Abstract
Purpose
The purpose of this study was to assess the influence of iodine concentration on diagnostic efficacy in multi-detector-row computed tomography (MDCT) angiography of the abdominal aorta and abdominal arteries.
Methods
IRB approval and informed consent were obtained. In this double-blind trial, patients were randomised to undergo MDCT angiography of the abdominal arteries during administration of iobitridol (350 mgI/ml) or iomeprol (400 mgI/ml). Each centre applied its own technique for delivery of contrast medium, regardless of iodine concentration. Diagnostic efficacy, image quality, visualisation of the arterial wall and arterial enhancement were evaluated. A total of 153 patients received iobitridol and 154 received iomeprol.
Results
The ability to reach a diagnosis was “satisfactory” to “totally satisfactory” in 152 (99.3%) and 153 (99.4%) patients respectively. Image quality was rated as being “good” to “excellent” in 94.7 and 94.8% segments respectively. Similar results were observed for image quality of arterial walls (84.3 vs. 83.2%). The mean relative changes in arterial enhancement between baseline and arterial phase images showed no statistically significant differences.
Conclusion
This study demonstrated the non-inferiority of the 350 versus 400 mgI/ml iodine concentration, in terms of diagnostic efficacy, in abdominal MDCT angiography. It also confirmed the high robustness and reliability of this technique across multi-national practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multi-detector-row computed tomography (MDCT) has been established as the method of choice for the diagnosis, treatment planning and follow-up of most diseases of the abdominal arteries, including the renal [1–4] and visceral arteries [5, 6] and the aorta [7–9]. In these anatomical regions, diagnostic invasive arteriography (digital subtraction angiography, DSA) has steadily been replaced over the past few years by non-invasive CT angiography and, in some cases, by non-invasive magnetic resonance (MR) angiography [10–12].
However, the broad use of MDCT in the clinical routine, as well as the choice of contrast agent and administration technique thereof, still remains controversial. Many authors have shown that the enhancement of the abdominal arteries is directly correlated to the amount of iodine (not contrast agent) per second [5, 13], also known as the “iodine flux”. The higher the iodine flux, the higher the density will be within the region of interest. On the other hand, increasing the total amount of iodine could raise safety issues such as concern of contrast-induced nephropathy (CIN) in at-risk patients. Because a lower total amount of iodine lowers the risk of CIN [14–16], protocols for CT angiography should always find a compromise between the required contrast enhancement and the amount of iodine injected.
Although many papers have been published about preferred methods of administering contrast medium, some questions remain unanswered. It is still unclear as to what degree of vascular opacification (in Hounsfield units, HU) is really necessary to obtain diagnostic images; in other words, does higher density, which can possibly be reached with more highly concentrated agents and higher iodine flux, really further improve diagnostic accuracy? Finally, even injection parameters still need to be standardised. Fleischmann and Hittmair [5, 17–19] have shown the advantages of using a biphasic contrast injection, calculated individually for every patient, in CT angiography of the abdominal aorta. However, these studies were performed on single-slice systems with long acquisition times. With fast imaging, using 64-slice CT systems, it seems that injection protocols could be made much easier, and the benefit of a contrast medium with higher iodine concentrations could be of great importance. In addition, optimum injection rate, optimum iodine concentration and optimum total volume of contrast medium for abdominal CT angiography still need to be determined.
In the present trial, patients were investigated during the injection of two different contrast agents with different iodine concentrations (350 vs. 400 mgI/ml). In contrast to some recently published papers [20], where patients were examined with different iodine concentrations but constant iodine flux rates, the injection protocol in the present study was not adapted to the iodine concentration of the agent used. In addition, the various centres participating in the present trial were to use their own injection protocols. A lack of relevant differences in diagnostic efficacy, image quality and arterial contrast enhancement among the centres will emphasise how stable and easy to perform abdominal CT angiography is in clinical settings if state-of-the-art equipment is used.
The aim of this prospective, randomised study was, therefore, to assess the influence of iodine concentration on diagnostic efficacy in CT angiography of the abdominal and visceral arteries.
Materials and methods
Study design and patient enrolment conditions
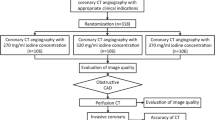
This study was a non-inferiority, randomised, double-blind, prospective, multi-centre trial in patients referred for CT angiography of the abdominal aorta and its branches. Nine European centres were involved in this trial between August 2006 and February 2008. The study protocol was approved by the local Ethics Committees and Competent Authorities.
As this study was designed to reflect the conditions of daily routine, patients were included in a consecutive manner, regardless of the indication for the examination. Patients between 18 and 85 years of age were eligible for study participation. A history of previous (open surgical or endovascular) vascular treatment in the abdomen was not an exclusion criterion. Patients with haemodynamic instability, non-compensated heart failure or hypertension (systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg) were not included. In addition, patients with severe renal insufficiency [defined as estimated creatinine clearance (Cockroft and Gault) below 30 ml/min], treatment with diuretics or biguanides within 48 h before CT angiography, known thyrotoxicosis or a history of hypersensitivity to iodinated contrast agents were not included. Breast-feeding or pregnant women were also excluded from participation in this trial.
After providing written, informed consent, patients were randomised into two examination groups according to a randomisation list stratified on centres and balanced every four patients. Patients in the first group were to undergo the abdominal CT angiography with injection of iobitridol 350 mgI/ml (Xenetix®, Guerbet, Roissy, France); patients in the second group were to be examined by means of CT angiography with injection of iomeprol 400 mgI/ml (Iomeron®, Bracco, Italy).
CT angiography
All patients included in the present evaluation were scheduled to undergo CT angiography of the abdominal aorta and abdominal arteries. All examinations were performed on 64-slice single-source CT systems (two Siemens SOMATOM Sensation, four General Electrics LightSpeed VCT, two Philips Brillance 64), and on a dual-source Siemens SOMATOM Definition used as a single-source.
Imaging protocol
To allow direct measurement of abdominal arterial enhancement, the study examination consisted of two steps including unenhanced imaging of the abdomen followed by abdominal CT angiography during the arterial first pass. If clinically indicated, additional venous or late-phase imaging could also be performed outside the study protocol.
Study centres were advised to use their own routinely used imaging protocol for abdominal CT angiography. Only a maximum allowed volume of 150 ml was predefined by the study protocol; all additional parameters, including injection rate and volume, were to be determined by the investigators. No compensations based on the different iodine concentrations of the contrast agents being compared were performed, as the investigators were blinded to both products. Thus, iodine flux, meaning the iodine administration per unit of time, was lower in the group receiving iobitridol than in the group receiving iomeprol.
MDCT angiography was performed using automatic bolus detection software (Smart Prep®, Care Bolus®, or equivalent) to identify peak contrast enhancement and to launch the acquisition. The whole abdomino-iliac system from above the suprarenal aorta to the femoral bifurcation was to be covered in one imaging procedure.
As the study centres were asked to use their own clinically established examination protocols, the kilovoltage (kV) and milliampere seconds (mAs) values differed among centres as did the average duration of the examination.
Injection of contrast medium
To ensure contrast agent administration in double-blind conditions, allocation of patients to one of the two groups (iobitridol or iomeprol group) and the preparation of the contrast agent, pump and injection were performed by a radiographer or nurse who was not involved in the subsequent evaluation. The contrast agent used was not identified on the images to allow blinded reading.
Patient follow-up
Clinical safety monitoring started at inclusion and lasted until 1 h after the end of the examination.
Evaluation
All CT images were assessed on-site by one local investigator at each participating hospital. The investigator was blinded to the contrast material used for each patient.
Diagnostic efficacy
Diagnostic efficacy (primary endpoint) of the CT examination was defined as the level of available information for diagnosis provided by the MDCT examination. Readers rated the diagnostic efficacy of the CT angiographies using a four-point scale ranging from 0, i.e. not satisfactory (“not providing enough information = complementary examination recommended”), to 3, i.e. totally satisfactory (“providing the expected information”), with level 1 indicating not satisfactory (“not providing all the expected information = could need a complementary examination”) and 2 representing satisfactory (“providing sufficient information”). The primary endpoint was calculated within both product groups by a cumulative proportion of those patients who presented with satisfactory (level 2) to totally satisfactory (level 3) images, and then the two product groups were compared.
Image quality according to vascular territories
As one of the secondary study criteria, the quality of images was evaluated in 16 pre-specified segments from the aorto-ilio-femoral axis: the suprarenal, the juxtarenal and the infrarenal aorta; the coeliac axis and its branches; the superior and inferior mesenteric artery; the right and left renal artery; the right and left common and external iliac artery; the right and left hypogastric artery; and the right and left common femoral artery.
In all of these 16 arterial segments, image quality was rated according to a four-point scale, i.e. 0 (null), 1 (poor), 2 (good) and 3 (excellent). If the segment was not delineated by the field of view (FOV), it was counted as “not applicable”. In the case of a 0 rating or poor quality, the reason for insufficient quality was indicated. This endpoint was calculated within both product groups by a cumulative proportion of vascular segments that presented with good (2) and excellent (3) image quality, and then the two product groups were compared.
Quality of vascular wall visualisation
Additionally, the quality of arterial wall visualisation within the 16 segments defined above was assessed. This evaluation was focused on changes within the vascular wall, including internal deposition of thrombotic material or inflammatory reaction, if present, and used the four-point scale mentioned above. In the case of a 0 rating or poor quality, the reason for this insufficient quality was indicated. This criterion was calculated within both product groups by a cumulative proportion of vascular wall segments that displayed with good (2) and excellent (3) image quality, and then the two groups were compared.
Quantification of arterial enhancement
As an additional endpoint, arterial enhancement was measured in eight predefined areas, including the suprarenal and the infrarenal aorta, the right and left renal artery, the right and left common iliac artery, and the right and left common femoral artery, and compared between the two groups. For measurement purposes, regions of interest (ROIs) were defined on unenhanced as well as enhanced images in the same table position (Fig. 1). According to the study protocol, the circumference of the ROI should not extend beyond the internal limit of the arterial wall. For the aorta, the ROI was to be located in the middle of the vessel and for its branches in the first 2 cm (Fig. 1). The two groups’ average absolute and relative enhancements were compared.
Principle of the measurement of arterial enhancement in the region of the suprarenal aorta, demonstrated in a patient examined with administration of 100 ml iobitridol because of infrarenal aortic aneurysm. Region of interest (ROI) is defined at the same table position in the baseline examination (a) as well as in the arterial phase images (b), as round surfaces in the lumen of the vessel, the circumference of which should not extend beyond the internal limit of the arterial wall
Clinical safety
In addition to the efficacy evaluation, the safety profile of both contrast agents was investigated, and any event, starting from inclusion until the end of the follow-up period (1 h after the end of CT angiography), was collected and reported.
Statistical evaluation
Assuming that in the iobitridol group and the iomeprol group at least 90% of patients would provide CT images with a satisfactory to totally satisfactory level of diagnostic information [21, 22], non-inferiority was defined as a difference between the two products (Δ = Piobitridol - Piomeprol) that was statistically higher than the clinical non-inferiority limit set at Δ0 = −10% in the study (maximum negative difference allowed between iobitridol and iomeprol). The study was to be demonstrative if the 95% confidence interval of the difference excluded the Δ0 = −10% clinical non-inferiority limit.
Before starting the trial, the sample size of the study (310 patients) was calculated so that the non-inferiority of iobitridol (350 mgI/ml) in providing the same level of diagnostic information as iomeprol (400 mgI/ml) could be statistically demonstrated with 80% power and 5% type-one error.
Confidence intervals and associated P values were computed for the estimated parameters based on the asymptotic normality of maximum likelihood estimators and using the Wald χ2 statistics in the SAS Genmod procedure. This procedure computes a generalised linear model, particularly suitable for adjusting on covariates and modelling clustered data (assessments at segment level nested in patients in the present study). In addition, the exact confidence interval and exact P value were also computed for the primary endpoint using StatXact software (Cytel Statistical Software & Services).
Results
Baseline characteristics and examination protocol
In total, 310 patients (247 men and 63 women; mean age: 66.5 years old) referred for CT angiography of the abdominal aorta and/or the abdominal arteries were enrolled in this trial. The baseline characteristics of the study population were well balanced between groups (Table 1).
Indications for CT included post-surgical follow-up (56.5%), pre-therapeutic assessment (36.1%) or other indications (7.4%), such as staging of lymphomas or gastric carcinomas. At the time of examination, 92 patients had various clinical symptoms, including hypertension (11.9%), limb ischaemia (10.7%), abdominal pain (9.4%) or other (5.5%). Moreover, 129 patients had a history of endovascular and/or surgical treatment of abdominal arteries (66 in the iobitridol group and 63 in the iomeprol group).
Of the 310 patients included, 154 were randomly assigned into the iobitridol group, and 156 into the iomeprol group, and only 1 patient was not injected for technical reasons.
Most of the centres (n = 6) performed the CT angiographies at a fixed tube voltage of 120 kV; the others at either 100 or 120 kV. The mean mAs used was 319.0 (range 63–896 mAs). No differences could be noticed between groups for those two parameters.
As shown in Table 2, four centres (accounting for 149 patients or 48% overall) injected the same fixed volume of contrast material at a fixed injection rate, regardless of the patient’s weight or indication for the examination. The other five centres adjusted the injection protocol to the patient’s condition. Between 63 and 140 ml of contrast agent were injected at a rate of 2.5–6 ml/s. On average, patients received 101.0 ml in the iobitridol group and 101.5 ml in the iomeprol group corresponding respectively to 35.4 and 40.6 g iodine, indicating that there was no additional volume injected to balance the lack of iodine in the iobitridol group.
The average duration of the examination (from time of injection to the end of CT) ranged from less than 1 min up to 14 min (mean of all centres: 1 min 22 s; standard deviation: 1 min 37 s).
Overall diagnostic efficacy
Of the 310 patients included, 3 were excluded from the efficacy analysis for technical reasons (1 in the iobitridol group and 2 in the iomeprol group).
Of the 307 patients available for further evaluation, diagnostic efficacy was rated satisfactory or totally satisfactory in 152 patients (99.3%) of the iobitridol group and in 153 patients (99.4%) of the iomeprol group. Non-inferiority was statistically demonstrated between the two products (exact 95% confidence interval [−2.98%; 3.01%] excluding the −10% clinical non-inferiority limit; Table 3).
Non-inferiority still holds if the maximum grade (totally satisfactory) of both products is considered and compared, as the exact 95% CI (−4.8%; 3.2%) still excludes the −10% clinical non-inferiority limit (Table 3).
The two examinations which failed to provide sufficient diagnostic information were performed in patients who had a history of endovascular and/or surgical treatment of abdominal arteries.
Image quality according to vascular territories
Of the 2,448 vascular segments assessed in the iobitridol group and 2,464 in the iomeprol group, 2,319 (94.7%) and 2,336 (94.8%) were rated as being of “good” to “excellent” image quality respectively. Differences between the two groups were statistically not significant (P = 0.5769), and 95% confidence intervals were small enough to conclude the equivalence of both contrast agents (Table 4; Fig. 2).
Two examples of abdominal aortic angiograms (maximum intensity projections, MIP) obtained during the administration of two contrast agents. The patient in a was examined during administration of 100 ml iobitridol 350 mgI/ml, and the patient in b received 100 ml iomeprol 400 mgI/ml. Regardless of the different total amounts of iodine, both MIPs show excellent and homogeneous arterial enhancement without visualised differences
Eighty-three segments were not assessable (out of the FOV, not applicable).
Quality of vascular wall visualisation
The quality of vascular wall visualisation was assessed in 2,448 segments in the iobitridol group and in 2,464 in the iomeprol group. It was rated good to excellent in 2,064 (84.3%) and 2,049 (83.2%) segments respectively. The differences between the two groups in this regard were statistically not significant (P = 0.8316), and 95% confidence intervals were small enough to conclude the equivalence of the two contrast agents (asymptotic 95% CI [−6.33%; 7.87%]) (Table 5).
Quantification of arterial enhancement
As expected, there was no difference in the mean baseline values between the two groups (40.0 HU in the iobitridol group; 40.8 HU in the iomeprol group). After contrast injection, mean arterial enhancement was 320.4 HU (SD, 93.54; range, 42–638) in the iobitridol group and 353.6 HU (SD, 95.45; range, 25–770) in the iomeprol group. The enhancement variation statistically differed between groups when considering the absolute variation (P = 0.0014; 95% CI [−53.47; −12.85]). Comparing the relative changes from baseline to arterial phase, no statistical difference was observed (P = 0.0673; 95% CI [−1.53; 0.053]) (Table 6).
Safety
Three patients reported a total of five non-serious adverse events, all occurring after contrast agent injection (diarrhoea, pain, neck rash and swelling in the iobitridol group; nausea in the iomeprol group). Two adverse events (neck rash and diarrhoea) experienced by the same patient were reported after the safety follow-up time required (9 and 33 h after the injection of the contrast agent respectively). All the adverse events had a favourable outcome, and relationship to the contrast agent was assumed for three of them (nausea, rash in neck and diarrhoea).
Discussion
Although non-invasive MDCT angiography has become the method of choice for most indications involving the abdominal and visceral arteries, no general contrast injection protocol has been established to date [13]. A number of papers have evaluated the influence of different parameters for contrast administration, including iodine concentration, total volume of contrast, injection rate, and other factors [20, 23–25], on arterial and/or parenchymal enhancement [13], but very limited general recommendations on optimised parameters could be gleaned from these publications. The very simple and basic relation, expressed as “the higher the iodine application per unit of time, the higher the enhancement” seems to be an established general rule for MDCT angiography following most of these publications [13, 19]. However, the question of whether this higher iodine flux should be reached by using a higher iodine concentration or a higher injection flow remains unclear, despite the number of studies recently published in this field [20, 25]. A study conducted by Awai et al. [24] concluded that arterial enhancement could be improved by increasing the iodine concentration of the contrast agent. In this study, the injection rate was the same for both products (0.56 ml/s/kg). Despite the fact that the results of the latter study are concordant with the study results reported by Bae et al. [26], it is apparently still unclear whether a higher concentration of iodine is really beneficial or not in abdominal MDCT angiography, as an additional study by Awai et al. [27] reported that faster injection (4 ml/s) of a lower-concentration agent (300 mgI/ml) led to greater enhancement compared with a slower injection (3.6 ml/s) of a higher-concentration agent (350 mgI/ml).
In fact, from a clinical point of view, the question of how to reach the amount of iodine per time needed for diagnostic images is of limited interest; on the other hand, the question of how much iodine per unit of time is really needed for abdominal MDCT angiography seems to be of greater relevance. Surprisingly, very few recommendations about the minimum iodine flux and minimum total amount of iodine necessary for abdominal CT angiography of sufficient image quality and diagnostic confidence have been published to date [13]. Roos and co-authors have shown that an iodine concentration of at least 300 mgI/ml should be recommended [28]. Ho et al. have shown that the volume of contrast medium can be reduced to a mean quantity of 107 ml (300 mgI/ml) in abdominal aortic CT angiography for aortic aneurysms without a significant decrease in aortic attenuation [29].
From a patient’s point of view, reducing the amount of iodine as much as possible would help to avoid CIN in patients at risk, as the probability of CIN is mainly determined by the amount of iodine delivered to patients [14, 16].
In the present study, two contrast agents (different with regard to their iodine concentration - iobitridol 350 mgI/ml vs. iomeprol 400 mgI/ml) were compared in a large population. The aim of this study was expressly not to test whether a higher-concentration contrast agent is better for arterial enhancement, as published previously in many other papers [20, 24, 25], but to demonstrate that the total amount of iodine can be reduced by more than 12% without significant disadvantages for diagnostic efficacy, image quality, or attenuation of MDCT angiography.
The present study statistically demonstrates the non-inferiority of the 350 mgI/ml iodine concentration vs. 400 mgI/ml in terms of diagnostic efficacy, confirming that a lower total amount of iodine and a reduced iodine flux for abdominal CT angiography does not yield inferior diagnostic contribution results. Moreover, in a per-patient, as well as in the more detailed per-segment evaluation, no significant differences between the two groups were found with regard to image quality (vessels and vascular walls levels) and relative arterial enhancement, emphasising the equality of the contrast agents injected for abdominal MDCT angiography.
The selected study protocol, in which no adaptation of injection parameters to the iodine rate led to different iodine fluxes and a broad range of iodine doses being used (from 24.5 to 56 g, i.e. more than 100%), and the very large number of examinations rated as being of good or almost excellent quality in both groups, exemplifies the limited influence of high iodine concentration and iodine flux on diagnostic efficacy and image quality of ultra-fast MDCT angiography using state-of-the-art 64-slice or dual-source systems (used as single source).
A comparison of these findings with those of previously published studies is difficult, as most of the other comparisons between two iodine concentrations have been performed with a constant iodine flux [20, 24, 25]. The results presented herein are in contradiction to the reports, for example, by Awai [24]. One reason for this difference could be the faster system used in the present study. Performance of a shorter imaging procedure, which is initiated slightly later, could lead to a lesser influence of higher iodine concentrations with regard to image quality.
These findings allow two major conclusions and recommendations to be made for the future development of injection protocols in abdominal MDCT angiography. Firstly, the role played by contrast material selection seems to be limited. Secondly, regarding the distribution of total iodine volumes used by the various investigators in the present study, there seems to be a general trend towards using a larger contrast dose than necessary to obtain images of good or almost excellent quality and high diagnostic efficacy. Additionally, other parameters become more important in the case of ultrafast CT angiographies. Reducing the total amount of contrast to a minimum, optimizing timing of contrast injection and CT scanning, and adapting to the extremely short examination times are mandatory to ensure diagnostic images. Thus, the data published herein should lead us to revise the examination protocols used for abdominal MDCT angiography with the clearly defined purpose of reducing the total amount of iodine. This will help to reduce the potential for the development of CIN in patients at risk.
This study is not free of limitations. First, no correlation with a reference standard was performed. However, as the aim of the study was to assess the diagnostic efficacy, image quality and contrast enhancement, rather than the sensitivity and specificity values in the detection of a dedicated pathological condition, the lack of difference between the two groups seems to be reliable even without a reference standard. As MDCT angiography is now well-established as the method of choice for the diagnosis of most of the different arterial pathological conditions in the abdomen [4], it would have been difficult to examine all included patients with an additional intra-arterial DSA for the purposes of our study.
Another limitation is that only two different iodine concentrations were used with a limited difference in iodine concentration. It would be of great interest to know the exact concentration at which no relevant difference could be observed, in other words, to assess the minimum iodine volume the injection could be reduced to without sacrificing diagnostic efficacy and image quality. Finally, a limitation of this study could be inherent in the different injection protocols; the relevant potential confounding factors (injection rate, volume injected, iodine flux, stent presence, imaging parameters and clinical indication for CT) were well-balanced between both groups and had no statistical impact on the difference between the two products, leading us to consider that the influence of iodine concentration has been addressed.
In conclusion, the present study demonstrates the limited influence of iodine concentration in the range between 350 and 400 mgI/ml on diagnostic efficacy and image quality in abdominal MDCT angiography with 64-slice and dual-source systems (used as single source), and the lack of additional benefit with the use of highly concentrated agents for this purpose. Furthermore, we showed that more iodine than necessary is typically injected into patients. In times of ultrafast CT scanning, continuous adaptation of injection parameters to the acquisition speed seems to be necessary to avoid unneeded high amounts of contrast material on the one hand while still ensuring sufficient arterial enhancement on the other hand. The results of the present study underline the limited influence of high iodine concentration on diagnostic image quality and exemplify the still existing space for improvement in contrast injection protocols. Based on the results of the present study, the amount of contrast can be reduced by using ultrafast equipment without sacrificing image quality and/or diagnostic efficacy. Further studies would be of interest to evaluate the minimum amount of iodine needed for diagnostic abdominal MDCT angiograms. Finally, the excellent safety, robustness and reproducibility of abdominal MDCT angiography was shown in a clinical setting by the very small number of adverse events and very few examinations of limited quality. This underlines the outstanding role played by MDCT angiography in the diagnosis of diseases of the abdominal arteries.
References
Glockner JF, Vrtiska TJ (2007) Renal MR and CT angiography: current concepts. Abdom Imaging 32:407–420
Puchner S, Stadler A, Minar E, Lammer J, Bucek RA (2007) Multidetector CT angiography in the follow-up of patients treated with renal artery stents: value of different reformation techniques compared with axial source images. J Endovasc Ther 14:387–394
Saba L, Caddeo G, Sanfilippo R, Montisci R, Mallarini G (2007) Multidetector-row CT angiography diagnostic sensitivity in evaluation of renal artery stenosis: comparison between multiple reconstruction techniques. J Comput Assist Tomogr 31:712–716
Rau MM, Setty BN, Blake MA, Ouellette-Piazzo K, Hahn PF, Sahani DV (2007) Evaluation of renal transplant donors with 16-section multidetector CT angiography: comparison of contrast media with low and high iodine concentrations. J Vasc Interv Radiol 18:603–609
Fleischmann D (2003) Multiple detector-row CT angiography of the renal and mesenteric vessels. Eur J Radiol 45:S79–S87
Horton KM, Fishman EK (2007) Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am 45:275–288
Willmann JK, Baumert B, Schertler T, Wildermuth S, Pfammatter T, Verdun FR, Seifert B, Marincek B, Böhm T (2005) Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology 236:1083–1093
Yu T, Zhu X, Tang L, Wang D, Saad N (2007) Review of CT angiography of aorta. Radiol Clin North Am 45:461–483
Teutelink A, Muhs BE, Vincken KL, Bartels LW, Cornelissen SA, van Herwaarden JA, Prokop M, Moll FL, Verhagen HJ (2007) Use of dynamic computed tomography to evaluate pre- and postoperative aortic changes in AAA patients undergoing endovascular aneurysm repair. J Endovasc Ther 14:44–49
Michaely HJ, Kramer H, Dietrich O, Nael K, Lodemann KP, Reiser MF, Schoenberg SO (2007) Intraindividual comparison of high-spatial-resolution abdominal MR angiography at 1.5 T and 3.0 T: initial experience. Radiology 244:907–913
Sutter R, Nanz D, Lutz AM, Pfammatter T, Seifert B, Struwe A, Heilmaier C, Weishaupt D, Marincek B, Willmann JK (2007) Assessment of aortoiliac and renal arteries: MR angiography with parallel acquisition versus conventional MR angiography and digital subtraction angiography. Radiology 245:276–284
Attenberger UI, Michaely HJ, Wintersperger BJ, Sourbron SP, Lodemann KP, Reiser MF, Schoenberg SO (2008) Three-dimensional contrast-enhanced magnetic-resonance angiography of the renal arteries: interindividual comparison of 0.2 mmol/kg gadobutrol at 1.5 T and 0.1 mmol/kg gadobenate dimeglumine at 3.0 T. Eur Radiol 18:1260–1268
Johnson PT, Fishman EK (2006) IV contrast selection for MDCT: current thoughts and practice. AJR Am J Roentgenol 186:406–415
Katzberg RW, Barrett BJ (2007) Risk of iodinated contrast material-induced nephropathy with intravenous administration. Radiology 243:622–628
Thomsen HS, Morcos SK, Barrett BJ (2008) Contrast-induced nephropathy: the wheel has turned 360 degrees. Acta Radiol 49:646–657
Nyman U, Almén T, Aspelin P, Hellström M, Kristiansson M, Sterner G (2005) Contrast-medium-induced nephropathy correlated to the ratio between dose in gram iodine and estimated GFR in ml/min. Acta Radiol 46:830–842
Fleischmann D, Rubin GD, Bankier AA, Hittmair K (2000) Improved uniformity of aortic enhancement with customized contrast medium injection protocols at CT angiography. Radiology 214:363–371
Hittmair K, Fleischmann D (2001) Accuracy of predicting and controlling time-dependent aortic enhancement from a test bolus injection. J Comput Assist Tomogr 25:287–294
Fleischmann D (2005) How to design injection protocols for multiple detector-row CT angiography (MDCTA). Eur Radiol 15:E60–E65
Behrendt FF, Mahnken AH, Keil S, Das M, Hohl C, Bauer D, Seidensticker P, Jost E, Wildberger JE, Günther RW, Mühlenbruch G (2008) Contrast enhancement in multidetector-row computed tomography (MDCT) of the abdomen: intraindividual comparison of contrast media containing 300 mg versus 370 mg iodine per ml. Eur Radiol 18:1199–1205
Petersein J, Peters CR, Wolf M, Hamm B (2003) Results of the safety and efficacy of iobitridol in more than 61,000 patients. Eur Radiol 13:2006–2011
Valentini AL, Tartaglione T, Monti L, Marano P (1994) Iomeprol versus iopamidol in contrast-enhanced computed tomography of thoracic and abdominal organs. Eur J Radiol 18:S88–S92
Furuta A, Ito K, Fujita T, Koike S, Shimizu A, Matsunaga N (2004) Hepatic enhancement in multiphasic contrast-enhanced MDCT: comparison of high- and low-iodine-concentration contrast medium in same patients with chronic liver disease. AJR Am J Roentgenol 183:157–162
Awai K, Takada K, Onishi H, Hori S (2002) Aortic and hepatic enhancement and tumor-to-liver contrast: analysis of the effect of different concentrations of contrast material at multi-detector row helical CT. Radiology 224:757–763
Suzuki H, Oshima H, Shiraki N, Ikeya C, Shibamoto Y (2004) Comparison of two contrast materials with different iodine concentrations in enhancing the density of the aorta, portal vein and liver at multi-detector row CT: a randomized study. Eur Radiol 14:2099–2104
Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic peak enhancement at CT: effect of contrast medium injection rate-pharmacokinetic analysis and experimental porcine model. Radiology 206:455–464
Awai K, Inoue M, Yagyu Y, Watanabe M, Sano T, Nin S, Koike R, Nishimura Y, Yamashita Y (2004) Moderate versus high concentration of contrast material for aortic and hepatic enhancement and tumor-to-liver contrast at multi-detector row CT. Radiology 233:682–688
Roos JE, Desbiolles LM, Weishaupt D, Wildermuth S, Hilfiker PR, Marincek B, Boehm T (2004) Multi-detector row CT: effect of iodine dose reduction on hepatic and vascular enhancement. Rofo 176:556–563
Ho LM, Nelson RC, Thomas J, Gimenez EI, DeLong DM (2004) Abdominal aortic aneurysms at multi-detector row helical CT: optimization with interactive determination of scanning delay and contrast medium dose. Radiology 232:854–859
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented as a scientific paper at ECR 2009.
This clinical trial was supported by Guerbet, Roissy, France.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Loewe, C., Becker, C.R., Berletti, R. et al. 64-Slice CT angiography of the abdominal aorta and abdominal arteries: comparison of the diagnostic efficacy of iobitridol 350 mgI/ml versus iomeprol 400 mgI/ml in a prospective, randomised, double-blind multi-centre trial. Eur Radiol 20, 572–583 (2010). https://doi.org/10.1007/s00330-009-1600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1600-6