Abstract
Objectives
To investigate whether T1-mapping allows assessment of acute kidney injury (AKI) and prediction of chronic kidney disease (CKD) in mice.
Methods
AKI was induced in C57Bl/6N mice by clamping of the right renal pedicle for 35 min (moderate AKI, n = 26) or 45 min (severe AKI, n = 23). Sham animals served as controls (n = 9). Renal histology was assessed in the acute (day 1 + day 7; d1 + d7) and chronic phase (d28) after AKI. Furthermore, longitudinal MRI-examinations (prior to until d28 after surgery) were performed using a 7-Tesla magnet. T1-maps were calculated from a fat-saturated echoplanar inversion recovery sequence, and mean and relative T1-relaxation times were determined.
Results
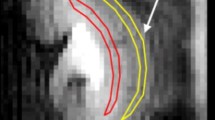
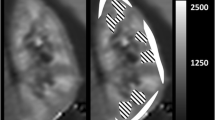
Renal histology showed severe tubular injury at d1 + d7 in both AKI groups, whereas, at d28, only animals with prolonged 45-min ischemia showed persistent signs of AKI. Following both AKI severities T1-values significantly increased and peaked at d7. T1-times in the contralateral kidney without AKI remained stable. At d7 relative T1-values in the outer stripe of the outer medulla were significantly higher after severe than after moderate AKI (138 ± 2 % vs. 121 ± 3 %, p = 0.001). T1-elevation persisted until d28 only after severe AKI. Already at d7 T1 in the outer stripe of the outer medulla correlated with kidney volume loss indicating CKD (r = 0.83).
Conclusion
T1-mapping non-invasively detects AKI severity in mice and predicts further outcome.
Key Points
• Renal T1-relaxation times are increased after ischemia-induced acute kidney injury.
• Renal T1-values correlate with subsequent kidney volume loss.
• T1-mapping detects the severity of acute kidney injury and predicts further outcome.






Similar content being viewed by others
Abbreviations
- AKI:
-
acute kidney injury
- CKD:
-
chronic kidney disease
- IRI:
-
ischemia reperfusion injury
- ISOM:
-
inner stripe of the outer renal medulla
- MRI:
-
magnetic resonance imaging
- OSOM:
-
outer stripe of the outer renal medulla
References
Wald R, Quinn RR, Luo J et al (2009) Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302:1179–1185
Leung KC, Tonelli M, James MT (2013) Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9:77–85
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334:1448–1460
Srisawat N, Kellum JA (2011) Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care 17:548–555
Li X, Hassoun HT, Santora R, Rabb H (2009) Organ crosstalk: the role of the kidney. Curr Opin Crit Care 15:481–487
Basile DP (2007) The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72:151–156
Sutton TA, Molitoris BA (1998) Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol 18:490–497
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–4221
McCullough PA, Shaw AD, Haase M et al (2013) Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 182:13–29
Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K (2012) Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156:785–795, W-270, W-271, W-272, W-273, W-274, W-275, W-276, W-277, W-278
Lanzman RS, Wittsack HJ, Martirosian P et al (2010) Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol 20:1485–1491
Prasad PV (2006) Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Ren Physiol 290:F958–974
Pohlmann A, Hentschel J, Fechner M et al (2013) High temporal resolution parametric MRI monitoring of the initial ischemia/reperfusion phase in experimental acute kidney injury. PLoS One 8:e57411
Lanzman RS, Ljimani A, Pentang G et al (2013) Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology 266:218–225
Thoeny HC, De Keyzer F (2011) Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology 259:25–38
Hueper K, Gutberlet M, Rodt T et al (2011) Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol 21:2427–2433
Shah B, Anderson SW, Scalera J, Jara H, Soto JA (2011) Quantitative MR imaging: physical principles and sequence design in abdominal imaging. Radiographics 31:867–880
Kiricuta IC Jr, Simplaceanu V (1975) Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res 35:1164–1167
Kundel HL, Schlakman B, Joseph PM, Fishman JE, Summers R (1986) Water content and NMR relaxation time gradients in the rabbit kidney. Investig Radiol 21:12–17
de Miguel MH, Yeung HN, Goyal M et al (1994) Evaluation of quantitative magnetic resonance imaging as a noninvasive technique for measuring renal scarring in a rabbit model of antiglomerular basement membrane disease. J Am Soc Nephrol 4:1861–1868
Puntmann VO, Voigt T, Chen Z et al (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6:475–484
Messroghli DR, Walters K, Plein S et al (2007) Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med 58:34–40
Won S, Davies-Venn C, Liu S, Bluemke DA (2013) Noninvasive imaging of myocardial extracellular matrix for assessment of fibrosis. Curr Opin Cardiol 28:282–289
Salerno M, Kramer CM (2013) Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging 6:806–822
Hueper K, Rong S, Gutberlet M et al (2013) T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol 48:834–842
Liu AS, Xie JX (2003) Functional evaluation of normothermic ischemia and reperfusion injury in dog kidney by combining MR diffusion-weighted imaging and Gd-DTPA enhanced first-pass perfusion. J Magn Reson Imaging 17:683–693
Gueler F, Rong S, Mengel M et al (2008) Renal urokinase-type plasminogen activator (uPA) receptor but not uPA deficiency strongly attenuates ischemia reperfusion injury and acute kidney allograft rejection. J immunol (Baltimore, Md : 1950) 181:1179-1189
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205
Lu X, Li N, Shushakova N et al (2012) C57BL/6 and 129/Sv mice: genetic difference to renal ischemia-reperfusion. J Nephrol 25:738–743
Hueper K, Gutberlet M, Rong S et al (2014) Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice--Comparison with Histopathologic Results and Renal Function. Radiology 270:117–124
Karlberg L, Norlen BJ, Ojteg G, Wolgast M (1983) Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand 118:11–17
Haase VH (2013) Mechanisms of Hypoxia Responses in Renal Tissue. J Am Soc Nephrol. doi:10.1681/ASN.2012080855
Hricak H, Terrier F, Demas BE (1986) Renal allografts: evaluation by MR imaging. Radiology 159:435–441
Yuasa Y, Kundel HL (1985) Magnetic resonance imaging following unilateral occlusion of the renal circulation in rabbits. Radiology 154:151–156
London DA, Davis PL, Williams RD, Crooks LE, Sheldon PE, Gooding CA (1983) Nuclear magnetic resonance imaging of induced renal lesions. Radiology 148:167–172
Acknowledgements
The scientific guarantor of this publication is Prof. Dr. Faikah Gueler. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by REBIRTH Cluster of Excellence of Hannover Medical School (Germany). No complex statistical methods were necessary for this paper. Institutional Review Board approval was not required because it was an experimental study without the use of human data. Approval from the institutional animal care committee was obtained. Some study subjects or cohorts have been previously reported in: Hueper K, Rong S, Gutberlet M, et al. (2013) T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol, 48(12):834-842.
Hueper K, Gutberlet M, Rong S, et al. (2014) Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice--Comparison with Histopathologic Results and Renal Function. Radiology, 270(1):117-124
Methodology: prospective, experimental, performed at one institution.
Katja Hueper and Matti Peperhove contributed equally as first authors.
Dagmar Hartung and Faikah Gueler contributed equally as last authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Examples of inversion recovery EPI-images at different inversion times Shown are inversion recovery EPI-images at different inversion times (TI = 30, 700, 1200, 1500, 8000 ms). In the upper row EPI-images at baseline (d0) and in the lower row images of the same kidney at d7 after severe AKI are shown. (JPEG 1129 kb)
Rights and permissions
About this article
Cite this article
Hueper, K., Peperhove, M., Rong, S. et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur Radiol 24, 2252–2260 (2014). https://doi.org/10.1007/s00330-014-3250-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3250-6