Abstract
Objective
To evaluate, with the use of magnetic resonance imaging (MRI), whether aortic pulse wave velocity (PWV) is associated with cardiac left ventricular (LV) function and mass as well as with cerebral small vessel disease in patients with type 1 diabetes mellitus (DM).
Materials and methods
We included 86 consecutive type 1 DM patients (49 male, mean age 46.9 ± 11.7 years) in a prospective, cross-sectional study. Exclusion criteria included aortic/heart disease and general MRI contra-indications. MRI of the aorta, heart and brain was performed for assessment of aortic PWV, as a marker of aortic stiffness, systolic LV function and mass, as well as for the presence of cerebral white matter hyperintensities (WMHs), microbleeds and lacunar infarcts. Multivariate linear or logistic regression was performed to analyse the association between aortic PWV and outcome parameters, with covariates defined as age, gender, mean arterial pressure, heart rate, BMI, smoking, DM duration and hypertension.
Results
Mean aortic PWV was 7.1 ± 2.5 m/s. Aortic PWV was independently associated with LV ejection fraction (ß = -0.406, P = 0.006), LV stroke volume (ß = -0.407, P = 0.001), LV cardiac output (ß = -0.458, P = 0.001), and with cerebral WMHs (P < 0.05). There were no independent associations between aortic stiffness and LV mass, cerebral microbleeds or lacunar infarcts.
Conclusion
Aortic stiffness is independently associated with systolic LV function and cerebral WMHs in patients with type 1 DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes mellitus (DM) patients show functional and structural alterations of the arterial vessel wall, resulting in arterial stiffness [1, 2]. Arterial stiffening has been described as an early phenomenon in subjects with type 1 DM, already apparent before clinical onset of cardiovascular (CV) complications, and also as an independent predictor of overt CV disease and mortality [3]. Therefore, arterial stiffening may be related to the pathogenesis of CV complications in type 1 DM. This notion could be substantiated if an independent relationship could be established between arterial stiffness and cardiac function in type 1 DM. Furthermore, CV complications in type 1 DM also involve small vessel disease in the brain, and if a relationship between arterial stiffness and cerebral small vessel disease could be established as well, this would support the importance of arterial stiffness in CV complications in type 1 DM. An integrated study investigating the relationship between arterial stiffness, cardiac function and cerebral small vessel disease has not been performed in type 1 DM so far.
Stiffening of the aorta affects cardiac function by increasing the cardiac afterload and reducing diastolic coronary artery perfusion [4]. Myocardial perfusion might fail to compensate for the increased metabolic energy demand, resulting in an impaired myocardial contractility function [5]. Furthermore, stiffness of the central large arteries results in deficient absorption of the pulse wave. This high pulsatile flow is transmitted from the aorta to the brain causing damage to the endothelial and smooth muscle cells, disrupting the cerebral small vessels [6, 7]. Also, aortic stiffness may represent coronary and cerebral endothelial dysfunction or wall thickening caused by shared underlying mechanisms.
As aortic function plays a central role in maintaining adequate perfusion of both the heart and the brain, we hypothesized that aortic stiffness is associated with cardiac function as well as with cerebral small vessel disease in DM patients. To our knowledge, no previous magnetic resonance imaging (MRI) studies have evaluated the relationship between aortic function, cardiac function and cerebral small vessel disease in one comprehensive protocol. MRI is a non-invasive tool for the accurate assessment of aortic pulse wave velocity (PWV) [8] as a marker of aortic stiffness [9]. Notably, MRI is not dependent on geometric assumptions about the aortic path length unlike ultrasound techniques [10]. Furthermore, MRI is well suited to assess cardiac LV function [11]. Moreover, MRI is a validated technique for the detection of cerebral small vessel disease manifesting as white matter hyperintensities (WMHs) [12], microbleeds [13] and lacunar infarcts [14].
Therefore, the purpose of the present study was to use a comprehensive MRI protocol to evaluate whether aortic PWV is associated with cardiac LV function and mass as well as with cerebral small vessel disease in patients with type 1 DM.
Materials and methods
Study participants
Between February 2008 and February 2009, consecutive patients with type 1 DM were recruited from our local outpatient clinic of the university medical centre. For inclusion, patients had to be older than 18 years and diagnosed with type 1 DM. A total of 86 patients (49 men, 37 women; mean age 46.9 ± 11.7 years) gave written informed consent to participate in the study and were prospectively included. Exclusion criteria included congenital aortic/heart disease, evidence of aortic or heart disease as evaluated by means of cardiac auscultation and ECG, and standard contra-indications to MRI such as claustrophobia, pacemaker and metal implantations.
Information about patient characteristics was obtained by means of a standardised interview and physical and laboratory examinations. Diabetes duration was estimated as the time passed between the reported age of diagnosis and the MRI examination. Body mass index (BMI) was calculated from body length and mass at the time of MRI. Blood pressure and heart rate were measured after MRI using a semi-automated sphygmomanometer (Dinamap, Critikon, Tampa, FL, USA, validated to ANSI/AAMI SP10 criteria). Pulse pressure was defined as the difference between systolic and diastolic blood pressure. Mean arterial pressure (MAP) was calculated by adding diastolic blood pressure to one-third of the pulse pressure. Smoking was defined as non-smoker or a current smoker. Hypertension was defined as the use of anti-hypertensive medication. Glycated haemoglobin (HbA1c), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglycerides and C-reactive protein were determined. The albumin excretion ratio was calculated using the microalbumin and creatinine concentrations in the urine.
The study was approved by the local medical ethics committee, and the study was conducted according to the principles in the Declaration of Helsinki.
Magnetic resonance imaging
Aortic and cardiac imaging was performed using 1.5 Tesla MRI (NT 15 Gyroscan Intera; Philips Medical Systems, Best, the Netherlands). All brain examinations were performed on 3.0 Tesla MRI (Achieva; Philips Medical Systems, Best, the Netherlands).
For the evaluation of aortic stiffness, aortic PWV was determined using a previously described protocol [15]. In short, a scout view of the aorta was performed. Two velocity-encoded images were obtained: one perpendicular to the aorta at the level of the pulmonary trunk, and one at the level of the abdominal descending aorta 7.5 cm beneath the diaphragm. This resulted in through-plane flow measurements of the ascending and descending aorta at those levels. Aortic PWV was calculated for the aorta as Δx/Δt, where Δx is the aortic path length between the two measurement sites and Δt is the time delay between the arrivals of the foot of the pulse wave at the respective measurement sites. Data were analysed with MASS/FLOW (Medis, Leiden, the Netherlands) by a single observer (S.v.E., 2 years of experience in cardiac MRI) supervised by a senior researcher (J.W., 13 years of experience in cardiac MRI).
For the assessment of systolic LV function and LV mass, the entire heart was imaged in short-axis orientation as described previously [11]. Endocardial and epicardial LV borders were manually traced on short-axis cine images using the software package MASS. Ejection fraction (EF), stroke volume (SV), cardiac output (CO), LV end-diastolic volume (LV ED volume), LV end-systolic volume (LV ES volume) and LV end-diastolic mass (LV ED mass) were assessed. Volumes and mass were indexed (i) for body surface area (BSA). All manual contour drawings were performed by a researcher (S.v.E., 2 years of experience in cardiac MRI), with supervision of a radiologist (L.K., 12 years of experience in cardiac MRI).
For evaluation of cerebral small vessel disease a spin-echo T2-weighted image, a fluid-attenuated inversion recovery (FLAIR) image and a T2*-weighted gradient echo sequence were assessed. WMHs were defined as areas of brain parenchyma with increased signal on T2-weighted and FLAIR images without mass effect. WMHs were distinguished as either periventricular (pv) WMHs or subcortical (sc) WMHs because of the different pathogenesis involved [16]. WMHs were classified according to Fazekas et al. [16]. For statistical analysis subjects were divided into those with normal (0) versus abnormal (1) amounts of pv and sc WMHs. Figure 1 demonstrates a case example with presence of pv and sc WMHs on a FLAIR sequence. Microbleeds were defined as focal, nodular areas of signal loss in brain parenchyma on T2* images that are invisible or smaller on T2-weighted spin echo images [17]. Microbleeds were scored as absent (0) or present (1). Lacunar brain infarcts were defined as small (but >3 mm in size) cavities within the brain parenchyma, with similar signal intensity to that of cerebrospinal fluid on all pulse sequences, surrounded by an area of high signal intensity on T2 and FLAIR images [18, 19]. Their presence was defined on a binary scale: absent (0) or present (1). WMHs, microbleeds and lacunar infarcts were visually scored by consensus reading by a researcher (S.v.E., 1 year of experience in neuroradiology) and a senior neuroscientist (J.v.d.G., 15 years of experience in neuroradiology) or a neuroradiologist (M.v.B., 15 years of experience in neuroradiology).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 16.0; SPSS, Chicago, IL, USA). Data are expressed as mean ± standard deviation, unless stated otherwise. Aortic PWV values were non-normally distributed (Kolmogorov-Smirnov test of normality, P = 0.002). Spearman correlation analysis was performed to analyse the association between aortic PWV and continuous outcome parameters. Spearman correlation coefficients (r) and P values are reported. Univariate logistic regression was performed to analyse the association between aortic PWV and dichotomous outcome parameters. Odds ratios (OR), 95% confidence intervals (CI) and P values are reported. For adjustment of confounding factors, defined as age, gender, MAP, heart rate, BMI, smoking, DM duration and hypertension, these covariates were entered into a multivariate linear or logistic regression model. Associations between cardiac parameters indexed for BSA were not corrected for BMI.
Results
The characteristics of the study population are described in Table 1. All MRI examinations were successfully performed without any adverse events. Eighty-six patients with type 1 DM were included, with a mean age of 46.9 ± 11.7 years. The mean HbA1c values were 7.7 ± 1.0%.
Mean aortic PWV was 7.11 ± 2.51 m/s. Aortic PWV was significantly associated with age (r = 0.674, P < 0.001), DM duration (r = 0.299, P = 0.006), BMI (r = 0.317, P = 0.003), systolic blood pressure (r = 0.484, P < 0.001), pulse pressure (r = 0.509, P < 0.001), MAP (r = 0.339, P = 0.001) and use of antihypertensive medication (OR = 1.278, P = 0.017). There were no statistically significant associations between aortic PWV and laboratory markers (Table 1).
Associations between aortic PWV and cardiac LV function and mass
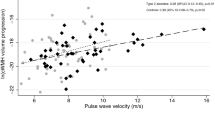
The mean values of cardiac LV parameters and the associations with aortic PWV are summarised in Table 2. After statistical correction for confounder’s age, gender, MAP, heart rate, BMI, smoking, DM duration and hypertension, aortic PWV was found to be significantly associated with systolic LV parameters: LV EF (ß = -0.402, P = 0.006), LV SVi (ß = -0.407, P = 0.001) and LV COi (ß = -0.458, P = 0.001). The inverse association between aortic PWV and LV SVi is illustrated in Fig. 2. There was no statistically significant association between aortic PWV and LV mass.
Associations between aortic PWV and cerebral small vessel disease
According to their Fazekas score, 16 (18.6%) patients showed pv WMHs and 38 (44.2%) patients were diagnosed with sc WMHs. In 7 (8.1%) DM patients, microbleeds were detected. Two patients (2.3%) showed lacunar infarcts on brain MRI. The associations between aortic PWV and parameters of cerebral small vessel disease are summarised in Table 2. Univariate logistic regression analysis showed that aortic PWV was significantly associated with all MRI parameters of cerebral small vessel disease.
After statistical adjustment for confounding factors, aortic PWV was significantly associated with pv WMHs (OR = 1.425, 95% CI = 1.003–2.026, P < 0.05) and sc WMHs (OR = 1.479, 95% CI = 1.063–2.058, P = 0.02). In Fig. 3, aortic PWV in patients with type 1 DM with and without pv WMHs is presented in boxplots showing higher aortic PWV in patients with pv WMHs compared with patients without pv WMHs.
Discussion
The purpose of the present study was to evaluate whether aortic PWV is associated with cardiac LV function and mass as well as with cerebral small vessel disease in patients with type 1 DM using a comprehensive MRI protocol.
The main findings of our study are that aortic stiffness in patients with type 1 DM is inversely associated with systolic LV function and is associated with cerebral WMHs, independently of age, gender, MAP, heart rate, BMI, smoking, DM duration and hypertension. No independent association between aortic stiffness and LV mass, cerebral microbleeds or lacunar infarcts was found.
This study is the first to report an integrated approach for detecting the relationship among arterial stiffness, cardiac function and cerebral small vessel disease. Indeed, in this cohort of relatively young type 1 DM patients, aortic stiffness was found to be inversely associated with systolic LV function and with WMHs. Patients with type 1 DM are at increased risk of developing systolic LV dysfunction, with subsequent progressive heart failure and premature death [20]. In addition, it is known that WMHs may progress and become manifest over time [21, 22] but also that individuals with WMHs benefit from secondary stroke prevention therapies [23]. We hypothesized that PWV as measured in the aorta by MRI may be a marker or risk factor for generalised vascular disease that might potentially be treated. Interestingly, this association between PWV and MRI manifestations of cardiac function and cerebral small vessel disease was independent of many major risk factors such as age and hypertension, suggesting that aortic stiffness might have an independent pathophysiological mechanism.
The present MRI data show that in type 1 DM patients aortic stiffness was inversely related to systolic LV function. To our knowledge, this is the first study to report a relationship between systolic LV function and aortic stiffness in a type 1 DM population. In our study population, the LV ejection fraction was in the normal range [24], which might emphasise an important role of aortic stiffness in cardiac function already manifesting before occurrence of cardiac dysfunction or failure and compensatory remodelling. High blood pressure is strongly associated with LV hypertrophy [25]. However, in type 1 DM patients the presence of LV hypertrophy is not a well-known phenomenon [26, 27]. Therefore it is more likely that in patients with hypertension increased aortic stiffness is associated with LV hypertrophy [28, 29]. Our study on type 1 DM patients is in line with these findings; no relationship between aortic PWV and LV mass was found in a relatively young type 1 DM population with well-treated hypertension. Furthermore, a statistical correction for hypertension was performed.
In the present study, aortic PWV was independently associated with pv and sc WMHs. Although increased arterial stiffness in DM was related to microvascular complications of the kidneys and the retina in a previous study [3], the current study is the first to report on the potential relation between aortic stiffness and small vessel disease in the brain in patients with type 1 DM. Interestingly, this relationship between aortic stiffness and WMHs was independent of hypertension, a finding that has been reported recently [30], and independent of age as described before [31], suggesting an independent pathophysiological mechanism for aortic stiffness and WMHs in patients with type 1 DM.
No independent relationship was found between aortic stiffness and microbleeds. It is plausible that instead of a haemodynamic effect, other mechanisms including altered glucoregulation and microvascular changes by advanced glycation end products are involved in the development of microbleeds in DM patients [32, 33]. The low prevalence of lacunar infarcts (n = 2) in our study population may have hampered the statistical analyses.
In addition to arterial stiffness being a predictor of (fatal) myocardial infarction and stroke [3], our study results show that aortic stiffness is associated with reduced cardiac function and cerebral small vessel disease as shown by MRI. Of note, assessment of aortic PWV is currently not part of the clinical routine assessment in DM patients. Our study results suggest that aortic PWV could be useful in the cardiovascular risk screening of patients with type 1 DM, if longitudinal studies confirm our initial observations and establish their prognostic implications in patients with type 1 DM.
As the study design is cross-sectional, a causal relationship between aortic PWV and systolic LV function or WMHs cannot be determined. However, the present study reveals that aortic stiffness reflects both cardiac function and cerebral small vessel disease. In our study design, we chose to measure aortic PWV with some distance of the aortic bifurcation to avoid wave reflections from the aortic bifurcation, which can corrupt the observer-independent automatic assessment of the PWV [34]. However, more refined analysis of thoracic and abdominal aortic segments may allow local identification of aortic vessel wall condition, and its relationship with cardiac function and cerebral small vessel disease. A possible limitation of this study is the lack of a healthy control group. However, the main purpose of this study was to investigate the possible relationship between aortic PWV and cardiac function and mass as well as cerebral small vessel disease in type 1 DM patients.
In conclusion, this study shows that in patients with type 1 DM stiffness of the aorta is independently associated with systolic LV function as well as with cerebral WMHs. By documenting that aortic stiffness reflects stages of both cardiac function and cerebral small vessel disease, our study results suggest that aortic PWV assessment might be a useful marker of cardiac and cerebrovascular disease in patients with type 1 DM. Future studies are needed to assess the prognostic implications of our observations.
References
Giannattasio C, Failla M, Piperno A, Grappiolo A, Gamba P, Paleari F, Mancia G (1999) Early impairment of large artery structure and function in type I diabetes mellitus. Diabetologia 42:987–994
Oxlund H, Rasmussen LM, Andreassen TT, Heickendorff L (1989) Increased aortic stiffness in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 32:748–752
Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD (2003) Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. J Hypertens 21:2035–2044
Leite-Moreira AF, Correia-Pinto J, Gillebert TC (1999) Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res 43:344–353
Brooks B, Molyneaux L, Yue DK (1999) Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care 22:1722–1727
Byrom FB, Dodson LF (1948) The causation of acute arterial necrosis in hypertensive disease. J Pathol Bacteriol 60:357–368
O’Rourke MF, Safar ME (2005) Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46:200–204
Grotenhuis HB, Westenberg JJM, Steendijk P, van der Geest RJ, Ottenkamp J, Bax JJ, Jukema W, de Roos A (2009) Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. J Magn Reson Imaging 30:521–526
O’Rourke MF (2007) Arterial aging: pathophysiological principles. Vasc Med 12:329–341
Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605
Lamb HJ, Doornbos J, van der Velde EA, Kruit MC, Reiber JH, de Roos A (1996) Echo planar MRI of the heart on a standard system: validation of measurements of left ventricular function and mass. J Comput Assist Tomogr 20:942–949
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Cordonnier C, Al-Shahi SR, Wardlaw J (2007) Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 130:1988–2003
Lastilla M (2006) Lacunar infarct. Clin Exp Hypertens 28:205–215
van der Meer RW, Diamant M, Westenberg JJ, Doornbos J, Bax JJ, de Roos A, Lamb HJ (2007) Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson 9:645–651
Fazekas F, Schmidt R, Scheltens P (1998) Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord 9 Suppl 1:2–5
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi SR, Warach S, Launer LJ, Van Buchem MA, Breteler MM (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8:165–174
Vermeer SE, Longstreth WT Jr, Koudstaal PJ (2007) Silent brain infarcts: a systematic review. Lancet Neurol 6:611–619
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Bell DS (2003) Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26:2433–2441
Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM (2004) Cerebral white matter lesions and the risk of dementia. Arch Neurol 61:1531–1534
van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM (2008) Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke 39:2712–2719
Ovbiagele B, Saver JL (2006) Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis 22:83–90
Maceira AM, Prasad SK, Khan M, Pennell DJ (2006) Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8:417–426
Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G (2004) Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). Am J Cardiol 93:40–44
Di Cori A, Di Bello V, Miccoli R, Talini E, Palagi C, delle Donne MG, Penno G, Nardi C, Bianchi C, Mariani M, Del Prato S, Balbarini A (2007) Left ventricular function in normotensive young adults with well-controlled type 1 diabetes mellitus. Am J Cardiol 99:84–90
Palmieri V, Capaldo B, Russo C, Iaccarino M, Pezzullo S, Quintavalle G, Di Minno G, Riccardi G, Celentano A (2008) Uncomplicated type 1 diabetes and preclinical left ventricular myocardial dysfunction: insights from echocardiography and exercise cardiac performance evaluation. Diabetes Res Clin Pract 79:262–268
Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL (1997) Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension 30:654–659
Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB (2000) Impact of arterial stiffening on left ventricular structure. Hypertension 36:489–494
Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW (2008) Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52:1120–1126
Ohmine T, Miwa Y, Yao H, Yuzuriha T, Takashima Y, Uchino A, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T (2008) Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res 31:75–81
Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3:169–178
Qiu C, Cotch MF, Sigurdsson S, Garcia M, Klein R, Jonasson F, Klein BE, Eiriksdottir G, Harris TB, Van Buchem MA, Gudnason V, Launer LJ (2008) Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes 57:1645–1650
Stevanov M, Baruthio J, Gounot D, Grucker D (2001) In vitro validation of MR measurements of arterial pulse-wave velocity in the presence of reflected waves. J Magn Reson Imaging 14:120–127
Acknowledgements
The authors declare that there is no conflict of interest associated with this manuscript. This research was performed within the framework of the Center for Translational Molecular Medicine (CTMM; www.ctmm.nl), project PREDICCt (grant 01C-104), and supported by the Netherlands Heart Foundation (NHS2003B136 and NHS2007B81 to P.C.N. Rensen), Dutch Diabetes Research Foundation and Dutch Kidney Foundation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Elderen, S.G.C., Brandts, A., Westenberg, J.J.M. et al. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol 20, 1132–1138 (2010). https://doi.org/10.1007/s00330-009-1655-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1655-4