Abstract
Objectives
T o evaluate the value of multiparametric MRI (mpMRI) for the prediction of prostate cancer (PCA) aggressiveness.
Methods
In this single center cohort study, consecutive patients with histologically confirmed PCA were retrospectively enrolled. Four different ISUP grade groups (1, 2, 3, 4–5) were defined and fifty patients per group were included. Several clinical (age, PSA, PSAD, percentage of PCA infiltration) and mpMRI parameters (ADC value, signal increase on high b-value images, diameter, extraprostatic extension [EPE], cross-zonal growth) were evaluated and correlated within the four groups. Based on combined descriptors, MRI grading groups (mG1–mG3) were defined to predict PCA aggressiveness.
Results
In total, 200 patients (mean age 68 years, median PSA value 8.1 ng/ml) were analyzed. Between the four groups, statistically significant differences could be shown for age, PSA, PSAD, and for MRI parameters cross-zonal growth, high b-value signal increase, EPE, and ADC (p < 0.01). All examined parameters revealed a significant correlation with the histopathologic biopsy ISUP grade groups (p < 0.01), except PCA diameter (p = 0.09). A mixed linear model demonstrated the strongest prediction of the respective ISUP grade group for the MRI grading system (p < 0.01) compared to single parameters.
Conclusions
MpMRI yields relevant pre-biopsy information about PCA aggressiveness. A combination of quantitative and qualitative parameters (MRI grading groups) provided the best prediction of the biopsy ISUP grade group and may improve clinical pathway and treatment planning, adding useful information beyond PI-RADS assessment category. Due to the high prevalence of higher grade PCA in patients within mG3, an early re-biopsy seems indicated in cases of negative or post-biopsy low-grade PCA.
Key Points
• MpMRI yields relevant pre-biopsy information about prostate cancer aggressiveness.
• MRI grading in addition to PI-RADS classification seems to be helpful for a size independent early prediction of clinically significant PCA.
• MRI grading groups may help urologists in clinical pathway and treatment planning, especially when to consider an early re-biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The combination of clinical parameters such as PSA testing and multiparametric magnetic resonance imaging (mpMRI) of the prostate using PI-RADS (Prostate Imaging–Reporting and Data System) is highly sensitive in prostate cancer (PCA) detection of not only large aggressive PCA but also small, early-stage and/or low-risk PCA in the clinical routine. Although PI-RADS is designed for assessing the likelihood of clinically significant PCA (ISUP ≥ 2) being present, especially when using a low threshold for biopsy, e.g. patients in PI-RADS overall assessment category 3, and/or if patients receive additional systematic biopsies, the rate of low-grade, non-significant PCA (ISUP 1) increases as high as 90% of all diagnosed PCA [1, 2]. Since there are many different therapy options for PCA, MRI can provide helpful assistance for treatment decisions, as established in the EAU guidelines. Whereas aggressive PCA demands urgent, extensive treatment, i.e. radical resection of the prostate, including the seminal vesicles and the pelvic lymph nodes, other therapy options are available for lower risk PCA [3, 4]. More invasive procedures have certain risks and may entail complications like incontinence or erectile dysfunction [5]. In cases of less aggressive PCA, nerve-sparing surgery and radiotherapy, including brachytherapy or other focal therapies, can be an option, among others. For PCA within ISUP 1 (in studies ISUP 2), active surveillance is also a possible strategy. Since serial follow-up mpMRI is part of this strategy, it is necessary to evaluate and define more specific imaging descriptors indicating PCA aggressiveness and PCA progress so that re-biopsy may be avoided or delayed in stable cases [6].
Different risk calculators are available to predict initial PCA aggressiveness and the risk of possible PCA progression, including parameters like Gleason score, PSA value, or demographic aspects [7, 8]. There is evidence that mpMRI is also helpful in estimating the initial PCA aggressiveness and supporting the clinical decision-making process [9,10,11,12,13]. Studies revealed that ADC values are negatively correlated with the Gleason score [14,15,16]. Additionally, it has been shown that the combination of functional and anatomic MRI sequences cannot only differentiate between cancerous areas and benign prostate tissue but also clearly define PCA margins so that tumour size and potential extraprostatic extensions (EPE) can be assessed. However, as of now, there is no consensus of MRI-based prediction of PCA aggressiveness [17].
The aim of this study is the systematic evaluation of clinical and MRI parameters for the prediction of PCA aggressiveness in biopsies next to the PI-RADS evaluation and suggestion of an MRI-based grading system to assist in the choice of the appropriate therapy regime.
Materials and methods
Study design
The local ethics committee approved the study (Medical Faculty of the Heinrich-Heine-University Düsseldorf; Study-ID: 2,017,034,171). Written informed consent was obtained from every patient. Four groups (biopsy ISUP grade group 1, 2, 3, and 4 combined with 5) were defined and 50 consecutive patients per group were included to ensure equal group size. All patients received mpMRI of the prostate at our institution between January 2016 and March 2020. Subsequently, targeted MRI/ultrasound fusion-guided biopsy combined with systematic 12-core transrectal ultrasound-guided prostate biopsy was conducted. Only patients with biopsy-confirmed PCA were included. None of the patients had a known prostate cancer. Exclusion criteria were previous treatment for prostate cancer and incomplete or non-diagnostic MRI. Clinical and MRI parameters were defined and retrospectively correlated with different biopsy ISUP grade groups. Clinical information contained age, PSA, PSAD, and percentage of infiltration in biopsy. MRI parameters included ADC values of PCA lesions, PCA diameter, EPE, cross-zonal growth, and signal increase on high b-value images. The primary study endpoint was to prove if there are significant differences in the mentioned parameters between the histopathologic biopsy ISUP grade groups. Secondary objective was the biopsy ISUP grade group correlation with defined MRI grading groups (mG1 to mG3), based on the combination of different descriptors.
Imaging acquisition
All mpMRI scans were conducted on 3-T MRI scanners (Magnetom TIM Trio: n = 164, Prisma: n = 61, or Skyra: n = 23; Siemens Healthineers) using either an 18-channel phased-array surface coil combined with a 32-channel spine coil or a 60-channel phased-array surface coil. MRI parameters were chosen according to international recommendations and contained T2-weighted turbo spin echo (TSE) sequences in 3 planes (T2WI; axial: voxel size 0.5 × 0.5 × 3.0 mm; FOV 130 mm), diffusion-weighted imaging (DWI; ss-EPI [single-shot EPI DWI] and rs-EPI [readout-segmented multi-shot EPI DWI, RESOLVE; Siemens Healthineers]; voxel size 1.4 × 1.4 × 3.0 mm; b-values 0, 500, 1000 s/mm2 plus calculated 1800s/mm2), and dynamic contrast-enhanced imaging (DCE; T1 vibe; voxel size 0.8–1.5 × 0.8–1.5 × 3.0 mm, scan time 3 min, temporal resolution 7 s) [18]. Apparent diffusion coefficient (ADC) parameter maps were calculated by the scanner using the standard monoexponential model. Ss-EPI was acquired in 125 and rs-EPI in 96 of 200 patients. Further details of acquisition parameters are provided in the supplementary data (Supp. Tables 2–4).
Biopsy
Targeted MRI/ultrasound fusion-guided biopsy and subsequent systematic 12-core TRUS-GB were conducted on an MRI/US fusion-guided biopsy system with elastic registration (UroNAV, Invivo) using an 18-G fully automatic biopsy gun (Bard Medical) by experienced urologists with over 5 years of experience. Two targeted cores were taken from each lesion.
Image analysis
MpMRI data were retrospectively evaluated by three readers in consensus (M.B., T.U., and L.S.) with 3, 7, and 10 years of experience. By the time of the imaging analysis, the readers were blinded towards the histopathologic ISUP groups. Prostate volume was measured by software volumetric (DynaCAD, Philips Healthcare) and PSA density (PSAD) was calculated by dividing PSA blood levels by prostate volume. First, it was evaluated if a PCA index lesion (IL) was visible on mpMRI, defined as PIRADS (v2.1) assessment category 3, 4, or 5. Only one IL was assessed per patient. If there was more than one lesion, the one with the highest PI-RADS v2.1 assessment category or the one with EPE, if present, or the largest lesion was chosen. For all visible PCA IL, maximum diameter was measured in T2w sequences. EPE or seminal vesicle infiltration (cT3 stage) was present if PCA crossed the prostate pseudocapsule (≥ 3 mm) or extended per continuitatem into the seminal vesicles. Cross-zonal growth was defined as growth in the peripheral zone (PZ) and expansion into the transition zone (TZ) or vice versa indicating invasive behaviour. In DWI, lesions were classified as positive if they were visible and different from the background in high b-values (calculated b1800). ADC values were measured by placing a circular region of interest (ROI) into the visually darkest PCA area. The PCA were assigned to defined MRI grading groups (mG1 to mG3) containing quantitative and qualitative information of patients and PCA lesions (Table 1). The definition of the different groups was based on previous studies showing that ADC values using a threshold from 750 to 900 µm2/s may help to estimate lesion aggressiveness and on clinical experience [16, 19, 20].
Statistical analysis
Statistics were performed using IBM SPSS® Statistics (Version 27, IBM Corp). p values < 0.05 (marked in bold) were defined as statistically significant. Bonferroni-corrected analysis of variance (ANOVA) was used to compare clinical and mpMRI parameters between different ISUP grade groups. For correlation analyses, the Kendall Tau correlation coefficient τ was calculated. Correlation strengths were graded as suggested by Cohen: small (< 0.3), moderate (0.3–0.5), and large (> 0.5) [21]. For the prediction of ISUP group based on MRI images, a multivariable statistical analysis was performed using a linear mixed model (LMM). The performance of combinations of parameters in discriminating between different ISUP grade groups was analyzed using restricted maximum likelihood (REML) to account for within-patient correlations. Models for rs-EPI and ss-EPI were calculated, respectively.
Results
Patients
Of the entire patient cohort of 200 patients (mean age 68 ± 8 years; median PSA 9.3 ng/ml, IQR 6.2–12 ng/ml; median PSAD 0.21 ng/ml/cm3, IQR 0.15–0.33 ng/ml/cm3), 50 patients had PCA classified as biopsy ISUP 1, 50 patients ISUP 2, 50 patients ISUP 3, and 50 patients ISUP 4 or 5.
Comparison of clinical and MRI parameters among biopsy ISUP grade groups
In 187 of all 200 patients, a PCA suspicious IL was found on mpMRI. In 13 patients, PCA was indistinct or masked. In ISUP 1, only 40/50 of the PCA were visible in mpMRI, whereas all PCA were visible in ISUP 4 and 5. 39/50 of the IL in ISUP 1 were localized in the peripheral zone, 35/50 in ISUP 2, 39/50 in ISUP 3, and 39/50 in ISUP 4 and 5. 7/50 of the IL in ISUP 1 were localized in the transition zone (ISUP 2: 9/50; ISUP 3: 9/50; ISUP 4–5: 7/50), and 4/50 of the IL in ISUP 1 were localized in the anterior stroma (ISUP 2: 6/50; ISUP 3: 2/50; ISUP 4–5: 4/50). Except for PCA diameter (p = 0.092), ANOVA analysis showed significant differences for the means of all examined clinical and MRI-based parameters among the different ISUP grade groups with p = 0.004 for signal increase on high b-value images, p = 0.044 for ADC values using ss-EPI (n = 125 patients), and p < 0.001 for all others, respectively (Table 2).
MRI grading groups
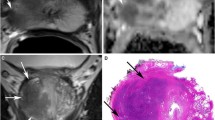
Thirty-one lesions were classified as mG1 (17/50 ISUP 1; 10/50 ISUP 2, 4/50 ISUP 3, and 0/50 ISUP 4–5), 78 lesions as mG2 (20/50 ISUP 1; 24/50 ISUP 2, 20/50 ISUP 3, and 14/50 ISUP 4–5), and 78 lesions as mG3 (3/50 ISUP 1; 13/50 ISUP 2, 26/50 ISUP 3, and 36/50 ISUP 4–5) (Table 3). The risk of having a PCA with ISUP ≥ 2 was 45% in mG1, 74% in mG2, and 96% in mG3. The risk of ISUP ≥ 3 PCA was 13% in mG1, 44% in mG2, and 80% in mG3. Examples of cases of each MRI grading group are illustrated in Fig. 1.
MRI grading groups 1 to 3 and estimated risk of higher grade prostate cancer with examples of mpMRI. The first column shows T2w sequences, second column ADC-maps (rs-EPI), third column high b-value DWI (b1800), fourth column DCE, and last column the risk of ISUP grade group ≥ 2 or ≥ 3 PCA. In the first line of images, a representative patient within mG1 is shown and the second line presents a patient within mG2 with focally PCA and only moderate ADC value reduction (rs-EPI). The last patient in line 3 was graded within mG3 with marked reduction in ADC value, dark focal appearance on T2w images, and bright signal on high b-value DWI
Correlation and predictive power of the ISUP grade group among clinical and MRI parameters
All clinical parameters and almost all mpMRI parameters showed a significant correlation with the PCA ISUP grade group (Table 4; Supp. Figure 1) with the strongest association for the parameters MRI grading group (τ = 0.534; p < 0.001) and ADC values (rs-EPI) (τ = − 0.468; p < 0.001). No significant correlation was found for PCA diameter (τ = 0.132; p = 0.71). The PI-RADS scoring system also correlated significantly with the ISUP grade groups (p < 0.001).
In the linear mixed model analysis, the MRI grading group was the only parameter that showed a significant effect in prediction of the ISUP grade group in direct comparison to the other MRI parameters using ADC from rs-EPI (Table 5) (p < 0.001) or ADC from ss-EPI (Supp. Tab. 1).
Discussion
MpMRI can deliver important information about PCA aggressiveness [22, 23], especially using ADC values [24, 25]. In this study, we could demonstrate that clinical and MRI-based quantitative and qualitative parameters can provide comprehensive, reliable information about the PCA ISUP grade group, potentially facilitating even more individualized, suitable therapy planning. In direct comparison with the single MRI-based parameters, a defined MRI grading group system (mG1 to mG3), incorporating different MRI descriptors, revealed the strongest effect in prediction of the final biopsy ISUP grade group, which might be useful in addition to PI-RADS.
In our multivariate analysis, almost all defined clinical and MRI parameters showed significant differences between the ISUP grade groups except for PCA diameter. Lesion size plays e.g. a role for differentiating PI-RADS 4 from 5 lesions based on tumour volume calculations of prostatectomy specimens focusing the differentiation of significant and non-significant PCA [26]. However, also smaller lesions can contain high-grade cancer, especially in early detection [27]. Somehow contrary results might be likewise due to the more detailed grading with differentiation of single ISUP grade groups and/or affected by size measuring on MRI. The parameter cross-zonal growth of PCA showed a good performance in differentiating the various biopsy ISUP groups in our analysis. As the majority of cancers are localized in the PZ and infiltrative behaviour into the TZ has been reported to occur in more aggressive cancers [28], this parameter seems to be an interesting aspect in PCA characterization and grading. Besides, the crossing of anatomical borders, not only between the different intraprostatic zones but also into extraprostatic tissue, especially occurs in higher ISUP grade groups [29] and is a general measure of PCA aggressiveness. Focusing on ADC values, in ss-EPI, technical aspects could play a role for the poorer performance compared to readout-segmented multi-shot EPI (rs-EPI). In previous studies, it has been shown that advanced DWI technology, e.g. parallel transmit EPI (ptx-EPI) or rs-EPI, delivers a significantly higher ADC reduction of PCA lesions versus healthy tissue compared to standard ss-EPI DWI [18]. The PI-RADS scoring system, designed to detect PCA with an ISUP ≥ 2, also showed a positive correlation with the pathologic biopsy ISUP groups in our analysis which also served as a measure of consistency given that PI-RADS assessment category 5 lesions contain significantly more higher ISUP grade PCA compared to the other PI-RADS categories. However, in PI-RADS 4 and 5 also ISUP 1 PCA were detected [1, 2].
Head-to-head comparison of the single MRI parameters for MRI-based grading of PCA using a linear mixed model demonstrated the best performance for a combination of parameters within MRI grading groups. This seems logical as most of the MRI parameters already demonstrated significant correlation with the ISUP grade groups and incorporating as many cancer characteristics as possible should lead to the clearest results. Other parameters failed to reach the level of significance in a LMM although they showed good correlation with the ISUP grade group distribution. A possible explanation is the outshining effect of the parameter grading group in the LMM, which possibly masks the effect of other parameters in this mixed model.
The presented MRI grading group system partly uses similar imaging features of PI-RADS for its classification, but some descriptors are not part of the PI-RADS evaluation, e.g. cross zonal growth, and single features are weighed and combined differently in comparison to the PI-RADS system. However, it has to be emphasized that the two systems have a different focus. PI-RADS primarily provides a likelihood for the presence of clinically significant PCA (ISUP ≥ 2) and the MRI grading groups providing information about PCA aggressiveness. PI-RADS differentiates category 4 and 5 lesions mainly based on the largest tumour diameter under or ≥ 1.5 mm. But in clinical settings, there are also small higher grade PCA lesions with high ADC reduction and clear imaging features or large low-grade PCA lesions with mild to moderate ADC reduction and discreet imaging features. Our results confirmed no significant correlation of the tumour diameter with the ISUP grade groups. In comparison, the MRI grading group score can express the higher or less aggressiveness to complement the PI-RADS classification and can e.g. suggest early re-biopsy in mG3 or primary follow-up in mG1 cases. This might reduce an unnecessary therapy delay and potential development of metastases due to late detection of high-grade PCA.
During active surveillance, it is important to evaluate MRI features of PCA aggressiveness and development of these features over time to trigger or postpone re-biopsy or definite treatment. The PRECISE criteria provide assistance in evaluation of lesion development in follow-up scans [30]. Our results showed that the MRI grading groups correlate with the histopathologic findings and thus might be useful to observe cancer progression or PCA aggressiveness assessment in patients planned for or within AS in an objective way. In this regard, an early re-biopsy should be considered if targeted biopsy of an mG3 lesion yields no or low-grade PCA (ISUP 1) given the high prevalence of higher grade PCA in this group. Patients with mG1 lesions (also ≥ 1.5 cm) may receive primarily follow-up mpMRI if they qualify for active surveillance. Therefore, it might increase early detection of higher grade PCA and on the other hand, it might reduce re-biopsy rates in patients with ISUP 1 PCA within mG1 and thus increase AS safeness. For lesions in mG2, imaging is not unequivocal and may be impaired, for example due to coexistent signs of prostatitis. Follow-up mpMRI instead of prompt re-biopsy may be justified if the clinical setting allows.
Some limitations of this study, besides the retrospective design, need to be discussed. First, the definition of ADC value thresholds for the MRI grading groups was based on the literature and clinical- and scanner-specific experience [15, 31] The assessed values seem reasonable in our circumstances and clinical settings. Nevertheless, the values may be subjective to a certain extent, so that different thresholds may be defined at other institutions. Therefore, before using MRI grading groups, it has to be verified that the definitions are suitable for the individual settings. Second, the defined parameters offer a good aggregation of PCA characteristics and should be considered and evaluated in image analysis. However, there is no guarantee for completeness and more parameters may have an influence on MRI grading. Third, even if the examined parameters correlated with the different ISUP groups, a definite distinction is not possible merely based on this information, so that other (clinical) factors always need to be taken into account. Further research is required to evaluate performance and accuracy of MRI grading between non-significant and significant prostate cancer in detail. This means differentiation between ISUP groups 1–2 and ISUP groups 3–5 as conducted for ADC before [32]. Fourth, cancer detection on T2w sequences is qualitatively conducted in a subjective way, differentiating between discreet and clear lesions. As there is no quantitative approach established, this probably leads to low interreader and intrareader variability. Finally, we have no radical prostatectomy results or follow-up data included to determine the final patient outcome. It might be interesting to prove MRI grading in a prospective study design.
In conclusion, our study indicates that several mpMRI parameters correlate with the biopsy ISUP grade groups. Combination of these parameters using defined MRI grading groups (mG1 to mG3) seems to be helpful in addition to the standard PI-RADS classification for the better prediction of PCA aggressiveness and may offer more certainty for clinicians in further clinical pathway and their individual treatment selection or monitoring. Moreover, our data reveal that in patients within mG3 and no or low-grade PCA detection after biopsy an early re-biopsy should be considered, due to the high prevalence of higher grade PCA. Patients with mG1 lesions may receive follow-up mpMRI first if they qualify for active surveillance.
Change history
17 August 2022
The original version of this article was updated. Funding note "Open Access funding enabled and organized by Projekt DEAL" has been added.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DCE:
-
Dynamic contrast enhancement
- DWI:
-
Diffusion-weighted imaging
- EPE:
-
Extraprostatic extension
- EPI:
-
Echoplanar imaging
- IL:
-
Index lesion
- ISUP:
-
International Society of Urological Pathology
- LMM:
-
Linear mixed model
- mpMRI:
-
Multiparametric magnetic resonance imaging
- PCA:
-
Prostate cancer
- PIRADS:
-
Prostate Imaging and Reporting Archiving Data System
- PSA:
-
Prostate-specific antigen
- PSAD:
-
Prostate-specific antigen density
- PZ:
-
Peripheral zone
- ROI:
-
Region of interest
- TZ:
-
Transition zone
References
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378(19):1767–1777. https://doi.org/10.1056/NEJMoa1801993
Kim M, Ryu H, Lee HJ, Hwang SI, Choe G, Hong SK (2020) Who can safely evade a magnetic resonance imaging fusion-targeted biopsy (MRIFTB) for prostate imaging reporting and data system (PI-RADS) 3 lesion? World J Urol. https://doi.org/10.1007/s00345-020-03352-3
Litwin MS, Tan H-J (2017) The diagnosis and treatment of prostate cancer: a review. JAMA 317(24):2532–2542. https://doi.org/10.1001/jama.2017.7248
Briganti A, Fossati N, Catto JWF et al (2018) Active surveillance for low-risk prostate cancer: the European Association of Urology Position in 2018. Eur Urol 74(3):357–368. https://doi.org/10.1016/j.eururo.2018.06.008
Pompe RS, Beyer B, Haese A et al (2018) Postoperative complications of contemporary open and robot-assisted laparoscopic radical prostatectomy using standardised reporting systems. BJU Int 122(5):801–807. https://doi.org/10.1111/bju.14369
Ullrich T, Arsov C, Quentin M et al (2020) Multiparametric magnetic resonance imaging can exclude prostate cancer progression in patients on active surveillance: a retrospective cohort study. Eur Radiol 30:6042. https://doi.org/10.1007/s00330-020-06997-1
Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD (2001) Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 58(6):843–8. https://doi.org/10.1016/s0090-4295(01)01441-8
D’Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11):969–974. https://doi.org/10.1001/jama.280.11
de Rooij M, Israël B, Tummers M et al (2020) ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur Radiol 30(10):5404–5416. https://doi.org/10.1007/s00330-020-06929-z
Borofsky MS, Rosenkrantz AB, Abraham N, Jain R, Taneja SS (2013) Does suspicion of prostate cancer on integrated T2 and diffusion-weighted MRI predict more adverse pathology on radical prostatectomy? Urology 81(6):1279–1283. https://doi.org/10.1016/j.urology.2012.12.026
Itatani R, Namimoto T, Atsuji S et al (2014) Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 83(10):1740–1745. https://doi.org/10.1016/j.ejrad.2014.06.026
Shukla-Dave A, Hricak H, Akin O et al (2012) Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int 109(9):1315–1322. https://doi.org/10.1111/j.1464-410X.2011.10612.x
Turkbey B, Mani H, Aras O et al (2013) Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 268(1):144–152. https://doi.org/10.1148/radiol.13121325
Boesen L, Chabanova E, Løgager V, Balslev I, Thomsen HS (2015) Apparent diffusion coefficient ratio correlates significantly with prostate cancer Gleason score at final pathology. J Magn Reson Imaging 42(2):446–453. https://doi.org/10.1002/jmri.24801
Nowak J, Malzahn U, Baur ADJ et al (2016) The value of ADC, T2 signal intensity, and a combination of both parameters to assess Gleason score and primary Gleason grades in patients with known prostate cancer. Acta Radiol 57(1):107–114. https://doi.org/10.1177/0284185114561915
Turkbey B, Shah VP, Pang Y et al (2011) Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 258(2):488–495. https://doi.org/10.1148/radiol.10100667
Sun C, Chatterjee A, Yousuf A et al (2019) Comparison of T2-weighted imaging, DWI, and dynamic contrast-enhanced MRI for calculation of prostate cancer index lesion volume: correlation with whole-mount pathology. AJR Am J Roentgenol 212(2):351–356. https://doi.org/10.2214/AJR.18.20147
Klingebiel M, Ullrich T, Quentin M et al (2020) Advanced diffusion weighted imaging of the prostate: comparison of readout-segmented multi-shot, parallel-transmit and single-shot echo-planar imaging. Eur J Radiol 130:109161. https://doi.org/10.1016/j.ejrad.2020.109161
Hambrock T, Somford DM, Huisman HJ et al (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–61. https://doi.org/10.1148/radiol.11091409
Kitajima K, Takahashi S, Ueno Y et al (2012) Clinical utility of apparent diffusion coefficient values obtained using high b-value when diagnosing prostate cancer using 3 tesla MRI: comparison between ultra-high b-value (2000 s/mm2) and standard high b-value (1000 s/mm2). J Magn Reson Imaging 36(1):198–205. https://doi.org/10.1002/jmri.23627
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, Lawrence Erlbaum Associates, Publishers
Turkbey B, Pinto PA, Mani H et al (2010) Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology 255(1):89–99. https://doi.org/10.1148/radiol.09090475
Donati OF, Mazaheri Y, Afaq A et al (2014) Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 271(1):143–152. https://doi.org/10.1148/radiol.13130973
Hambrock T, Hoeks C, Hulsbergen-van de Kaa C et al (2012) Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 61(1):177–184. https://doi.org/10.1016/j.eururo.2011.08.042
Felker ER, Margolis DJ, Nassiri N, Marks LS (2016) Prostate cancer risk stratification with magnetic resonance imaging. Urol Oncol 34(7):311–319. https://doi.org/10.1016/j.urolonc.2016.03.001
Wolters T, Roobol MJ, van Leeuwen PJ et al (2011) A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 185(1):121–125. https://doi.org/10.1016/j.juro.2010.08.082
Ullrich T, Schimmöller L (2020) Perspective: a critical assessment of PI-RADS 2.1. Abdom Radiol (NY) 45(12):3961–3968. https://doi.org/10.1007/s00261-020-02424-7
Kayhan A, Fan X, Oommen J, Oto A (2010) Multi-parametric MR imaging of transition zone prostate cancer: Imaging features, detection and staging. World J Radiol 2(5):180–187. https://doi.org/10.4329/wjr.v2.i5.180
Park KJ, Kim M-H, Kim JK (2020) Extraprostatic tumor extension: comparison of preoperative multiparametric MRI criteria and histopathologic correlation after radical prostatectomy. Radiology 296(1):87–95. https://doi.org/10.1148/radiol.2020192133
Moore CM, Giganti F, Albertsen P et al (2017) Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE recommendations-a report of a European School of Oncology Task Force. Eur Urol 71(4):648–655. https://doi.org/10.1016/j.eururo.2016.06.011
Bonekamp D, Kohl S, Wiesenfarth M et al (2018) Radiomic machine learning for characterization of prostate lesions with MRI: comparison to ADC values. Radiology 289(1):128–137. https://doi.org/10.1148/radiol.2018173064
Maas MC, Litjens GJS, Wright AJ et al (2019) A single-arm, multicenter validation study of prostate cancer localization and aggressiveness with a quantitative multiparametric magnetic resonance imaging approach. Invest Radiol 54(7):437–447. https://doi.org/10.1097/RLI.0000000000000558
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Lars Schimmöller.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic and prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boschheidgen, M., Schimmöller, L., Arsov, C. et al. MRI grading for the prediction of prostate cancer aggressiveness. Eur Radiol 32, 2351–2359 (2022). https://doi.org/10.1007/s00330-021-08332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08332-8