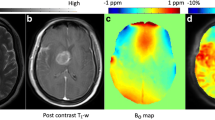
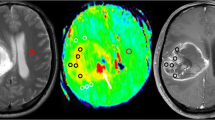
Abstract
Objectives
Differentiation of glioblastomas (GBMs) and solitary brain metastases (SBMs) is an important clinical problem. The aim of this study was to determine whether amide proton transfer–weighted (APTW) imaging is useful for distinguishing GBMs from SBMs.
Methods
We examined 31 patients with GBM and 17 with SBM. For each tumor, enhancing areas (EAs) and surrounding non-enhancing areas with T2-prolongation (peritumoral high signal intensity areas, PHAs) were manually segmented using fusion images of the post-contrast T1-weighted and T2-weighted images. The mean amide proton transfer signal intensities (APTSIs) were compared among the EAs, PHAs, and contralateral normal appearing white matter (NAWM) within each tumor type. Furthermore, we analyzed APTSI histograms to compare the EAs and PHAs of GBMs and SBMs.
Results
In GBMs, the mean APTSI in EAs (2.92 ± 0.74%) was the highest, followed by that in PHAs (1.64 ± 0.83%, p < 0.001) and NAWM (0.43 ± 0.83%, p < 0.001). In SBMs, the mean APTSI in EAs (1.85 ± 0.99%) and PHAs (1.42 ± 0.45%) were significantly higher than that in NAWM (0.42 ± 0.30%, p < 0.001), whereas no significant difference was found between EAs and PHAs. The mean and 10th, 25th, 50th, 75th, and 90th percentiles for APT in EAs of GBMs were significantly higher than those of SBMs. However, no significant difference was found between GBMs and SBMs in any histogram parameters for PHA.
Conclusions
APTSI in EAs, but not PHAs, is useful for differentiation between GBMs and SBMs.
Key Points
• Amide proton transfer–weighted imaging and histogram analysis in the enhancing tumor can provide useful information for differentiation between glioblastomas and solitary brain metastasis.
• Amide proton transfer signal intensity histogram parameters from peritumoral areas showed no significant difference between glioblastomas and solitary brain metastasis.
• Vasogenic edema alone can substantially increase amide proton transfer signal intensity which may mimic tumor invasion.




Similar content being viewed by others
Abbreviations
- APTSI:
-
Amide proton transfer signal intensity
- APTW:
-
Amide proton transfer–weighted
- EA:
-
Enhancing area
- GBM:
-
Glioblastoma
- ICC:
-
Intraclass correlation coefficient
- NAWM:
-
Normal appearing white matter
- PHA:
-
Peritumoral high signal intensity area
- SBM:
-
Solitary brain metastasis
References
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G; ESMO guidelines working group (2014) High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:93–101
Tsao MN, Rades D, Wirth A et al (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225
Nussbaum ES, Djalilian HR, Cho KH, Hall WA (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78:1781–1788
Schiff D (2001) Single brain metastasis. Curr Treat Options Neurol 3:89–99
Cha S, Lupo JM, Chen MH et al (2007) Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 28:1078–1084
Ma JH, Kim HS, Rim NJ, Kim SH, Cho KG (2010) Differentiation among glioblastoma multiforme, solitary metastatic tumor, and lymphoma using whole-tumor histogram analysis of the normalized cerebral blood volume in enhancing and perienhancing lesions. AJNR Am J Neuroradiol 31:1699–1706
Bauer AH, Erly W, Moser FG, Maya M, Nael K (2015) Differentiation of solitary brain metastasis from glioblastoma multiforme: a predictive multiparametric approach using combined MR diffusion and perfusion. Neuroradiology 57:697–703
Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592
Wen Z, Hu S, Huang F et al (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51:616–622
Togao O, Kessinger CW, Huang G et al (2013) Characterization of lung cancer by amide proton transfer (APT) imaging: an in-vivo study in an orthotopic mouse model. PLoS One Available via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3797134. Accessed 10 Mar 2018
Togao O, Hiwatashi A, Yamashita K et al (2017) Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion weighted imaging. Eur Radiol 27:578–588
Sakata A, Okada T, Yamamoto A et al (2015) Grading glial tumors with amide proton transfer MR imaging: different analytical approaches. J Neurooncol 122:339–348
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ (2017) Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res 23:3667–3675
Jiang S, Yu H, Wang X et al (2016) Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur Radiol 26:64–71
Keupp J, Baltes C, Harvey PR, van den Brink J (2011) Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain at 3T. Proc Intl Soc Mag Reson Med 19:710
Togao O, Hiwatashi A, Keupp J et al (2015) Scan-rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J Magn Reson Imaging 42:1346–1353
Thévenaz P, Ruttimann UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7:27–41
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol 16:441–448
Park JE, Kim HS, Park KJ, Choi CG, Kim SJ (2015) Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: increased accuracy of MR perfusion. Radiology 277:151–161
Yu H, Lou H, Zou T et al (2017) Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol 27:4516–4524
Zamecnik J (2005) The extracellular space and matrix of gliomas. Acta Neuropathol 110:435–442
Emblem KE, Nedregaard B, Nome T et al (2008) Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 247:808–817
Law M, Young R, Babb J, Pollack E, Johnson G (2007) Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 28:761–766
Togao O, Hiwatashi A, Keupp J et al (2016) Amide proton transfer imaging of diffuse gliomas: effect of saturation pulse length in parallel transmission-based technique. PLoS One Available via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881971. Accessed 10 Mar 2018
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Reifenberger G, Hentschel B, Felsberg J et al (2012) Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 131:1342–1350
Jiang S, Rui Q, Wang Y et al (2018) Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur Radiol 28:2115–2123
Acknowledgments
The authors thank Dr. Hajime Yonezawa, MD, PhD, Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, for providing the clinical information for this article.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Takashi Yoshiura.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Jochen Keupp is an employee of Philips Research, and Yuta Akamine is an employee of Philips Japan.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic study
• Performed at one institution
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Kamimura, K., Nakajo, M., Yoneyama, T. et al. Histogram analysis of amide proton transfer–weighted imaging: comparison of glioblastoma and solitary brain metastasis in enhancing tumors and peritumoral regions. Eur Radiol 29, 4133–4140 (2019). https://doi.org/10.1007/s00330-018-5832-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5832-1