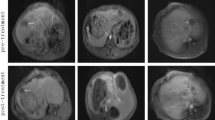
Abstract
High intensity focused ultrasound (HIFU) has been introduced to treat cancers. However, this therapy is a time-consuming procedure; destructing a deeper volume is also difficult as ultrasonic energy attenuates exponentially with increasing depth in tissues. The aim of the present study was to investigate the effects of introducing microbubbles on liver HIFU ablation. Seventeen goats were divided into groups A (n=8) and B (n=9). The livers in both groups were ablated using HIFU (1.0 MHz, 22,593 W/cm2) performed in the manner of a clinical regime using a clinical device. A microbubble agent was bolus-injected intravenously before HIFU exposure in group B. All animals in group A and seven goats in group B were euthanased to evaluate the ablation efficiency 24 h after HIFU. The necrosis rate (mm3/s), which was the volume of necrosis tissue per second of HIFU exposure, was used to judge the ablation efficiency. Pathological examinations were performed to determine whether there were residual intact tissues within the exposed volume. The other two goats in group B were used to determine the delayed pathological changes 7 days after ultrasonic ablation. The necrosis rate (mm3/s) was increased in group B (14.4647±4.1960 versus 33.5302±12.4484, P=0.0059). Pathological examinations confirmed that there were no residual unaffected tissue focuses within the exposed volume. Two remarkable changes occurred in the other two goats in group B 7 days after HIFU: there were ghost-cell islands at the periphery of the ablated tissues, and surrounding adjacent tissues outside the reactive zone necrotized. These findings showed that microbubbles could be used to assist liver HIFU ablation.





Similar content being viewed by others
References
Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, Cranston D (2004) High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 42:931–935
Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, Li KQ, Jin CB, Xie FL, Su HB (2004) Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol 11:1061–1069
Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB (2005) Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 235:659–667
Kennedy JE, ter Haar GR, Cranston D (2003) High intensity focused ultrasound: surgery of the future? Br J Radiol 76:590–599
Halpern EJ (2005) High-intensity focused ultrasound ablation: will image-guided therapy replace conventional surgery? Radiology 235:345–346
Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5:321–327
ter Haar G (2001) Acoustic surgery. Phys Today 54(12):29–34
Blomley MJK, Cooke JC, Unger EC, Monaghan MJ (2001) Microbubble contrast agents: a new era in ultrasound. BMJ 322:1222–1225
Stride E, Saffari A (2003) Microbubble ultrasound agents: a review. Proc Inst Mech Eng [H] 217:429–447
Lindner JR (2004) Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 3:527–532
Feinstein SB (2004) The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic deliver system, and beyond. Am J Physiol Heart Circ Physiol 287:H450–H457
Dijkmansa PA, Juffermansa LJM, Mustersb RJP, van Wamelc A, ten Catec FJ, van Gilstd W, Vissera CA, de Jong N, Kamp O (2004) Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiography 5:245–256
Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z (2004) A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study. Urol Res 32:14–19
Yu T, Xiong S, Mason TJ, Wang Z (2006) The use of a microbubbble agent to enhance rabbit liver destruction using high intensity focused ultrasound. Ultrason Sonochem 13:143–149
Takegami K, Kaneko Y, Watanabe T, Maruyama T, Matsumoto Y, Nagawa H (2005) Erythrocytes, as well as microbubble contrast agents, are important factors in improving thermal and therapeutic effects of high-intensity focused ultrasound. Ultrasound Med Biol 31:385–390
Kaneko Y, Maruyama T, Takegami K, Watanabe T, Mitsui H, Hanajiri K, Nagawa H, Matsumota Y (2005) Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol 15:1415–1420
Wang W, Zhou J, Liu W, Bai L, Ye H, Gai L, Tan Y, Yang T (2002) Treatment of unresectable nodular hepatocellular carcinoma with high-intensity focused ultrasound combined transarterial oily chemoembolization: preliminary clinical outcomes. In: Andrew MA, Crum LA, Vaezy A (eds) Proceedings of 2nd International Symposium on Therapeutic Ultrasound, University of Washington, pp 44–50
Chen L, ter Haar G, Hill CR (1997) Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol 23:921–931
ter Haar G (1995) Ultrasound focal beam surgery. Ultrasound Med Biol 21:1089–1100
Fry FJ (1991) Intense focused ultrasound in medicine. Eur Urol 23 (Suppl 1):2–7
Chapelon JY, Margonari J, Theillere Y, Gorry F, Vernier F, Blanc E, Gelet A (1992) Effects of high-energy focused ultrasound on kidney tissue in the rat and the dog. Eur Urol 22:147–152
Chen WS, Lafon C, Matula TJ, Vaezy S, Crum LA (2003) Mechanisms of lesion formation in high intensity focused ultrasound therapy. Acoust Res Lett Online 4:41–46
Holt RG, Roy RA (2001) Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 27:1399–1412
Umemura S, Kawabata K, Sanghvi N, Sasaki K (2002) Enhancement of ultrasonic adsorption by microbubble agent for HIFU. In: Andrew MA, Crum LA, Vaezy A (eds). Proceedings of 2nd International Symposium on Therapeutic Ultrasound, University of Washington, pp 527–538
Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R (2002) Therapeutic applications of microbubbles. Eur J Radiol 42:160–168
Fry FJ, Sanghvi NT, Foster RS, Bihrle R, Hennige C (1995) Ultrasound and microbubbles: their generation, detection and potential utilization in tissue and organ therapy-experimental. Ultrasound Med Biol 21:1227–1237
Bailey MR, Couret LN, Sapozhnikov OA, Khokhlova VA, ter Haar G, Vaezy S, Shi X, Martin R, Crum LA (2001) Use of overpressue to asses the role of bubbles in focused ultrasound lesion shape in vitro. Ultrasound Med Biol 27:695–708
Sokka SD, King R, Hynynen K (2003) MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 48:223–241
Van Leenders GJLH, Beerlage HP, Ruijter ETh, de la Rosette JJMCH, van de Kaa CA (2000) Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol 53:391–394
Chen L, ter Haar G, Robertson D, Bensted JP, Hill CR (1999) Histological study of normal and tumor-bearing liver treated with focused ultrasound. Ultrasound Med Biol 25:847–856
Chen L, ter Haar G, Hill CR, Dworkin M, Carnochan P, Young H, Bensted JP (1993) Effects of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys Med Biol 38:1661–1673
Huber PE, Debus J (2001) Tumor cytotoxicity in vivo and radical formation in vitro depend on the shock wave-induced cavitation dose. Radiat Res 156:301–309
Hohmann J, Albrecht T, Oldenburg A, Skrok J, Wolf KJ (2004) Liver metastases in cancer: detection with contrast-enhanced ultrasonography. Abdom Imaging 29:669–681
Nicolau C, Brú C (2004) Focal liver lesions: evaluation with contrast-enhanced ultrasonography. Abdom Imaging 29:348–359
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, T., Fan, X., Xiong, S. et al. Microbubbles assist goat liver ablation by high intensity focused ultrasound. Eur Radiol 16, 1557–1563 (2006). https://doi.org/10.1007/s00330-006-0176-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0176-7