Abstract
Objectives
To evaluate the feasibility of contrast-enhanced mammography (CEM)-guided biopsy at Hospital del Mar, a Spanish university hospital.
Methods
We retrospectively reviewed all consecutive women with a suspicious enhancing finding eligible for CEM-guided biopsy, who were prospectively enrolled in a pre-marketing clinical validation and feasibility study (October 2019 to September 2021). CEM-guided biopsy is a stereotactic-based procedure that, by using intravenous iodinated contrast media administration and dual-energy acquisition, provides localisation of enhancing lesions. All the biopsies were performed using a vacuum-assisted device. We collected procedural characteristics (patient position and type of approach), and histopathological results. Feasibility endpoints included success (visualisation of the enhancing lesion, post-procedural biopsy changes and clip placement), procedural time, number of scout acquisitions and complications.
Results
A total of 66 suspicious enhancing lesions (18.0% foci, 44.0% mass, 38.0% non-mass enhancement; median size 8.5 mm) in 64 patients (median age 59 years, mostly minimal [48.4%] or mild [32.8%] background parenchymal enhancement) were referred for CEM-guided biopsy in the study period. The success rate was 63/66 (95.4%). Amongst successful procedures, patients were most frequently seated (52/63, 82.5%) and the preferred approach was horizontal (48/63, 76.2%). Median total time per procedure was 15 min. Median number of acquisitions needed before targeting was 2 (range 1–4). Complications consisted of hematoma (17/63, 27%) and vasovagal reaction (2/63, 3.2%). At histology, the malignancy rate was 25/63 (39.7%).
Conclusion
In this first patient series, CEM-guided breast biopsy was feasible, with success and complication rates similar to those previously reported for magnetic resonance guidance.
Key points
• CEM may be used to guide biopsy of enhancing lesions through a stereotactic-based procedure combined with intravenous iodinated contrast media administration and dual-energy acquisition.
• In this first patient series (n = 64), the success rate of CEM-guided biopsy was above 95%, the only complications were hematoma (22.2%) and vasovagal reaction (3.2%), and median total time per procedure was 15 min.
• CEM-guided biopsy is feasible and could potentially be a widely available biopsy technique for enhancing-only lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast biopsy can be guided by different imaging techniques. The preferred first-line modality is ultrasound (US) but when suspicious lesions, such as architectural distortions or calcifications, are not visible on US, stereotactic or digital breast tomosynthesis (DBT) guidance is used.
Some breast lesions can be depicted only by imaging modalities able to detect tumour neoangiogenesis, visualised as suspicious contrast enhancement after the injection of intravenous contrast media [1]. For these ‘enhancing-only’ lesions, magnetic resonance imaging (MRI) guidance has, to date, been the only option in percutaneous biopsy, if there is no correlating finding in conventional second-look examinations [2,3,4,5]. However, MRI-guided biopsy is not widely available, is expensive and is not feasible in patients with contraindications such as claustrophobia, anthropometric limitations or metal devices.
Contrast-enhanced mammography (CEM) is an emerging imaging tool that, similar to MRI, allows visualisation of breast tumour neovascularisation [6, 7]. The technique consists of the acquisition of a dual-energy mammogram 2 min after intravenous injection of iodinated contrast media [8]. CEM displays both a low-energy image, similar to regular digital mammography, providing morphologic information, and a recombined image, depicting contrast media distribution, including local changes in breast perfusion presumably caused by tumour angiogenesis. The most common indications for CEM are inconclusive findings [9, 10], pre-operative staging [11, 12] and response monitoring [13]. Another potential indication is as a screening tool for women with increased risk of breast cancer [14,15,16]. Compared with MRI, CEM is less expensive [17] and seems to be preferred by women as it is faster and offers more patient comfort [18, 19].
There is scarce information in the literature on enhancing-only lesions detected by CEM. Amongst 839 women who underwent CEM after a screening recall, 70 enhancing-only lesions were found in 65 (7.7%) women. Of these 70 lesions, 38 (54.3%) subsequently proved to be malignant [10].
CEM guidance is possible due to a specific biopsy hardware add-on to the mammographic equipment, in addition to a dedicated software upgrade that applies the dual-energy acquisition to the stereotactic technique; currently, only one device is commercially available.
Due to the above-mentioned similarities between the 2 techniques, it is reasonable to assume that CEM-guided biopsy may be a valid alternative to MRI guidance. The purpose of this study was to evaluate the feasibility of CEM-guided biopsy in terms of its success rate (visualisation of the suspicious lesion during the procedure, allowing adequate targeting and sampling), procedure time and complication rate amongst the first described series of patients undergoing this procedure.
Methods
Study design and population
This was a retrospective review of a cohort prospectively enrolled in a pre-marketing clinical validation and feasibility study performed at Hospital del Mar, a university hospital in Barcelona, Spain (ClinicalTrials.gov ID: NCT05250674). We included all consecutive adult women presenting with a clinical indication for CEM-guided breast biopsy and considered eligible for this procedure as per standard of care between October 2019 and September 2021. CEM-guided biopsy was clinically indicated for suspicious enhancing lesions, not reliably identified by other morphological breast imaging techniques, including ‘second-look’ US or FFDM. Exclusion criteria were contraindications to iodinated contrast media. The study was approved by the hospital ethics committee (protocol number 2019/8890) and all patients provided written informed consent. A pre-marketing version of the Pristina Serena Bright™ GE Healthcare system had been under clinical validation in this study since 2019. The system received CE mark and Food and Drug Administration (510k) pre-marketing notification [20]. All the biopsy procedures were carried out using a 7- or 10-gauge (G) vacuum-assisted biopsy (VAB) needle.
CEM-guided biopsy
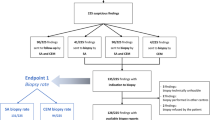
The procedure workflow is depicted in Fig. 1.
The first step of any mammographic-guided procedure should be a prior complete review of the case to define the optimal approach, based on the location of the lesion in the breast and the patient’s characteristics, such as breast thickness and any potential physical limitations. As the evaluated equipment is an upright biopsy add-on unit, the patient can be seated or lying in a lateral decubitus position, with the help of a decubitus breast imaging table. The same dedicated needle support can be used for both the vertical and horizontal approach. The optimal approach should be planned on the diagnostic CEM that leads to the indication for CEM-guided biopsy. When the suspicious enhancing finding was originally seen on MRI, a pre-procedural CEM was performed to allow correct planning.
CEM-guided biopsy uses the principle of conventional stereotactic guidance, with the additional preliminary step of intravenous iodinated contrast media administration. As with diagnostic CEM, a 2-min interval between contrast administration and compression is necessary to avoid interference with blood inflow to the breast, allowing adequate lesion enhancement. The focal compression applied during the biopsy procedure seems to minimise lesion washout and contrast uptake is visible for up to 10 min, which is sufficient for proper targeting.
At the end of the required 2-min interval, the patient is positioned, the area where the target lesion is expected to be found is centred on the compression paddle, and a dual-energy image is acquired at 0°. Once the inclusion of the enhancement in the scout image has been confirmed, a pair of dual-energy angled views is acquired at ± 15°. In each of the angled images, the target is identified and marked, so that the device can calculate the 3 location coordinates (X, Y and Z), allowing the biopsy needle holder to be automatically positioned.
After antisepsis and local anaesthesia, the needle is inserted into the breast, until reaching the limit point defined by the support itself, when the tip of the needle would face the target. A pre-fire stereo pair of dual-energy views is then performed to confirm that the target is in front of the needle tip; otherwise, minimal coordinate adjustments may improve precision. Next, once the fire-forward is activated, the sampling is carried out with the available vacuum system device. After sampling, the biopsy bed will be contaminated by blood with contrast media, so the pre-fire image will be the last to benefit from the dual technique.
It is highly important to place a radiological marker on the biopsy bed in order to evaluate procedural effectiveness and enable further localisation if the results reveal malignancy. It is recommended to check marker release with a DBT scout image, since this allows its precise location with a single acquisition, and therefore with a lower average glandular dose (AGD) than stereotactic acquisitions [21]. At the end of the procedure, the correct location of the radiological marker is confirmed, with the usual 2 mammographic views (mediolateral and craniocaudal), which are compared with the pre-procedural low-energy images.
Data collection
Clinical data of the included patients and suspicious lesions were collected, including patient age, breast density, background parenchymal enhancement (BPE) and the size and type of enhancement of the target lesion. Breast density was visually assessed on the low-energy image of the diagnostic CEM according to breast composition categories of the American College of Radiology (ACR) BI-RADS® mammography lexicon [22] and collected from the radiology report. BPE was visually graded in the diagnostic recombined image into 4 categories (minimal, mild, moderate and marked), according to the BI-RADS® MRI lexicon [23], applied for CEM [24]. Similarly, the reported size of the suspicious lesion was collected and the type of enhancement was classified in focus or foci (< 5 mm), mass enhancement (> 5 mm) and non-mass enhancement [23, 24].
Procedural characteristics, i.e. patient position (seated/lying), the kind of approach (horizontal/vertical) and needle gauge, were collected for each procedure.
For successful procedures, we collected the histopathological results on the biopsy specimen, as well as the kind of treatment undergone by patients. For patients who underwent surgery, we collected the pathological results of the surgical specimen.
Endpoints and data analysis
To evaluate the feasibility of CEM-guided biopsy, various endpoints were taken into account.
The success rate was calculated. Procedural success was defined as the visualisation of the suspicious enhancing lesion during procedural CEM, allowing adequate targeting and sampling, together with post-procedure imaging showing biopsy changes at the site of the intended lesion and with the clip in place or nearby in sequential mammography. Other cases were classified as cancelled, when the enhancing lesion visible at diagnostic CEM was not visible on procedural CEM, leading to non-performance of biopsy and short-term interval follow-up. Biopsies were classified as unsuccessful when the enhancing lesion was less visible on all procedural CEM views, limiting proper target selection, but the biopsy could still be performed by using another reference and guidance.
Procedural time was registered, considering the time span from contrast administration to clip placement scout view, immediately prior to breast decompression. Moreover, the number of scout acquisitions needed to represent the enhancing target in the field of view was collected. The complication rate was calculated, including immediately visible hematomas, vasovagal reactions, allergic reactions and late complications such as hematomas or infection.
For patients who underwent surgery or percutaneous VAE, the upgrade rate was calculated as the percentage of cases originally graded as B3 on the biopsy specimen, according to the NHS Breast Screening Programme pathology classification [25, 26], and then found to be malignant on the surgical or excision specimen, as well as cases diagnosed as ductal carcinoma in situ (DCIS) on biopsy and upstaged to invasive ductal carcinoma (IDC) on the surgical specimen.
Results
Study population
From October 2019 to September 2021, 64 patients were referred for CEM-guided breast biopsy. Of these, 2 underwent 2 biopsies for 2 different suspicious lesions (performed on the same day, in one patient with only one contrast administration), making a total of 66 procedures (Table 1). The median age of the included patients was 59 years, and breast density was predominantly classified as B (35.9%) and C (59.4%), whilst BPE was mostly minimal (48.4%) or mild (32.8%). Suspicious enhancing lesions were mass enhancement in 43.9% of the procedures, focus/foci in 18.2% and non-mass enhancement in 37.9% (Figs. 2 and 3). The median size of the suspicious lesion was 8.5 mm. Median time from diagnostic CEM to CEM-guided biopsy was 12 (6–21) days. For the 10 patients with an MRI-detected suspicious lesion, the CEM-guided biopsy was performed 15 (10–23) days after the MRI.
An asymptomatic 77-year-old patient with a history of right breast-conserving surgery was referred for complementary evaluation after detection of a suspicious scar in a routine external centre ultrasound (images not available). Low-energy CEM MLO view (A) of the right breast shows a stable architectural distortion related to the postsurgical scar (rectangle) with no enhancement on the recombined image (B). Left breast recombined images (D and F) show an incidental 7-mm enhancing mass (arrow) not seen on low-energy images (C and E, respectively) or ultrasound. Left breast CEM-guided biopsy was performed (G and H). The correct location of the radiological marker was confirmed, with the usual 2 mammographic craniocaudal and mediolateral views (I and J). Pathology report: intraductal papilloma. The lesion was further assessed with a DBT-guided vacuum-assisted excision (VAE)
A 53-year-old patient with a history of left breast–conserving surgery was referred for complementary evaluation after questionable changes in clinical examination of the left breast. Diagnostic CEM was performed, together with ultrasound. Low-energy CEM mediolateral oblique (E) and craniocaudal (F) views of the left breast show a stable postsurgical scar (rectangle) with no enhancement at recombined images (G and H, respectively). Right breast recombined images (C and D) show an incidental 26-mm non-mass enhancing finding (arrow) not clearly seen on low-energy images (A and B, respectively), DBT or ultrasound (not shown). Right breast CEM-guided biopsy was performed (I and J). Pathology report: High-grade triple negative ductal carcinoma in situ (DCIS). The lesion was further assessed with wire needle-guided surgical conservative treatment
Procedural characteristics and feasibility
Of the 66 suspicious lesions with an indication for CEM-guided biopsy, the procedure was successful in 63 (95.4%) lesions (Table 2). For the remaining 3 lesions, the enhancement classified as suspicious at diagnostic CEM was not clearly visible at procedural CEM. In 2 of these 3 patients, the biopsy was cancelled. In the remaining patient, who had a mild BPE, although the enhancement was not clearly visible in all views, a small distortion was seen at the time of the procedure with a complementary DBT scout, and biopsy was performed under DBT guidance, resulting in IDC (Fig. 4). Of the 2 patients with cancelled biopsies, one had a further follow-up CEM after 6 months showing no suspicious findings, whilst the second had no available follow-up CEM, although there was no evident suspicious enhancement at the CEM retest performed on the same day as the planned procedure (Supplementary Fig. 1). The original suspicious findings in these 2 patients were a 55-mm non-mass enhancement and a 6-mm mass enhancement, and BPE was moderate in the first and mild in the second.
Unsuccessful CEM guidance due to decreased enhancement. A 50-year-old asymptomatic patient was referred from the breast cancer screening programme due to a mass in the upper outer quadrant of the right breast (square). A CEM was performed (A–H), and an intense mass enhancement (square in B and D) led to an ultrasound-guided biopsy of this lesion, which was diagnosed as a complex fibroadenoma. As an incidental finding, there was a mild 15-mm non-mass enhancement in the inferior inner quadrant of the left breast (arrow in G and H), with no clear correlation in low-energy images (E and F). The patient was scheduled for a CEM-guided procedure. In the scout at 0° (I), there was mild enhancement but the finding was less visible in recombined angled views (J and K). A DBT scout confirmed a small distortion in the location of the enhancement, allowing biopsy (M and N). Pathology reported a grade I invasive ductal carcinoma
As for the procedural characteristics of successful CEM-guided biopsies, the patients were most frequently seated (n = 52, 82.5%) and the preferred approach was horizontal (n = 48, 76.2%). The median total time per procedure was 15 min. The number of acquisitions needed before the targeting was between 1 and 4 (median 2 acquisitions). In most procedures (n = 4, 69.8%), no complications were registered. Self-limiting early hematomas were recorded in 14 (22.2%) patients and vasovagal reactions in 2 (3.2%). Late complications consisted of only moderate hematomas (3/63, 4.8%). No severe complications or allergic reactions were registered.
Pathology results of successful procedures
Of the 63 lesions that underwent successful CEM-guided biopsy, 2 (3.2%) were B1 on biopsy specimen, 26 (41.3%) were B2 (predominantly fibroadenomas, fat necrosis and adenosis/sclerosis), 10 (15.9%) were B3 (predominantly atypical ductal hyperplasia) and 25 (39.7%) were B5, including IDC (n = 10), DCIS (n = 7), a combination of IDC and DCIS (n = 1) and invasive lobular carcinoma (n = 7) (Fig. 5). The median size of the suspicious enhancement according to histopathological diagnosis is reported in Table 3. After radiological-pathological correlation, only 2 patients with discordant results were referred to short-term follow-up; one of these showed no suspicious enhancement after 6 months and the other showed remaining enhancement at 6 months, resulting in a re-biopsy close to the clip, still with B2 histopathological results. Of the 35 lesions that were treated, whether surgically or through percutaneous VAE, 6 (17.1%) were upgraded, including 4/10 B3 lesions which were upgraded to malignancy, and 2/7 DCIS which were upstaged to IDC (Table 3).
A 47-year-old patient with a history of right breast–conserving surgery 11 years ago and bilateral fibroadenomas (squares in A, C and E). Routine MRI follow-up (sagittal 3D Maximum Intensity Projection subtraction, B) showed a new right breast mass (arrow) not seen on second-look ultrasound or DBT (mediolateral oblique planes, A). Right breast recombined images (D and F) show the 8-mm enhancing mass (arrow), not seen on low-energy images (C and E). Right breast CEM-guided biopsy was performed (G–J). Pathology report: invasive lobular carcinoma. The patient underwent a mastectomy
Discussion
This is the first report of a series of patients who received CEM-guided breast biopsy, a new approach that allows targeting of enhancing lesions and which may be a potential alternative to MRI guidance. In this series of 64 patients (with a total of 66 suspicious lesions), the success rate was above 95%. The preferred patient position was seated, and the preferred approach was horizontal. The median total time per procedure was 15 min, and the median number of acquisitions before the targeting was 2. The only complications were early or late hematoma (27%) and vasovagal reaction (3.2%).
MRI-guided biopsy is a safe and accurate technique but requires experience and the localisation process can be laborious and time-consuming, with a reported median of 35–41 min for imaging time and room occupancy of around 60–70 min [27,28,29,30,31]. Other major drawbacks are its limited availability and high costs [30].
The MRI-guided VAB success rate reported in the literature ranges from 87 to 98%, with some degree of procedure deferral due to absent enhancement or decreased visualisation [32,33,34,35,36]. The CEM-guided biopsy success rate in our case series was similar (95.4%). Of the 3 findings with absent or decreased visualisation at the time of the procedure, one was an IDC, whilst the follow-up available in one of the 2 patients with cancelled biopsies showed no suspicious lesions. Non-visualisation of a previously detected enhancing finding has been described in approximately 8–13% of MRI-guided biopsies [37,38,39], and possible explanations for these events, both for MRI and for CEM, are noncyclical hormonal factors, qualifying as BPE or focal inflammatory or fibrocystic changes that may be cleared out or scattered by the time of the procedure [39]. Despite the low cancer detection rate amongst the lesions leading to deferred MRI-guided biopsy (0–10%) [40], ACR Practice Parameters recommend a ‘short-term’ follow-up [4], and this practice was also applied to the 2 patients with cancelled biopsies.
In our case series, the malignancy rate at biopsy was almost 40%, falling within the range of the cancer detection rate reported in the literature for MRI-guided breast biopsy, ranging from 18 to 61% [33, 36, 41]. The rate of upgrade and upstaging to malignant disease after histological analysis of open surgical excision samples for the 35 treated patients was 17.1%. The upgrade rate for B3 lesions was 40% (4 of 10 cases), in line or slightly superior to rates described in the literature (12–38%) [37, 41, 42]. Of note, rates of upgrade to malignancy may be higher for MRI-guided biopsy, and presumably for CEM-guided biopsy, than for stereotactic and US-guided biopsy [4, 36].
The horizontal approach was preferred in our series. The use of a plane compressor paddle with the horizontal approach offers better image quality and target visualisation. Conversely, the use of a fenestrated compressor paddle, necessary for the vertical approach, may result in artefacts in recombined images, due to differences in compressed breast thickness. Similarly, vertical access also has implications in the pre-fire view as the anaesthesia and VAB needle itself may interfere with the original target (Fig. 6).
The main complications were hematomas and vasovagal reactions, occurring in rates similar to those reported in the literature for other upright mammography-guided procedures [43,44,45,46], i.e. 1–5% for vasovagal reactions [43,44,45], and 2–83% for hematomas [45, 46], which are very common although not usually clinically relevant. No severe allergic reactions were observed, but this may be due to patient selection, since previous allergies to iodinated contrast media, also during diagnostic CEM, were an exclusion criterion. Irrespective of this consideration, the risk of contrast reaction is low with the type of contrast media used, less than 1%, and most events are mild and self-limiting [8, 10].
A limitation of the present study is the lack of information on AGD, since it can be argued that the main drawback of CEM guidance is radiation exposure. It is plausible that levels of exposure are acceptable, since previous data on diagnostic CEM have shown that the radiation dose remains below the threshold set by the European guidelines for screening mammography [8, 47]. In this study, the median number of scout views before targeting was low and we avoided additional dual-energy imaging after sampling. Nevertheless, this issue requires further investigation. In addition, the relatively small sample size of included women may limit the generalizability of our findings. However, we believe that the number of procedures assessed in this study was sufficiently large to provide valid information on the feasibility of CEM-guided biopsy. Further cohort studies with a larger number of cases should focus on evaluation of its effectiveness and on the potential negative influence on procedural success induced by masking of the target lesion due to BPE.
In conclusion, in this first series of patients, CEM-guided breast biopsy was a feasible technique that was easy to implement and perform and which showed good success and complication rates and short procedural time (15 min on average). Since CEM-guided breast biopsy has the potential to be an alternative to MRI-guided biopsy for enhancing-only lesions, further studies are needed to directly compare the 2 techniques in terms of patient preference and cost-effectiveness.
Abbreviations
- AGD:
-
Average glandular dose
- BPE:
-
Background parenchymal enhancement
- CEM:
-
Contrast-enhanced mammography
- DBT:
-
Digital breast tomosynthesis
- DCIS:
-
Ductal carcinoma in situ
- FFDM:
-
Full field digital mammography
- IDC:
-
Invasive ductal carcinoma
- VAB:
-
Vacuum-assisted biopsy
- VAE:
-
Vacuum-assisted excision
References
Pinker K (2017) Advanced imaging for precision medicine in breast cancer: from morphology to function. Breast Care 12:208–210. https://doi.org/10.1159/000480397
Meissnitzer M, Dershaw DD, Lee CH, Morris EA (2009) Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol 193:1025–1029. https://doi.org/10.2214/AJR.09.2480
Spick C, Baltzer PAT (2014) Diagnostic utility of second-look US for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology 273:401–409. https://doi.org/10.1148/radiol.14140474
American College of Radiology (ACR) (2021) ACR practice parameter of the performance of magnetic resonance imaging-guided breast interventional procedures. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Guided-Breast.pdf. Accessed 22 Feb 2022
Bick U, Trimboli RM, Athanasiou A et al (2020) Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 11:12. https://doi.org/10.1186/s13244-019-0803-x
Jochelson MS, Lobbes MBI (2021) Contrast-enhanced mammography: state of the art. Radiology 299:36–48. https://doi.org/10.1148/RADIOL.2021201948
Neeter LMFH, Raat HPJ, Alcantara R et al (2021) Contrast-enhanced mammography: what the radiologist needs to know. 3:20210034. https://doi.org/10.1259/BJRO.20210034
Zanardo M, Cozzi A, Trimboli RM et al (2019) Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 10. https://doi.org/10.1186/s13244-019-0756-0
Lalji UC, Houben IPL, Prevos R et al (2016) Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 26:4371–4379. https://doi.org/10.1007/s00330-016-4336-0
Houben IPL, Van de Voorde P, Jeukens CRLPN et al (2017) Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur J Radiol 94:31–37. https://doi.org/10.1016/j.ejrad.2017.07.004
Fallenberg EM, Dromain C, Diekmann F et al (2014) Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 24:256–264. https://doi.org/10.1007/s00330-013-3007-7
Kim EY, Youn I, Lee KH et al (2018) Diagnostic value of contrast-enhanced digital mammography versus contrast-enhanced magnetic resonance imaging for the preoperative evaluation of breast cancer. J Breast Cancer 21:453–462. https://doi.org/10.4048/jbc.2018.21.e62
Iotti V, Ravaioli S, Vacondio R et al (2017) Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res 19:1–13. https://doi.org/10.1186/s13058-017-0899-1
Jochelson MS, Pinker K, Dershaw DD et al (2017) Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol 97:37–43. https://doi.org/10.1016/j.ejrad.2017.10.001
Sorin V, Yagil Y, Yosepovich A et al (2018) Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. 1–8.
Sung JS, Lebron L, Keating D et al (2019) Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 293:81–88. https://doi.org/10.1148/radiol.2019182660
Patel BK, Gray RJ, Pockaj BA (2017) Potential cost savings of contrast-enhanced digital mammography. AJR Am J Roentgenol 208:W231–W237. https://doi.org/10.2214/AJR.16.17239
Hobbs MM, Taylor DB, Buzynski S, Peake RE (2015) Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol 59:300–305. https://doi.org/10.1111/1754-9485.12296
Phillips J, Miller MM, Mehta TS et al (2017) Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging 42:193–197. https://doi.org/10.1016/j.clinimag.2016.12.011
U.S. Food and Drug Administration database (2020) 510(k) Premarket Notification. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K193334. Accessed 16 Feb 2022
Amir T, Zuckerman SP, Barufaldi B et al (2021) Comparison of radiation dose between 2D digital stereotactic versus digital breast tomosynthesis-guided breast biopsies. Eur J Radiol 134:109407. https://doi.org/10.1016/j.ejrad.2020.109407
Sickles EA, D’Orsi CJ, Bassett LW et al (2013) ACR BI-RADS® mammography. In: ACR BI-RADS® atlas, breast imaging reporting and data system, 5th ed. American College of Radiology, Reston, VA.
Morris EA, Comstock CE, Lee CH et al (2013) ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® atlas, breast imaging reporting and data system, 5th ed. American College of Radiology, Reston, VA.
Knogler T, Homolka P, Hoernig M et al (2017) Application of BI-RADS descriptors in contrast-enhanced dual-energy mammography: comparison with MRI. Breast Care 12:212–216. https://doi.org/10.1159/000478899
Lee AH, Anderson N, Carder P et al (2021) Guidelines for non-operative diagnostic procedures and reporting in breast cancer screening. R Coll Pathol G 150(1):1–74
Ellis IO, Humphreys S, Michell M et al (2004) Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol 57:897–902. https://doi.org/10.1136/jcp.2003.010983
Noroozian M, Gombos EC, Chikarmane S et al (2010) Factors that impact the duration of MRI-guided core needle biopsy. AJR Am J Roentgenol 194:150–157. https://doi.org/10.2214/AJR.09.2366
Tozaki M, Yamashiro N, Suzuki T et al (2009) MR-guided vacuum-assisted breast biopsy: is it an essential technique? Breast Cancer 16:121–125. https://doi.org/10.1007/s12282-008-0074-8
Perlet C, Heywang-Kobrunner SH, Heinig A et al (2006) Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer 106:982–990. https://doi.org/10.1002/cncr.21720
Clauser P, Mann R, Athanasiou A et al (2018) A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol 28:1909–1918. https://doi.org/10.1007/s00330-017-5121-4
Santiago L, Candelaria RP, Huang ML (2018) MR imaging–guided breast interventions. Magn Reson Imaging Clin N Am 26:235–246. https://doi.org/10.1016/j.mric.2017.12.002
Siegmann-Luz K, Bahrs S, Preibsch H et al (2013) Management of breast lesions detectable only on MRI. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgeb Verfahren 186:30–36. https://doi.org/10.1055/s-0033-1335972
Han BK, Schnall MD, Orel SG, Rosen M (2008) Outcome of MRI-guided breast biopsy. AJR Am J Roentgenol 191:1798–1804. https://doi.org/10.2214/AJR.07.2827
Malhaire C, El Khoury C, Thibault F et al (2010) Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol 20:1554–1562. https://doi.org/10.1007/s00330-009-1707-9
Heywang-Köbrunner SH, Heinig A, Schaumlöffel U et al (1999) MR-guided percutaneous excisional and incisional biopsy of breast lesions. Eur Radiol 9:1656–1665. https://doi.org/10.1007/s003300050905
Imschweiler T, Haueisen H, Kampmann G et al (2014) MRI-guided vacuum-assisted breast biopsy: comparison with stereotactically guided and ultrasound-guided techniques. Eur Radiol. https://doi.org/10.1007/s00330-013-2989-5
McGrath AL, Price ER, Eby PR, Rahbar H (2017) MRI-guided breast interventions. J Magn Reson Imaging 46:631–645. https://doi.org/10.1002/jmri.25738
Hefler R, Casselman J, Amaya B et al (2003) Follow-up of breast lesions detected by MRI not biopsied due to absent enhancement of contrast medium. Eur Radiol 13:344–346. https://doi.org/10.1007/s00330-002-1713-7
Brennan SB, Sung JS, Dershaw DD et al (2011) Cancellation of MR imaging-guided breast biopsy due to lesion nonvisualization: frequency and follow-up. Radiology 261:92–99. https://doi.org/10.1148/radiol.11100720
Niell BL, Lee JM, Johansen C et al (2014) Patient outcomes in canceled MRI-guided breast biopsies. AJR Am J Roentgenol 202:223–228. https://doi.org/10.2214/AJR.12.10228
Rauch GM, Dogan BE, Smith TB et al (2012) Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol 198:292–299. https://doi.org/10.2214/AJR.11.7594
Schiaffino S, Cozzi A, Sardanelli F (2020) An update on the management of breast atypical ductal hyperplasia. Br J Radiol 93:2–4. https://doi.org/10.1259/bjr.20200117
Kirshenbaum KJ, Voruganti T, Overbeeke C et al (2003) Stereotactic core needle biopsy of nonpalpable breast lesions using a conventional mammography unit with an add-on device. AJR Am J Roentgenol 181:527–531. https://doi.org/10.2214/ajr.181.2.1810527
Wunderbaldinger P, Wolf G, Turetschek K, Helbich TH (2002) Comparison of sitting versus prone position for stereotactic large-core breast biopsy in surgically proven lesions. AJR Am J Roentgenol 178:1221–1225. https://doi.org/10.2214/ajr.178.5.1781221
Georgian-Smith D, D’Orsi C, Morris E et al (2002) Stereotactic biopsy of the breast using an upright unit, a vacuum-suction needle, and a lateral arm-support system. AJR Am J Roentgenol 178:1017–1024. https://doi.org/10.2214/ajr.178.4.1781017
Lin LLY, Gao Y, Lewin AA, Toth HK, Heller SL, Moy L (2019) Overstated harms of breast cancer screening? A large outcomes analysis of complications associated with 9-gauge stereotactic vacuum-assisted breast biopsy. AJR Am J Roentgenol 212(4):925–932
Perry N, Broeders M, de Wolf C et al (2013) European guidelines for quality assurance in breast cancer screening and diagnosis, 4th ed.
Acknowledgements
We would like to thank all the women participating in the study, as well as the radiology team, radiographers and pathologists from the Hospital del Mar, Barcelona, Spain.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. R. Alcantara.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
R. A. declares to be a member of speakers’ bureau for Bard BD and GE Healthcare. V.I. declares to be a member of speakers’ bureau for GE Healthcare. All other authors declare that they have no competing interests.
General Electric provided the CEM-guided biopsy software to be validated. However, this provision played no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Statistics and biometry
One of the authors (M. Posso) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective review of prospectively enrolled cohort
• Pre-marketing clinical validation
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2356 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alcantara, R., Posso, M., Pitarch, M. et al. Contrast-enhanced mammography-guided biopsy: technical feasibility and first outcomes. Eur Radiol 33, 417–428 (2023). https://doi.org/10.1007/s00330-022-09021-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09021-w