist in the following databases: Cochrane Library (Wiley), Embase (Elsevier), Medline (OvidSP), Epistemonikos, KSR Evidence, and International HTA Database. The search strategy was developed and executed in close collaboration with the co-authors LSOM (radiologist) and JD (pediatrician).
A systematic review by Ding et al [25] was used as a starting point for the literature search. Our search was thus limited to studies published between January 2017 and March 2021; all studies included in the systematic review by Ding et al were evaluated for eligibility (see section “Study selection”).
In addition, a reference and citation search of the included studies was performed in the database Scopus (Elsevier). The complete search strategy is provided in Supplement 1.
Study selection
Two reviewers independently screened the titles and abstracts identified by the literature search strategy. All studies of potential relevance according to the inclusion criteria were obtained in full text, and two reviewers independently assessed them for inclusion. In addition, the articles included in the systematic review by Ding et al [25] were screened for relevance. Any disagreement was resolved by discussion. Excluded studies are shown in Supplement 2.
Risk of bias in individual studies
Quality assessment (risk of bias) of the included studies, both from the literature search and the systematic review by Ding et al [25], was performed by two independent reviewers using a modified version of Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 (Supplement 3). Any disagreement was resolved by discussion. Each study was rated as having low, moderate, or high risk of bias. Studies with high risk of bias were not included in the analysis.
Data collection process
Data was extracted and tabulated from each included study with low or moderate risk of bias by one reviewer. The extracted data was audited by a second reviewer. The extracted data were study design; how the index test, reference test, and outcome measured; population; and setting (Supplement 4).
Method of analysis
Data from each study was presented as a correlation between “mature knee” and age. In the studies where the number of subjects in each stage was presented for each age group, no recalculation was required [11, 14, 18, 21]. When data was presented using descriptive statistics regarding the age distribution conditional on being in a specific stage, i.e., mean age ± standard deviation (SD) for each stage, recalculation was performed. The mathematical method used for recalculation was based on a method described by Mostad et al [26] and Bleka et al [27], with the modification that we relaxed the assumption about normality and instead used the information about the exact age distribution of the subjects included in the study [23].
Rating the certainty of the evidence
The certainty in the estimated prevalence rates was supposed to be assessed using grading of recommendations assessment, development, and evaluation (GRADE) [28]. However, since the studies were too heterogenous to perform a meta-analysis, or even for a meaningful narrative review, we decided to refrain from a GRADE assessment.
Results
The literature search yielded 2529 references to be screened (see Fig. 1). Of these, 39 were reviewed in full text [10,11,12,13,14,15,16,17,18,19,20,21,22, 25, 26, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. A total of 16 articles were considered eligible since they met the inclusion and exclusion criteria. Of these, 13 had low or moderate risk of bias and are included in the analysis [10,11,12,13,14,15,16,17,18,19,20,21,22]. The risk of bias chart is shown in Supplement 5. Three articles had high risk of bias: one due to an inadequate description of the MRI method, one lacked complete information regarding birth date, and one due to several limitations. See Supplement 2 for the list of excluded studies. The characteristics of the included studies are presented in Supplement 4.
Study characteristics
Out of the 13 studies, 9 were retrospective [10, 11, 13,14,15,16,17, 19, 21] and 4 prospective [12, 18, 20, 22]. The studies were from Iran [13], Turkey [10, 11, 15, 16, 21], Germany [12, 17, 20, 22], Sweden [18], France [14], and USA [19]. The study population consisted of various subjects such as patients who underwent MRI for assessment of traumatic or degenerative changes to the knee joint [10, 11, 14, 16, 17, 19, 21], healthy volunteers (4 studies) [12, 18, 20, 22], and individuals assessed due to legal reasons (1 study) [13]. The information provided regarding the study population was sparse in most papers.
The studies used 6 different grading systems for evaluation of maturation stage of the knee on MRI: Schmeling and Kellinghaus [15, 17, 20], Vieth [10, 16, 22], Dedouit [11, 14, 19, 21], Dedouit and Kellinghaus (modified version of Schmeling) [18], Jopp [12], and Schmeling [13] (Table 1). The MRI grading systems differ both in terms of the number and the definition of stages of maturation. Stages varied between 3 and 5, with up to 11 substages. The stages were described with varying degrees of detail in the original publications, and there was a mix of quantitative and qualitative criteria. Therefore, the stages in the different grading systems were overlapping and could not be pooled. The MRI protocols used varied between the studies (see Table 1).
In 8 studies, two readers had reviewed all images, but in 4 studies, only 10–21% of the images were reviewed by two readers, and in one study, the information was unclear (Table 1).
The risk for misclassification
The studies were deemed too heterogeneous with regard to MRI protocols, grading system, and population; therefore, a meta-analysis was not performed (Table 1 and Supplement 4). Even in the studies using the same grading system, the population and MRI protocol were considered too heterogeneous to allow a meta-analysis. We therefore present the results from each study individually. The results are presented separately for males and females. We present the results for the most relevant classification stages for age determination, where individuals both over and under 18 years are represented (Table 2).
Of the 13 studies, 4 presented data as stage per age [11, 14, 18, 21] and no recalculations had to be performed. For the remaining nine studies [10, 12, 13, 15,16,17, 19, 20, 22], recalculation was performed for six of them [10, 15,16,17, 20, 22]. Two studies could not be included in the analysis since they lacked information about minimum and maximum age which is required for the recalculations [13, 19], and one study had only sufficient information for 6 participants [12].
In Fig. 2, the proportion of males or females with a mature knee per age (interval 15–23 years) is presented. The curves are estimated by using a logistic regression function, \((p(x)=1/1+e^{-(\beta_0+\beta_1X)}\), with age as the independent variable and mature/not-mature as the dependent variable. The model estimates the probability of having a mature knee as a function of age.
The data show clearly how the results differ between the studies. For example, the maturation of the knee for males seems to appear later in the studies by Kramer et al [17] and Dedouit et al [14] than in the studies by Vieth and Ottow et al [20, 22].
We calculated the risk for misclassification, i.e., the risk that a minor (an individual 17 years or younger) would be misclassified as an adult (an individual 18 years or older) or that an adult would be misclassified as a minor, in the age interval of 15 to 21 years for each of the studies. Figure 3 illustrates the results based on data from the study by Ottow et al [20]. In Table 3, the results for all grading systems and all studies are presented. The risk of misclassification varies between studies (Table 3). The risk for misclassification is generally much higher in females than in males until the age of 17, at which point males are more likely to be misclassified. For females, where the knee matures earlier, the highest risk for misclassification is between 16 and 18 years of age. For males, the risk is the highest between the ages of 17 and 18 years.
Positive predictive value, negative predictive value, sensitivity, and specificity
To be able to calculate positive predictive value (PPV), negative predictive value (NPV), specificity, and sensitivity, the frequency/ratio of mature and immature knee per age, as well as the age distribution of the population tested, needs to be known. The risk of misclassification depends on the actual age distribution of those tested. For example, if many individuals are tested in the age range 17 to 18 years, the risk of misclassification will be high. If the age range is widened, the proportion of errors will be lower.
To illustrate this issue, we have performed calculations on five hypothetical populations, each with a different age distribution (Table 4).
Using data from the study by Ottow et al (male) [20], we illustrate how the same datapoints give rise to different predictive values in response to variation in the age distribution of the population tested (Fig. 4). This is further elaborated in Supplement 6 where predictive values using datapoints from other included studies on the same five hypothetical populations are presented. The calculations show that NPV, PPV, sensitivity, and specificity for MRI of the knee as a test to determine whether a person is over or under 18 years old cannot be calculated in individuals between 15 and 21 years of age unless the exact age composition of the group is known.
Discussion
This systematic review shows that the proportion of individuals per age group deemed to have a mature knee on MRI varies considerably between the included studies. Women’s knees seem to mature earlier than men’s knees; however, the magnitude of the difference was not consistent between studies. The calculated risk for misclassification for each study also varied because it depends on the dataset from which the calculations are based. The variation in results of maturation according to age can be explained by the different grading systems, MRI protocols, and study populations in the published studies.
Further, we illustrate how calculating the risk of misclassification for the purpose of forensic age determination is paradoxical, as that risk can only be calculated in a population with a known age distribution. This fundamental statistical limitation cannot be overcome, no matter how advanced the measurement techniques are, or how well future studies are conducted.
Maturation of the knee is influenced by individual factors such as genetics, medical conditions, and nutrition [53]. This causes a wide, and somewhat unpredictable, variation in skeletal maturation rates. Since these factors naturally vary between individuals, the correlation between chronological age and bone maturation is influenced by the characteristics of the study population.
MRI is probably the modality within the field of radiology which is the most difficult to standardize, both in terms of image acquisition and image reading [54, 55]. Therefore, results from studies performed in different institutions, on different MRI machines, with different readers, different image scan parameters, and with different scoring systems, are not directly comparable, and to do so will result in substantial variability. For instance, Kvist et al [18] demonstrated that the use of different weightings applied on the same individuals influenced the grading of physeal maturation. Objective assessment of MRI signal is difficult because readers tend to perceive the same image intensity differently, depending on surrounding background intensities [56]. Reader experience and calibration of the reading structure also influence the interpretation of the images [18].
One strength of this systematic review is the strict adherence to international standards for systematic reviews. Studies were identified and selected according to the PRISMA statement which is internationally regarded as state of the art for performing and reporting systematic reviews (see Supplements 1–5). Another strength is our multidisciplinary approach where information specialists and statisticians worked in close collaboration with clinical experts, including pediatricians and a pediatric radiologist.
The main limitation of this systematic review is that relatively few studies were identified. Larger and more comparable studies may be able to show a stronger trend in terms of the proportion of individuals per age group deemed to have a mature knee on MRI. Still, we will not be able to able to predict the risk of misclassification in individuals with an unknown age due to the statistical paradox described above.
Forensic age estimations can have huge legal implications for the individual being evaluated, as well as for countries and authorities who use such methods. Therefore, the methods used should be reliable and reproducible and the statistical calculations of probability must exactly fit the population to which it is applied.
In conclusion, there is a considerable heterogeneity in the published studies on forensic age assessment based on MRI of the knee. Therefore, neither a meta-analysis nor a meaningful narrative review could be performed. Furthermore, the actual risk of misclassifying a minor as an adult and vice versa can never be calculated in a group of individuals with unknown age distribution.
Abbreviations
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
- SBU:
-
The Swedish Agency for Health Technology Assessment and Assessment of Social Services
- SD:
-
Standard deviation
References
EASO (2019) EASO practical guide on the best interests of the child in asylum procedures. In: EASO practical guides series. https://doi.org/10.2847/084111
EASO (2021) EASO age assessment practices in EU+ countries: updated findings. In: EASO practical guide series. https://doi.org/10.2847/565210
UNHCR, UNICEF, IOM (2019) Refugee and migrant children in Europe accompanied, unaccompanied and separated: Overview of trends January to December 2019. UNHCR, UNICEF and IOM. Available via https://www.unhcr.org/cy/wp-content/uploads/sites/41/2020/06/UNHCR-UNICEF-and-IOM_Refugee-and-Migrant-children-in-Europe-2019.pdf
EASO (2018) EASO practical guide on age assessment, Second edn. EASO Practical Guides Series. isbn:978-92-9494-647-8
Ording Müller L-S, Offiah A, Adamsbaum C et al (2019) Bone age for chronological age determination — statement of the European Society of Paediatric Radiology musculoskeletal task force group. Pediatr Radiol 49:979–982
VMA (2019) WMA statement on medical age assessment of unaccompanied minor asylym seekers. World Medical Association, Tbilisi
Dvorak J, George J, Junge A, Hodler J (2007) Age determination by magnetic resonance imaging of the wrist in adolescent male football players. Br J Sports Med 41:45–52
Hillewig E, De Tobel J, Cuche O, Vandemaele P, Piette M, Verstraete K (2011) Magnetic resonance imaging of the medial extremity of the clavicle in forensic bone age determination: a new four-minute approach. Eur Radiol 21:757–767
Heldring N, Larsson A, Rezaie AR, Råsten-Almqvist P, Zilg B (2022) A probability model for assessing age relative to the 18-year old threshold based on magnetic resonance imaging of the knee combined with radiography of third molars in the lower jaw. Forensic Sci Int 330:111108
Alatas O, Altınsoy HB, Gurses MS, Balci A (2021) Evaluation of knee ossification on 1.5 T magnetic resonance images using the method of Vieth et al.: A retrospective magnetic resonance imaging study. Rechtsmedizin 31:50–58
Altinsoy HB, Alatas O, Gurses MS, Turkmen Inanir N (2020) Forensic age estimation in living individuals by 1.5 T magnetic resonance imaging of the knee: a retrospective MRI study. Aust J Forensic Sci 52:439–453
Auf der Mauer M, Saring D, Stanczus B, Herrmann J, Groth M, Jopp-van Well E (2019) A 2-year follow-up MRI study for the evaluation of an age estimation method based on knee bone development. Int J Legal Med 133:205–215
Daghighi MH, Pourisa M, Javanpour-Heravi H et al (2021) Application of knee MRI in forensic age estimation: A retrospective cohort. Radiography (Lond) 27:108–114
Dedouit F, Auriol J, Rousseau H, Rouge D, Crubezy E, Telmon N (2012) Age assessment by magnetic resonance imaging of the knee: a preliminary study. Forensic Sci Int 217:232.e231–232.e237
Ekizoglu O, Er A, Bozdag M et al (2021) Forensic age estimation via magnetic resonance imaging of knee in the Turkish population: use of T1-TSE sequence. Int J Legal Med 135:631–637
Gurses MS, Altinsoy HB (2020) Evaluation of distal femoral epiphysis and proximal tibial epiphysis ossification using the Vieth method in living individuals: applicability in the estimation of forensic age. Aust J Forensic Sci 53:431–447
Kramer JA, Schmidt S, Jürgens KU, Lentschig M, Schmeling A, Vieth V (2014) Forensic age estimation in living individuals using 3.0 T MRI of the distal femur. Int J Legal Med 128:509–514
Kvist OF, Dallora AL, Nilsson O et al (2020) Comparison of reliability of magnetic resonance imaging using cartilage and T1-weighted sequences in the assessment of the closure of the growth plates at the knee. Acta Radiol Open 9:2058460120962732
Margalit A, Cottrill E, Nhan D et al (2019) The spatial order of physeal maturation in the normal human knee using magnetic resonance imaging. J Pediatr Orthop 39:e318–e322
Ottow C, Schulz R, Pfeiffer H, Heindel W, Schmeling A, Vieth V (2017) Forensic age estimation by magnetic resonance imaging of the knee: the definite relevance in bony fusion of the distal femoral- and the proximal tibial epiphyses using closest-to-bone T1 TSE sequence. Eur Radiol 27:5041–5048
Uygun B, Kaya K, Kose S, Ekizoglu O, Hilal A (2021) Applicability of magnetic resonance imaging of the knee in forensic age estimation. Am J Forensic Med Pathol 42:147–154
Vieth V, Schulz R, Heindel W et al (2018) Forensic age assessment by 3.0T MRI of the knee: proposal of a new MRI classification of ossification stages. Eur Radiol 28:3255–3262
SBU (2021) Åldersbedömning – magnetkameraundersökning av tillväxtzonen i lårbenets nedre del (knät) och röntgenundersökning av visdomständer i underkäkenSBU Bereder. Statens beredning för medicinsk och social utvärdering (SBU), Stockholm
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264−269):w264
Ding KY, Dahlberg PS, Rolseth V et al (2018) Development stages of the knee and ankle by computed tomography and magnetic resonance imaging for estimation of chronological age: a systematic review. Norwegian Institute of Public Health
Mostad P, Tamsen F (2019) Error rates for unvalidated medical age assessment procedures. Int J Legal Med 133:613–623
Bleka Ø, Wisløff T, Dahlberg PS, Rolseth V, Egeland T (2019) Advancing estimation of chronological age by utilizing available evidence based on two radiographical methods. Int J Legal Med 133:217–229
Guyatt G, Oxman A, Vist G et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924
Allen H, Liu F, Kijowski R, Nguyen J (2018) T2 mapping of articular cartilage of the normal pediatric knee at 3.0 t. Skeletal Radiol 47:453–454
Boeyer ME, Ousley SD (2017) Skeletal assessment and secular changes in knee development: a radiographic approach. Am J Phys Anthropol 162:229–240
Dallora AL, Berglund JS, Brogren M et al (2019) Age assessment of youth and young adults using magnetic resonance imaging of the knee: A deep learning approach. JMIR Med Inform 7:e16291
Dallora AL, Kvist O, Berglund JS et al (2020) Chronological age assessment in young individuals using bone age assessment staging and nonradiological aspects: Machine learning multifactorial approach. JMIR Med Inform 8:e18846
Ekizoglu O, Hocaoglu E, Can IO, Inci E, Aksoy S, Bilgili MG (2015) Magnetic resonance imaging of distal tibia and calcaneus for forensic age estimation in living individuals. Int J Legal Med 129:825–831
Ekizoglu O, Hocaoglu E, Inci E, Can IO, Aksoy S, Kazimoglu C (2016) Forensic age estimation via 3-T magnetic resonance imaging of ossification of the proximal tibial and distal femoral epiphyses: Use of a T2-weighted fast spin-echo technique. Forensic Sci Int 260:102.e101–102.e107
El-Din EAA, Mostafa HES, Tantawy EF, El-Shafei DA (2019) Magnetic resonance imaging of the proximal tibial epiphysis: could it be helpful in forensic age estimation? Forensic Sci Med Pathol 15:352–361
Gräwert S (2019) Forensic age assessment by means of MRI of the knee. Rofo 191:187–188
Herrmann J, Saring D, Auf der Mauer M, Groth M, Jopp-van Well E (2021) Forensic age assessment of the knee: proposal of a new classification system using two-dimensional ultrasound volumes and comparison to MRI. Eur Radiol 31:3237–3247
Konigsberg LW, Sgheiza V (2019) The use of Roche, Wainer, and Thissen's skeletal maturity of the knee. J Forensic Sci 64:1769–1775
Kvist O, Luiza Dallora A, Nilsson O et al (2021) A cross-sectional magnetic resonance imaging study of factors influencing growth plate closure in adolescents and young adults. Acta Paediatr 110:1249–1256
Laor T, Chun GF, Dardzinski BJ, Bean JA, Witte DP (2002) Posterior distal femoral and proximal tibial metaphyseal stripes at MR imaging in children and young adults. Radiology 224:669–674
Maggio A (2017) The skeletal age estimation potential of the knee: current scholarship and future directions for research. J Forensic Radiol Imaging 9:13–15
Mauer MA, Well EJ, Herrmann J et al (2021) Automated age estimation of young individuals based on 3D knee MRI using deep learning. Int J Legal Med 135:649–663
Meza B, LaValva S, Aoyama J et al (2020) Knee bone age using MRI: validation of a novel method to reduce hand bone age radiographs. Orthop J Sports Med 8(Suppl 6):1–2
Meza BC, LaValva SM, DeFrancesco CJ et al (2020) MRI knee bone age: a novel shorthand approach to reduce bone age radiographs in children. Orthop J Sports Med 8(Suppl 3):1–4
Nagrale N, Patond S, Ambad R, Bankar N, Jain K (2020) Forensic age estimation from proximal end of femur: A radiological study in living individuals. Indian J Forensic Med Toxicol 14:7117–7120
Pennock AT, Bomar JD (2017) Bone age assessment utilizing knee MRI. Orthop J Sports Med 5(Suppl 6):1–2
Pennock AT, Bomar JD, Manning JD (2018) The creation and validation of a knee bone age atlas utilizing MRI. J Bone Joint Surg Am 100:e20
Prove PL, Jopp-van Well E, Stanczus B et al (2019) Automated segmentation of the knee for age assessment in 3D MR images using convolutional neural networks. Int J Legal Med 133:1191–1205
Tamsen F (2017) Results of age determinations indicate errors in the method. Lakartidningen 21:114
Tamsen F (2017) A majority of girls near the age of 18 may be misjudged as adults with MRI-knee. Lakartidningen 26:114
Timme M, Karch A, Shay D, Ottow C, Schmeling A (2020) The relevance of body mass index in forensic age assessment of living individuals: an age-adjusted linear regression analysis using multivariable fractional polynomials. Int J Legal Med 134:1861–1868
Timme M, Karch A, Shay D, Ottow C, Schmeling A (2021) Age assessment of living individuals: the influence of socioeconomic status on skeletal and dental development in a German study cohort. Rechtsmedizin 31:35–41
Fleshman K (2000) Bone age determination in a paediatric population as an indicator of nutritional status. Trop Doct 30:16–18
Sharma PS, Saindane AM (2020) standardizing magnetic resonance imaging protocols across a large radiology enterprise: barriers and solutions. Curr Probl Diagn Radiol 49:312–316
Simmons A, Tofts PS, Barker GJ, Arridge SR (1994) Sources of intensity nonuniformity in spin echo images at 1.5 T. Magn Reson Med 32:121–128
Sinha P, Crucilla S, Gandhi T et al (2020) Mechanisms underlying simultaneous brightness contrast: early and innate. Vision Res 173:41–49
Acknowledgements
Fabian Soderdahl, Naama Kenan Moden, Jessika Degerhamn, and Anna Edling
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital) The literature search was conducted and funded by the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU), following an assignment by the Ministry of Health and Social Affairs in Sweden.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantors of this publication are Jenny Odeberg and Lil-Sofie Ording Müller.
Conflict of interest
Johan Bring is the owner, funder, and board member of the company Statisticon A.B.
Statistics and biometry
One of the authors (Johan Bring) has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because it is a literature review.
Ethical approval
Institutional review board approval was not required because the study is a literature review.
Study subjects or cohorts overlap
A commissioned online report, written in Swedish, published in October 2021 {SBU, 2021 #97}.
Methodology
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ording Muller, LS., Adolfsson, J., Forsberg, L. et al. Magnetic resonance imaging of the knee for chronological age estimation—a systematic review. Eur Radiol 33, 5258–5268 (2023). https://doi.org/10.1007/s00330-023-09546-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09546-8