Abstract
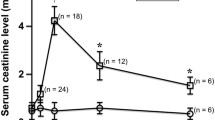
The primary goal of this study was to quantitatively assess regional changes in function and pathophysiological characteristics in renal ischemia/reperfusion (IR)-injured mice and compare data obtained at baseline and following injury. The second goal was to correlate blood oxygenation level-dependent (BOLD) and diffusion tensor imaging (DTI) data obtained in renal IR-injured mice at baseline using time-dependent scans. The analyses were conducted using a mouse model of IR injury. T2*, apparent diffusion coefficient (ADC), and fractional anisotropy (FA) values were acquired to pathophysiologically evaluate three renal regions. The T2* values obtained in the renal cortex and medulla demonstrated no significant differences between baseline and after IR injury. According to DTI results, ADC values in both renal regions were significantly lower at 0 h, and then gradually increased within 48 h. The FA values of both renal regions decreased at 0 h, and then gradually increased by 8 h after IR injury. BOLD and DTI correlations were not significant in renal cortex. However, significantly positive correlations were found between baseline and time-dependent data obtained from renal medulla of IR-injured kidneys. Our current findings suggest that magnetic resonance imaging could be used to obtain pathophysiological data from separate renal compartments in IR-injured mice. Hence, BOLD and DTI may also provide functional and pathophysiological data on allograft status following kidney transplant without the need to use a contrast agent.





Similar content being viewed by others
References
J.V. Bonventre, L. Yang, J. Clin. Invest. 121(11), 4210–4221 (2011)
A.M. Sheridan, J.V. Bonventre, Curr. Opin. Nephrol. Hypertens. 9(4), 427–434 (2000)
H. Boom, M.J.K. Mallat, J.W. de Fijter, A.H. Zwinderman, L.C. Paul, Kidney Int. 58, 859–866 (2000)
F. Moreso, D. Serón, S. Gil-Vernet, L. Riera, X. Fulladosa, R. Ramos, J. Alsina, J.M. Grinyó, Nephrol. Dial. Transplant. 14(4), 930–935 (1999)
R. Böhmová, O. Viklický, Folia Microbiol. 46, 267–276 (2001)
D. Dragun, U. Hoff, J.K. Park, Y. Qun, W. Schneider, F.C. Luft, H. Haller, Kidney Int. 60, 1173–1181 (2001)
M. Brezis, S. Rosen, N. Engl, J. Med. 332, 647–655 (1995)
K. Aukland, J. Krog, Nature 188, 671 (1960)
K.U. Eckardt, W.W. Bernhardt, A. Weidemann, C. Warnecke, C. Rosenberger, M.M. Wiesener, C. Willam, Kidney Int. 68, S46–S51 (2005)
R.R. Perrella, A.J. Duerinckx, F.N. Tessier, G.M. Danovitch, A. Wilkinson, S. Gonzalez, A.H. Cohen, E.G. Grant, Am. J. Kidney Dis. 15(6), 544–550 (1990)
Y. Agmon, H. Peleg, Z. Greenfeld, S. Rosen, M. Brezis, J. Clin. Invest. 94(3), 1069–1075 (1994)
J.R. Chapman, P.J. O’Connell, B.J. Nankivell, J. Am. Soc. Nephrol. 16(10), 3015–3026 (2005)
P.V. Prasad, R.R. Edelman, F.H. Epstein, Circulation 94, 3271–3275 (1996)
P.V. Prasad, A. Priatna, K. Spokes, F.H. Epstein, J. Magn. Reson. Imaging 13(5), 744–747 (2001)
L. Juillard, L.O. Lerman, D.G. Kruger, J.A. Haas, B.C. Rucker, J.A. Polzin, S.J. Riederer, J.C. Romero, Kidney Int. 65, 944–950 (2004)
M. Oostendorp, E.E. de Vries, J.M.G.M. Slenter, C.J. Peutz-Kootstra, M.G. Snoeijs, M.J. Post, L.W.E. van Heurn, W.H. Backes, NMR Biomed. 24(2), 194–200 (2011)
M. Haque, T. Franklin, P. Prasad, J. Magn. Reson. Imaging 33(4), 898–901 (2011)
W.J. Yin, F. Liu, X.M. Li, L. Yang, S. Zhao, Z.X. Huang, Y.Q. Huang, R.B. Liu, Eur. J. Radiol. 81(7), 1426–1431 (2012)
L.-P. Li, L.J. Ji, S. Lindsay, P.V. Prasad, J. Magn. Reson. Imaging 25, 635–638 (2007)
D. LeBihan, E. Breton, D. Lallemand, M.L. Aubin, J. Vignaud, M. Laval-Jeantet, Radiology 168(2), 497–505 (1988)
A.S. Liu, J.X. Xie, J. Magn. Reson. Imaging 17(6), 683–693 (2003)
J.S. Cheung, S.J. Fan, A.M. Chow, J. Zhang, K. Man, E.X. Wu, NMR Biomed. 23(5), 496–502 (2010)
A. Priatna, F.H. Epstein, K. Spokes, P.V. Prasad, J. Magn. Reson. Imaging 9(6), 842–846 (1999)
M. Ries, F. Basseau, B. Tyndal, R. Jones, C. Deminière, B. Catargi, C. Combe, C.W.T. Moonen, N. Grenier, J. Magn. Reson. Imaging 17(1), 104–113 (2003)
A. Djamali, E.A. Sadowski, R.J. Muehrer, S. Reese, C. Smavatkul, A. Vidyasagar, S.B. Fain, R.C. Lipscomb, D.H. Hullett, M. Samaniego-Picota, T.M. Grist, B.N. Becker, Am. J. Physiol. Renal. Physiol. 292, F513–F522 (2007)
L. Mannelli, J.H. Maki, S.F. Osman, H. Chandarana, D.J. Lomas, W.P. Shuman, K.F. Linnau, D.E. Green, G. Laffi, M. Moshiri, Curr. Urol. Rep. 13(1), 99–107 (2012)
B. Taouli, R.K. Thakur, L. Mannelli, J.S. Babb, S. Kim, E.M. Hecht, V.S. Lee, G.M. Israel, Radiology 251(2), 398–407 (2009)
K. Hueper, M. Gutberlet, T. Rodt, W. Gwinner, F. Lehner, F. Wacker, M. Galanski, D. Hartung, Eur. Radiol. 21(11), 2427–2433 (2011)
T.A. Powers, C.H. Lorenz, G.E. Holburn, R.R. Price, Radiology 178(2), 543–548 (1991)
M. Notohamiprodjo, M.F. Reiser, S.P. Sourbron, Eur. J. Radiol. 76(3), 337–347 (2010)
C. Santosh, D. Brennan, C. McCabe, I.M. Macrae, W.M. Holmes, D.I. Graham, L. Gallagher, B. Condon, D.M. Hadley, K.W. Muir, W. Gsell, J. Cer. Blood. Flow. Metab. 28, 1742–1753 (2008)
S. Pazahr, A. Boss, C. Rossi, Curr. Radiol. Rep. 1(2), 115–125 (2013)
M.I. Kettunen, O.H.J. Gröhn, M.J. Silvennoinen, M. Penttonen, R.A. Kauppinen, J. Cereb. Blood Flow Metab. 22, 262–270 (2002)
A. Pohlmann, K. Arakelyan, J. Hentschel, K. Cantow, B. Flemming, M. Ladwig, S. Waiczies, E. Seeliger, T. Niendorf, Invest. Radiol. 49(8), 547–560 (2014)
Acknowledgments
This study was supported by grants of 2014-602 and 2014-7004 from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number : HI14C1090).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woo, DC., Kim, N., Lee, DW. et al. Assessing Renal Ischemia/Reperfusion Injury in Mice Using Time-Dependent BOLD and DTI at 9.4 T. Appl Magn Reson 46, 709–722 (2015). https://doi.org/10.1007/s00723-015-0668-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-015-0668-1