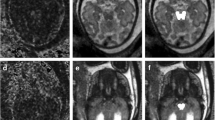
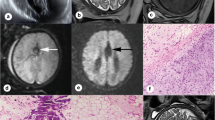
Abstract
Objectives
To explore the feasibility of single-direction diffusion-weighted imaging (DWI) for assessing the fetal corpus callosum (CC).
Methods
This prospective study included 67 fetuses with normal CC and 35 fetuses suspected with agenesis of the corpus callosum (ACC). The MR protocols included HASTE, TrueFISP, and single-direction DWI. Two radiologists independently evaluated the optimal visibility and the contrast ratio (CR) of the normal fetal CC. The Chi-squared test or Fisher’s exact test was used to compare the proportions of “good” visibility (score ≥ 3, and the CC was almost/entirely visible) between single-direction DWI and HASTE/TrueFISP. The CR difference between single-direction DWI and HASTE/TrueFISP was detected using the paired t-test. The diagnostic accuracies were determined by comparison with postnatal imaging. In fetuses suspected of ACC, we measured and compared the length and area of the mid-sagittal CC in the single-direction DWI images.
Results
The proportion of “good” visibility in single-direction DWI was higher than that in HASTE/TrueFISP, with p < 0.0001. The mean CR from single-direction DWI was also higher than that of TrueFISP and HASTE (both with p < 0.0001). The diagnostic accuracy of the single-direction DWI combined with HASTE/TrueFisp (97.1%, 34/35) was higher than that of the Haste/TrueFISP (74.3%, 26/35) (p = 0.013). The length and area of the PACC (p < 0.001, p = 0.001, respectively) and HCC (p < 0.001, p = 0.018, respectively) groups were significantly lower than those of the normal group.
Conclusions
The single-direction DWI is feasible in displaying fetal CC and can be a complementary sequence in diagnosing ACC.
Key Points
• We suggest a simple method for the display of the fetal CC.
• The optimal visibility and contrast ratio from single-direction DWI were higher than those from HASTE and TrueFISP.
• The diagnostic accuracy of the single-direction DWI combined with HASTE/TrueFISP sequences (97.1%, 34/35) was higher than that of the Haste/TrueFISP (74.3%, 26/35).





Similar content being viewed by others
Abbreviations
- ACC:
-
Agenesis of the corpus callosum
- ADC:
-
Apparent diffusion coefficient
- CACC:
-
Complete agenesis of the corpus callosum
- CC:
-
Corpus callosum
- CI:
-
Confidence interval
- CR:
-
Contrast ratio
- DTI:
-
Diffusion-tensor magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- HASTE:
-
Half-Fourier single-shot turbo spin-echo
- HCC:
-
Hypoplasia of the corpus callosum
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- PACC:
-
Partial agenesis of the corpus callosum
- ROI:
-
Region of interest
- TrueFISP:
-
True fast imaging with steady-state precession
References
Huang H, Xue R, Zhang J et al (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29:4263–4273. https://doi.org/10.1523/JNEUROSCI.2769-08.2009
Huang H, Zhang J, Jiang H et al (2005) DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage 26:195–205. https://doi.org/10.1016/j.neuroimage.2005.01.019
Manevich-Mazor M, Weissmann-Brenner A, Bar Yosef O et al (2018) Added value of fetal MRI in the evaluation of fetal anomalies of the corpus callosum: a retrospective analysis of 78 cases. Ultraschall Med 39:513–525. https://doi.org/10.1055/s-0043-113820
Achiron R, Achiron A (2001) Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study: fetal corpus callosum development. Ultrasound Obstet Gynecol 18:343–347. https://doi.org/10.1046/j.0960-7692.2001.00512.x
Cignini P, Padula F, Giorlandino M et al (2014) Reference charts for fetal corpus callosum length: a prospective cross-sectional study of 2950 fetuses. J Ultrasound Med 33:1065–1078. https://doi.org/10.7863/ultra.33.6.1065
Horsfield MA, Jones DK (2002) Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases - a review. NMR Biomed 15:570–577. https://doi.org/10.1002/nbm.787
Feng L, Li H, Oishi K et al (2019) Age-specific gray and white matter DTI atlas for human brain at 33, 36 and 39 postmenstrual weeks. Neuroimage 185:685–698. https://doi.org/10.1016/j.neuroimage.2018.06.069
Vasung L, Charvet CJ, Shiohama T et al (2019) Ex vivo fetal brain MRI: recent advances, challenges, and future directions. Neuroimage 195:23–37. https://doi.org/10.1016/j.neuroimage.2019.03.034
Ouyang A, Jeon T, Sunkin SM et al (2015) Spatial mapping of structural and connectional imaging data for the developing human brain with diffusion tensor imaging. Methods 73:27–37. https://doi.org/10.1016/j.ymeth.2014.10.025
Song JW, Gruber GM, Patsch JM et al (2018) How accurate are prenatal tractography results? A postnatal in vivo follow-up study using diffusion tensor imaging. Pediatr Radiol 48:486–498. https://doi.org/10.1007/s00247-017-3982-y
Mitter C, Prayer D, Brugger PC et al (2015) In vivo tractography of fetal association fibers. PLoS One 10:e0119536. https://doi.org/10.1371/journal.pone.0119536
Ouyang M, Dubois J, Yu Q et al (2019) Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage 185:836–850. https://doi.org/10.1016/j.neuroimage.2018.04.017
Huang H, Vasung L (2014) Gaining insight of fetal brain development with diffusion MRI and histology. Int J Dev Neurosci 32:11–22. https://doi.org/10.1016/j.ijdevneu.2013.06.005
Song L, Mishra V, Ouyang M et al (2017) Human fetal brain connectome: structural network development from middle fetal stage to birth. Front Neurosci 11:561. https://doi.org/10.3389/fnins.2017.00561
Huang H (2010) Structure of the fetal brain: what we are learning from diffusion tensor imaging. Neuroscientist 16:634–649. https://doi.org/10.1177/1073858409356711
Huang H, Zhang J, Wakana S et al (2006) White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage 33:27–38. https://doi.org/10.1016/j.neuroimage.2006.06.009
Milos R-I (2020) Developmental dynamics of the periventricular parietal crossroads of growing cortical pathways in the fetal brain - in vivo fetal MRI with histological correlation. Nuerolmage 210:116553
Khan S, Vasung L, Marami B et al (2019) Fetal brain growth portrayed by a spatiotemporal diffusion tensor MRI atlas computed from in utero images. Neuroimage 185:593–608. https://doi.org/10.1016/j.neuroimage.2018.08.030
Jakab A, Tuura R, Kellenberger C, Scheer I (2017) In utero diffusion tensor imaging of the fetal brain: a reproducibility study. Neuroimage Clin 15:601–612. https://doi.org/10.1016/j.nicl.2017.06.013
Hunt D, Dighe M, Gatenby C, Studholme C (2020) Automatic, age consistent reconstruction of the corpus callosum guided by coherency from in utero diffusion-weighted MRI. IEEE Trans Med Imaging 39:601–610. https://doi.org/10.1109/TMI.2019.2932681
Marami B, Mohseni Salehi SS, Afacan O et al (2017) Temporal slice registration and robust diffusion-tensor reconstruction for improved fetal brain structural connectivity analysis. Neuroimage 156:475–488. https://doi.org/10.1016/j.neuroimage.2017.04.033
Scola E, Sirgiovanni I, Avignone S et al (2016) Fetal development of the corpus callosum: insights from a 3T DTI and tractography study in a patient with segmental callosal agenesis. Neuroradiol J 29:323–325. https://doi.org/10.1177/1971400916665390
Tagliafico A, Succio G, Neumaier CE et al (2012) Brachial plexus assessment with three-dimensional isotropic resolution fast spin echo MRI: comparison with conventional MRI at 3.0 T. Br J Radiol 85:e110–e116. https://doi.org/10.1259/bjr/28972953
Yoneyama M, Takahara T, Kwee TC et al (2013) Rapid high resolution MR Neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci 12:111–119. https://doi.org/10.2463/mrms.2012-0063
Ghi T, Carletti A, Contro E et al (2010) Prenatal diagnosis and outcome of partial agenesis and hypoplasia of the corpus callosum. Ultrasound Obstet Gynecol 35:35–41. https://doi.org/10.1002/uog.7489
Takahara T, Hendrikse J, Kwee TC et al (2010) Diffusion-weighted MR neurography of the sacral plexus with unidirectional motion probing gradients. Eur Radiol 20:1221–1226. https://doi.org/10.1007/s00330-009-1665-2
Bydder GM, Rutherford MA, Hajnal JV (2001) How to perform diffusion-weighted imaging. Childs Nerv Syst 17:195–201. https://doi.org/10.1007/s003810000281
Liu B, Xin W, Tan J-R et al (2019) Myelin sheath structure and regeneration in peripheral nerve injury repair. Proc Natl Acad Sci 116:22347–22352. https://doi.org/10.1073/pnas.1910292116
Bando Y (2019) Roads to formation of normal myelin structure and pathological myelin structure. In: Sango K, Yamauchi J, Ogata T, Susuki K (eds) Myelin. Springer Singapore, Singapore, pp 257–264
Jiang S, Xue H, Counsell S et al (2009) Diffusion tensor imaging (DTI) of the brain in moving subjects: application to in-utero fetal and ex-utero studies. Magn Reson Med 62:645–655. https://doi.org/10.1002/mrm.22032
Diogo MC, Prayer D, Gruber GM et al (2019) Echo-planar FLAIR sequence improves subplate visualization in fetal MRI of the brain. Radiology 292:159–169. https://doi.org/10.1148/radiol.2019181976
Kasprian G, Brugger PC, Schöpf V et al (2013) Assessing prenatal white matter connectivity in commissural agenesis. Brain 136:168–179. https://doi.org/10.1093/brain/aws332
Mitter C, Jakab A, Brugger PC et al (2015) Validation of in utero tractography of human fetal commissural and internal capsule fibers with histological structure tensor analysis. Front Neuroanat 9:164. https://doi.org/10.3389/fnana.2015.00164
Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA et al (2012) Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal 16:1550–1564. https://doi.org/10.1016/j.media.2012.07.004
Kuklisova-Murgasova M, Lockwood Estrin G, Nunes RG et al (2018) Distortion correction in fetal EPI Using non-rigid registration with a Laplacian constraint. IEEE Trans Med Imaging 37:12–19. https://doi.org/10.1109/TMI.2017.2667227
Deprez M, Price A, Christiaens D et al (2020) Higher order spherical harmonics reconstruction of fetal diffusion MRI with intensity correction. IEEE Trans Med Imaging 39:1104–1113. https://doi.org/10.1109/TMI.2019.2943565
Gholipour A, Rollins CK, Velasco-Annis C et al (2017) A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep 7:476. https://doi.org/10.1038/s41598-017-00525-w
Acknowledgements
We thank P. Ellen Grant, MD, formally affiliated with the Department of Radiology and Fetal-Neonatal Neuroimaging & Developmental Science Center (FNNDSC), Boston Children’s Hospital, Harvard Medical School, for helping me revise the manuscript. We are very grateful to all the participants and their families who consented to participate in this study. Besides, we thank the radiography staff at the Shandong Medical Imaging Research Institute, Cheeloo College of Medicine, Shandong University, for their support in the imaging data collection.
Funding
This work was supported by the National Natural Science Foundation of China (81671668); and the Natural Science Foundation of Shandong Province (ZR201911150560).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Guangbin Wang.
Conflict of interest
One of the authors of this manuscript (Jinxia Zhu) is an employee of Siemens Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, C., Zhang, X., Chen, X. et al. Single-direction diffusion-weighted imaging may be a simple complementary sequence for evaluating fetal corpus callosum. Eur Radiol 32, 1135–1143 (2022). https://doi.org/10.1007/s00330-021-08176-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08176-2