Abstract
The summer climate in Maritime Antarctica is characterised by high humidity and cloudiness with slightly above zero temperatures. Under such conditions, photosynthetic activity is temperature-limited and plant communities are formed by a few species. These conditions could prevent the operation of the photoprotective xanthophyll (VAZ) cycle as low irradiance reduces the excess of energy and low temperatures limit enzyme activity. The VAZ cycle regulates the dissipation of the excess of absorbed light as heat, which is the main mechanism of photoprotection in plants. To test whether this mechanism operates dynamically in Antarctic plant communities, we characterised pigment dynamics under natural field conditions in two representative species: the moss Polytrichum juniperinum and the grass Deschampsia antarctica. Pigment analyses revealed that the total VAZ pool was in the upper range of the values reported for most plant species, suggesting that they are exposed to a high degree of environmental stress. Despite cloudiness, there was a strong conversion of violaxanthin (V) to zeaxanthin (Z) during daytime. Conversely, the dark-induced enzymatic epoxidation back to V was not limited by nocturnal temperatures. In contrast with plants from other cold ecosystems, we did not find any evidence of overnight retention of Z or sustained reductions in photochemical efficiency. These results are of interest for modelling, remote sensing and upscaling of the responses of Antarctic vegetation to environmental challenges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosynthesis is the main energy input in the vast majority of Earth’s ecosystems, and it is also the key to understanding the long-term changes in atmospheric CO2 concentrations that ultimately result in climate alterations. Although this process is reasonably well understood at the biochemical level, there are many uncertainties when the scale of observation is moved upwards. For example, it is well known that photosynthesis is dynamically regulated by the amount of energy that reaches the pigment–protein complexes; thus, that when light is in excess, the efficiency of energy conversion is downregulated, decreasing photosynthetic efficiency (Kromdijk et al. 2016). Conversely, under limited light, photochemical efficiency increases. This process is matched, and partially regulated, by the interconversions of three xanthophylls, violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z) within the light-harvesting antenna (Pinnola and Bassi 2018). Thus, under nonstress conditions, when light absorption activates proton pumping in chloroplasts, lumen acidification induces the activity of the enzyme violaxanthin de-epoxidase (VDE), which reduces diepoxide V to Z, via A (Pfündel and Dilley 1993). Under low light or darkness, the reverse reaction takes place and Z is converted into V by the action of the Z-epoxidase (ZE), which is active at neutral pH (Färber and Jahns 1998). These interconversions among the three xanthophylls constitute what is known as the xanthophyll, violaxanthin or VAZ cycle (Demmig-Adams and Adams 1996). Overall, when light is in excess, Z accumulates, downregulating the photochemical efficiency and the higher the energy excess is, the higher the Z content. The fact that this cycle correlates with light use efficiency and that it involves three pigments that consequently absorb visible light, has led to the development of methods for its remote detection by spectrometric measurements (Peñuelas et al. 1995). As a result, several optical indices that estimate photosynthetic efficiency have been developed, as is the case of the photochemical reflectance index (PRI) (Gamon et al. 1990). However, when we shift to larger scales of observation, a number of uncertainties appear, and the relationship between optical indices and photosynthetic efficiency is not straightforward (Garbulsky et al. 2011). Among other gaps in knowledge, the relationship between the operation of the VAZ cycle and the photochemical efficiency has not been characterised in all ecosystems and plant functional groups. In addition, it has been shown that particular conditions, such as desiccation, can lead to the accumulation of Z in darkness (Fernández-Marín et al. 2021). Additionally, low temperatures block ZE activity, impeding the reconversion of Z into V during the night (Verhoeven 2014). Additionally, the freezing of tissues activates the de-epoxidation of the xanthophyll cycle in evergreen plants (Fernández-Marín et al. 2018, 2021).
The dynamics of the VAZ cycle under natural conditions have been well characterised for a large series of plants mostly belonging to temperate (Niinemets et al. 2003), alpine (Streb et al. 2003) and tropical biomes (Matsubara et al. 2009), as well as for major crops (Falbel et al. 1994) and cultivated trees (Matsubara et al. 2011). However, the information available for high latitude and polar environments is scarce (Bascuñán-Godoy et al. 2010; Fernández-Marín et al. 2019), in part due to the inherent technical limitations of these “hard to reach” places. These limitations include the collection, storage and transport of samples. In fact a majority of the few studies that have focussed on the VAZ cycle in Antarctic plants have been performed ex situ with specimens collected in the field (Lovelock et al. 1995; Lovelock and Robinson 2002; Pérez-Torres et al. 2004; Bravo et al. 2007; Molina-Montenegro et al. 2016; Sáez et al. 2019), and only a few preliminary studies were carried out directly on field specimens (Post 1990). However, the particular conditions of these ecosystems, such as low average temperatures plus long photoperiods (i.e. > 20 h day or night) throughout the year, together with the global importance of polar areas, demands such field studies. In the Arctic tundra, for example, the effect of constant daylight on the VAZ cycle was studied in a few locations (Magney et al. 2017; Fernández-Marín et al. 2018, 2019). This phenomenon only occurs at very high latitudes and deserves further study to characterise its influence on photosynthetic dynamics. In contrast with the Arctic, higher plant communities in Antarctica are much less developed at latitudes where the sun never sets because of the much harsher environmental conditions. In fact, the only two angiosperm species occurring in Antarctica are almost exclusively found on the shores of the Antarctic Peninsula and the South Shetland Islands (Robinson et al. 2003; Peat et al. 2007), the so-called Maritime Antarctic region. The remaining Antarctic vegetation is basically cryptogamic, comprising approximately 130 bryophyte and 475 lichen species on the whole continent and islands (Øvstedal and Lewis Smith 2001; Robinson et al. 2003; Peat et al. 2007; Ochyra et al. 2008).
During the vegetative period, the climate in this region is typically moist and windy, with mean temperatures slightly higher than zero and frequent cloudiness (Robinson et al. 2003). Under such conditions, and considering the rare occurrence of clear skies and the low temperature-induced enzyme activity limitation, it is uncertain whether the VAZ cycle operates in the same way as it does in warmer ecosystems. To fill this gap, in the present study, we have characterised pigment dynamics under natural field conditions in two representative Antarctic plant species: the moss Polytrichum juniperinum (Hedwig) and the grass Deschampsia antarctica (É.Desv). Furthermore, given the scarcity of field studies under natural Antarctic conditions, this study provides a substantial contribution to our understanding of the ecophysiology of Antarctic plants.
Methods
Field site, plant material and experimental design
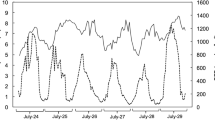
Measurements were performed in the surroundings of the Spanish Antarctic Research Station “Base Antártica Española Juan Carlos I” (BAE JCI) in Livingston Island, South Shetland (62°40′ S, 60°23′ W) during the first days of March 2018 (end of the Austral summer) (Fig. 1a, b). During the study period and the preceding week, the air temperature ranged between − 0.7 °C and + 5.6 °C with almost constant cloud coverage and an average daily rainfall of 1.57 mm day−1 (Fig. 1c). The cumulative daily irradiance was on average 8.44 mol photons day−1; however, it was 73% higher during the study period than during the previous week.
Weather conditions and geographical location of the study site. Upper panels include a map showing the location of the South Shetland Islands (a) and the study site adjacent to the Spanish Antarctic Station (BAE Juan Carlos I) in Livingston Island (b). Bottom panel show air temperature (red line), daily precipitation (grey bars) and photon flux density (blue line) during the study period and the previous week (c). Vertical dashed lines show sampling time points. Numbers in blue at the bottom of the figure represent values of daily cumulative irradiance (mol m−2 day−1)
Two plant species representative of the Antarctic tundra were studied: juniper haircap moss (Polytrichum juniperinum) and Antarctic hair grass (Deschampsia antarctica). The first is a cosmopolitan, widely distributed moss, while the second is a grass occurring along the Antarctic Islands and Peninsula and the southern tip of South America. Four cushions of each species were monitored every six hours during two consecutive day–night cycles. Thus, two consecutive midnights and three middays were studied. At each time point and cushion, chlorophyll fluorescence was measured in two adjacent leaves, one was dark-adapted with a Leaf-Clip and used for determination of the maximal efficiency of PSII (Fv/Fm), while the other was used for the determination of the actual efficiency of PSII (ΦPSII) (see description of the fluorescence protocols in the following paragraph). The latter was collected immediately after fluorescence measurements and frozen in liquid nitrogen, for additional photosynthetic pigment analyses. Samples were thereafter stored at − 80 °C in the BAE JCI and later transported to Spain at the same temperature in the research ship BIO Hesperides. Once the ship arrived in Spain the cold chain (− 80 °C) was always maintained until the samples were finally analysed at the University of the Basque Country.
Chlorophyll fluorescence
Chlorophyll a (Chl a) fluorescence was measured in the field using a portable modulated Plant Stress Kit fluorometer (Opti-Sciences, Hudson NH, USA). In dark-adapted samples (30 min) the maximum Chl a fluorescence (Fm) was induced with a saturating pulse (0.8 s duration, 7000 μmol m−2 s−1 PFD) while the initial fluorescence (Fo) was recorded with red modulated measuring light (ML < 1 μmol photons m−2 s−1). The Fv/Fm was then estimated by the ratio Fv/Fm = (Fm–Fo)/Fm. The actual efficiency of PSII (ΦPSII) was measured by using a Y(II) metre from the same manufacturer. In illuminated leaves (i.e. those directly exposed to natural solar radiation) the operating quantum efficiency of PSII was estimated as ΦPSII = (Fmʹ–Fs)/Fmʹ, where Fmʹ is the maximum Chl a fluorescence induced with a saturating pulse and Fs is the actual Chl a fluorescence under illumination. The photon flux density reaching the samples during each ΦPSII measurement (PFDΦPSII) was recorded with the Leaf-Clip built-in irradiance sensor.
Pigment analyses
Approximately 25–60 mg FW per replicate was used for photosynthetic pigment and tocopherol analyses. Plant material was ground under liquid N2. The metabolites were then extracted with an acetone solution buffered with 0.5 g CaCO3 L−1 and the extracts were centrifuged at 16,100g and 4 °C. The supernatant was filtered through a 0.2 µm pore size filter (Teknokroma, Barcelona, Spain). Pigment and tocopherol composition were finally analysed by HPLC using a reversed-phase C18 column (Waters, Milford, Massachusetts, USA) as described previously (García-Plazaola and Becerril 1999). We used a photodiode array detector (Waters Model 996) for pigments and a scanning fluorescence detector (Waters Model 474) for tocopherols.
From the analyses, several ratios were derived and used to characterise the structure and function of the photosynthetic apparatus:
-
Chlorophyll a/b (Chl a/b) was calculated as the molar ratio between chlorophyll a and b. Although Chl b is located in the light-harvesting complexes (LHCs) Chl a can be present both in the antennae and core complexes (CCs) and is a good indicator of the antenna size (Esteban et al. 2015a). This ratio can also be affected by the stoichiometry of PSI to PSII, as most of the Chl b pool is bound to LHCII. However, this relationship is not straightforward, as state transitions can derive energy from LHCII to PSI.
-
β-Carotene/neoxanthin (β-Car/Neo) was calculated as the molar ratio between neoxanthin (Neo) and β-carotene (β-Car). Since β-Car is mostly present in CCs in thylakoids (Cazzaniga et al. 2012) while Neo is only bound to antenna proteins (Formaggio et al. 2001), this parameter can be considered a plausible indicator of the CC/LHC ratio. However, this index should be considered with care since β-Car can also be found in the chloroplast envelope, thereby affecting this ratio.
-
Xanthophyll cycle pool (VAZ) per chlorophyll (VAZ/Chl): The total VAZ pool was calculated as the sum of violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z) and expressed on a chlorophyll (a + b) basis. As a consequence, the VAZ pool is usually proportional to the light environment where the plant or tissue develops, and is highly responsive to light fluctuations (Esteban et al. 2015a).
-
Total tocopherol pool (Toc) per chlorophyll (Toc/Chl): total Toc was calculated as the sum of α-Toc, γ-Toc and δ-Toc and expressed on a chlorophyll (a + b) basis. As the main plastidial lipophilic antioxidant, its content usually scales positively with irradiance (García-Plazaola et al. 2004).
-
De-epoxidation index (AZ/VAZ): The de-epoxidation index (AZ/VAZ) was calculated as the sum of A and Z divided by the sum of V, A and Z. It represents the state of operation of the VAZ cycle, which is proportional to light excess and/or to the extent of other stress factors (Demmig-Adams and Adams 1996; Fernández-Marín et al. 2021).
Statistical treatments
A t test was used to compare pigment ratios to chlorophyll between both species.
Linear regression was applied to analyse the relationships between the main parameters analysed in this study and the relationships between AZ/VAZ and irradiance. Calculated P values and regression lines are indicated on the figures whenever significant at P < 0.05. Statistical analyses were performed with IBM SPSS v24.0.
Results
The pigment content per unit of dry mass was threefold higher in D. antarctica than in P. juniperinum, but its composition was qualitatively similar in both species (Fig. 2). Thus, lutein (Lut) was the dominant carotenoid, followed by the total VAZ pool, β-carotene (β-Car) and neoxanthin (Neo). The carotenoid and tocopherol ratios to chlorophyll differed slightly, but significantly, between both species, with the exception of VAZ/Chl (data not shown, derived from Fig. 2). Thus, Neo/Chl, Lut/Chl and total tocopherol/Chl were 13%, 21% and 43% higher in P. juniperinum, respectively. In contrast β-Car/Chl was 4% higher in D. antarctica. Chla/b also differed between species, being 3.32 ± 0.06 in D. antarctica and 3.05 ± 0.05 in P. juniperinum.
Pigment composition in the leaves of Deschampsia antarctica and Polytrichum juniperinum (in µmol g−1 DW). Size of the pie charts is proportional to the total pigment content of each species (2.95 and 1.11 µmol g−1DW, respectively). Asterisks denote significant differences between both species in the ratios of each pigment to Chl a + b at P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***)
During the study period, differences between midday and midnight values were not significant (at P < 0.05) for any of the pigment to chlorophyll ratios studied, but they were for α-Toc/Chl in D. antarctica (Fig. 3). Despite this, significant longer-term trends (48 h) were found between the first midday and the last midday for several of the analysed parameters, in parallel to the overall decrease in temperature and irradiance observed during the sampling period (Fig. 1c). Thus Chl a/b decreased in both species while Neo/Chl showed the opposite pattern. In addition, α-Toc/Chl and β-Car/Neo decreased in P. juniperinum. These parallel responses resulted in the existence of significant correlations between pigment contents. Thus Chl a/b correlated positively with β-Car/Neo, VAZ/Chl and α-Toc/Chl in D. antarctica (Fig. 4). In the case of P. juniperinum the correlation was significant only for Chl a/b vs α-Toc/Chl (Fig. 4c).
Daily changes in pigment composition. Time-dependent changes during the study period in pigment ratios indicative of photosynthetic activity and regulation for Polytrichum juniperinum (triangles) and Deschampsia antarctica (circles): Chl a/b (a), β-Car/Neo (b), Toc/Chl (c), VAZ/Chl (d) and Neo/Chl (e). Chla/b and β-Car/Neo are expressed in mol mol−1 and Toc/Chl, VAZ/Chl and Neo/Chl are expressed in mmol mol−1. Darkened areas denote night periods. Values represent the mean of four replicates ± SE
Correlations between pigment ratios. Regression lines between Chl a/b vs β-Car/Neo (a), Chl a/b vs VAZ/Chl (b) and Chl a/b vs Toc/Chl (c) in Polytrichum juniperinum (triangles, dashed lines) and Deschampsia antarctica (circles, solid lines). Chla/b and β-Car/Neo are expressed in mol mol−1 and Toc/Chl and VAZ/Chl are expressed in mmol mol−1. Linear regression lines, r2 and P values are shown when statistically significant at P < 0.05
In contrast to the stability of VAZ/Chl, the de-epoxidation ratio (AZ/VAZ) varied rhythmically along the daily cycles in both species. This parameter peaked at noon and reached a minimum at midnight that was maintained until dawn, and its values were higher in P. juniperinum at all timepoints (Fig. 5). Midday values of AZ/VAZ were on average twofold higher in P. juniperinum and its daily amplitude of oscillation was on average 70% higher in this species. In contrast with this parameter, Fv/Fm did not show any clear pattern of temporal variation in either of the two species. As a consequence of this distinct behaviour, AZ/VAZ and Fv/Fm were not related in either of the two species studied (Fig. 6). In contrast, AZ/VAZ and ΦPSII showed a strong negative correlation in both species (Fig. 6), while AZ/VAZ correlated with the actual PFD at the time of sampling only in P. juniperinum. The linear correlation coefficient was markedly stronger in both species when AZ/VAZ was plotted against the irradiance accumulated during the two hours prior to sampling instead of to the actual PFD (Fig. 6c, d).
Daily changes in pigment composition. Time-dependent changes during the study period in Fv/Fm (a) and AZ/VAZ (b) for Polytrichum juniperinum (triangles) and Deschampsia antarctica (circles). AZ/VAZ is expressed in mol mol−1. Darkened areas denote night periods. Values represent the mean of four replicates ± SE
Correlations between photochemical parameters and light environment. Regression lines between AZ/VAZ vs Fv/Fm (a), AZ/VAZ vs ΦPSII (b), AZ/VAZ vs actual photosynthetic photon flux density (PFD) (c) and AZ/VAZ vs cumulative irradiance (d) received during the previous 2 h in Polytrichum juniperinum (triangles, dashed lines) and Deschampsia antarctica (circles, solid lines). Linear regression lines, r2 and P values are shown when statistically significant at P < 0.05
Discussion
Plant life in Antarctica encounters the environmental limits of its survival capacity (Robinson et al. 2003). Maritime Antarctica, which comprises the Antarctic Peninsula and Islands, is comparatively milder than the continent, but even though the growing season is very short, summer temperatures rarely surpass the threshold of ten degrees. Apart from the scarce ice-free area available on the continent and the obvious temperature limitation, the few plant species inhabiting Antarctica have to face an additional stress factor caused by the high cloudiness (80%) (Bañón et al. 2013), which potentially compromises the photosynthetic energy budget. In fact, during the study period, the values of cumulative daily irradiance were similar to those reported in the understorey of temperate forests (Niinemets 2007; Valladares et al. 2008). In addition, sun irradiance not only provides the energy for photosynthesis but also contributes to the temperature difference between air and leaves in Antarctic mosses. Even in Antarctica, moss leaves can easily reach + 20 °C during the infrequent sunny periods (Perera-Castro et al. 2020a). Consequently, low irradiance also implies a thermal limitation. During our study, lower irradiance and air temperatures (including sub-zero values) were registered at the end of the sampling time. This could be the reason behind the decreasing trend in the Chl a/b and Fv/Fm ratios obtained throughout the study.
The two species studied here can be considered good examples of contrasting strategies of Antarctic plants. Thus, while P. juniperinum is a poikilohydric moss that activates or deactivates metabolism in response to water availability, D. antarctica is a homeohydric grass. Their photosynthetic responses in Antarctic conditions have been characterised by a number of studies (Sáez et al. 2019; Clemente-Moreno et al. 2020; Perera-Castro et al. 2020a, b) that show that the optimum temperature for photosynthesis occurs at a range substantially higher than those experienced in their natural environment. This implies that even during frequent cloudy days and due to the low temperatures, leaves suffer an imbalance between the amount of light energy absorbed by chloroplasts and that actually used for photosynthesis. The imbalance could be potentially higher for P. juniperinum considering its relatively lower maximal net CO2 assimilation capacity if compared to D. antarctica: roughly 5 vs. 20 μmol m−2 s−1 (Clemente-Moreno et al. 2020; Perera-Castro et al. 2020b). This excess of absorbed light energy exacerbated by close to zero temperatures triggers the activation of photoprotective mechanisms. Among these mechanisms, several authors have highlighted the prominent role of antioxidant defences in Antarctic plants (Pérez-Torres et al. 2004; Clemente-Moreno et al. 2019, 2020). The activation of photoprotective thermal energy dissipation has also been observed in Antarctic species, but direct evidence linking this process to the operation of the xanthophyll cycle is missing (Perera-Castro et al. 2020b).
VAZ cycle activity seems to be higher in Arctic shrubs than in their lower latitude relatives (Magney et al. 2017), but the complete reversion of the cycle seems to be negatively affected by midnight sun (Fernández-Marín et al. 2019). The present study was performed close to the Equinox, meaning that the photoperiod was close to 12 h. Under these conditions, a strong xanthophyll interconversion was observed, particularly for P. juniperinum. Maximum values of AZ/VAZ in both species were observed at noon (0.335 with an amplitude of 0.224 and 0.669 with an amplitude of 0.393 in D. antarctica and P. juniperinum, respectively), and these values were remarkably similar to those reported in the literature for other Antarctic species: 0.28 (amplitude 0.10) in angiosperms (C. quitensis) (Bascuñán-Godoy et al. 2010) and 0.665 (amplitude 0.283) in mosses (Lovelock 1995). Unexpectedly, this ratio only correlated with the actual PFD in P. juniperinum (Fig. 6c), suggesting that the operation of the VAZ cycle responds faster to environmental conditions in the moss. However, in both the species, the correlation between AZ/VAZ and the cumulative dose of irradiance received during the previous two hours was much stronger than that with the actual irradiance at the time of sampling. This finding supports that the epoxidation state of the xanthophyll cycle represents a kind of “light memory” (Esteban et al. 2015b) and is not determined by the actual conditions but by the illumination history of the plant. Thus, being proportional to the irradiance received during the last hour, the epoxidation state itself represents a mechanism of information storage that modulates photosynthetic responses, fulfilling the classical definition proposed by Trewavas (2003) for “plant memory”. The time window of such a memory mechanism remains to be elucidated. In fact the reversible accumulation of A and Z did not have any photoinhibitory effect (decrease in Fv/Fm), but it controlled the actual photochemical efficiency (ΦPSII) of energy conversion (Fig. 6b).
The VAZ pool size, which integrates the level of light stress accumulated by the leaf during the previous days (García-Plazaola et al. 2012), was also consistent with the previous data on Antarctic plants. Thus, the mean values of VAZ/Chl were 126 mmol mol−1 and 119 mmol mol−1 in D. antarctica and P. juniperinum, respectively, while the VAZ/Chl content has been reported to be in the range of 85–144 mmol mol−1 in Antarctic bryophytes (Lovelock et al. 1995; Lovelock and Robinson 2002; Robinson et al. 2003; Schroeter et al. 2012) and 117 mmol mol−1 in C. quitensis (Bascuñán-Godoy et al. 2010). All these values are markedly higher than the average VAZ/Chl for unstressed plants (74 mmol mol−1) (Esteban et al. 2015a), suggesting that Antarctic plants are constitutively under stress.
Apart from daily changes in pigment composition, plants perform long-term adjustments in the structure of the photosynthetic apparatus. The present case study represented the transition from a period of low irradiance (average 5.9 mol photons m−2 day−1) to another period of moderate irradiance (average 13.4 mol photons m−2 day−1) (Fig. 1). A decrease in some parameters mimicked that change, such as Chl a/b in both species (Fig. 2a) and α-Toc/Chl and β-Car/Neo in P. juniperinum (Fig. 2b, c), but the direction was the opposite of what was expected, since these parameters usually correlate negatively with antenna size and/or photoprotective demand (Esteban et al. 2015a), but a wider time window is needed to understand the meaning of such changes. A process of desiccation-induced accumulation of tocopherols (Oliver et al. 2011) could contribute to such unexpected results, at least in P. juniperinum. The higher responsiveness of P. juniperinum supports the result that the physiological responses of moss are comparatively more dynamic than those of grass, probably because bryophytes are generally closer to their optimal range of performance under the environmental conditions of Maritime Antarctica.
Overall, the present study shows that even during the frequent cloudy days that characterise Maritime Antarctica, the VAZ cycle is operative and fully reversible in these two representative examples of the regional flora. Furthermore, we did not find any evidence of sustained Z accumulation or of sustained energy dissipation linked to the accumulation of Z, as is typically observed in the vegetation of other cold ecosystems. Given that the PRI is one of the most promising indices for the remote sensing of the photosynthetic performance (D’Odorico et al. 2021) and that this index captures changes in the state of operation of the VAZ cycle, the present results could be useful for the correct interpretation of remote sensing data in the Antarctic region.
Data availability
Available from corresponding author on reasonable request.
References
Bañón M, Justel A, Velázquez D, Quesada A (2013) Regional weather survey on Byers Peninsula, Livingston Island, South Shetland Islands, Antarctica. Antarct Sci 25:146–156
Bascuñán-Godoy L, García-Plazaola JI, Bravo LA, Corcuera LJ (2010) Leaf functional and micro-morphological photoprotective attributes in two ecotypes of Colobanthus quitensis from the Andes and Maritime Antarctic. Polar Biol 33:885–896
Bravo LA, Saavedra-Mella FA, Vera F, Guerra A, Cavieres LA, Ivanov AG, Huner NPA, Corcuera LJ (2007) Effect of cold acclimation on the photosynthetic performance of two ecotypes of Colobanthus quitensis (Kunth) Bartl. J Exp Bot 58:3581–3590
Cazzaniga S, Li Z, Niyogi KK, Bassi R, Dall’Osto L (2012) The Arabidopsis szl1 mutant reveals a critical role of β-carotene in photosystem I photoprotection. Plant Physiol 159:1745–1758
Clemente-Moreno MJ, Omranian N, Sáez PL, Figueroa CM, Del-Saz N, Elso M, Poblete L, Orf I, Cuadros-Inostroza A, Cavieres L, Bravo L, Fernie AR, Ribas-Carbó M, Flexas J, Nikoloski Z, Brotman Y, Gago J (2019) High cytochromic respiratory rates and sulphur metabolism sustain stress tolerance to low temperature in the Antarctic species Colobanthus quitensis. New Phytol 225:754–768
Clemente-Moreno MJ, Omranian N, Sáez PL, Figueroa CM, Del-Saz N, Elso M, Poblete L, Orf I, Cuadros-Inostroza A, Cavieres LA, Bravo L, Fernie A, Ribas-Carbó M, Flexas J, Nikoloski Z, Brotman Y, Gago J (2020) Low-temperature tolerance of the Antarctic species Deschampsia antarctica: a complex metabolic response associated with nutrient remobilization. Plant Cell Environ 6:1376–1393
D’Odorico P, Schönbeck L, Vitali V, Meusburger K, Schaub M, Ginzler C, Zweifel R, Velasco VME, Gisler J, Gessler A, Ensminger I (2021) Drone-based physiological index reveals long-term acclimation and drought stress responses in tress. Plant Cell Environ 44:3552–3570
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Esteban R, Barrutia O, Artetxe U, Fernández-Marín B, Hernández A, García-Plazaola JI (2015a) Internal and external factors affecting photosynthetic pigment composition in plants: a meta-analytical approach. New Phytol 206:266–280
Esteban R, Moran JF, Becerril JM, García-Plazaola JI (2015b) Versatility of carotenoids: an integrated view on diversity, evolution, functional roles and environmental interactions. Env Exp Bot 119:63–75
Falbel TG, Staehelin LA, Adams WW (1994) Analysis of xanthophyll cycle carotenoids and chlorophyll fluorescence in light intensity-dependent chlorophyll-deficient mutants of wheat and barley. Photosynth Res 42:191–202
Färber A, Jahns P (1998) The xanthophyll cycle of higher plants: influence of antenna size and membrane organization. Biochim Biophys Acta - Bioenerg 1363:47–58
Fernández-Marín B, Atherton J, Olascoaga B, Kolari P, Porcar-Castell A, García-Plazaola JI (2018) When the sun never sets: daily changes in pigment composition in three subarctic woody plants during the summer solstice. Trees 32:615–630
Fernández-Marín B, Gago J, Flexas J, Gulías J (2019) Plant pigment cycles in the high-Arctic Spitsbergen. Polar Biol 42:675–684
Fernández-Marín B, Roach T, Verhoeven AS, García-Plazaola JI (2021) Shedding light on the dark side of xanthophyll cycles. New Phytol 230:1336–1344
Formaggio E, Cinque G, Bassi R (2001) Functional architecture of the major light-harvesting complex from higher plants. J Molec Biol 314:1157–1166
Gamon JA, Field CB, Bilger W, Björkman O, Fredeen AL, Peñuelas J (1990) Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 85:1–7
Garbulsky MF, Peñuelas J, Gamon J, Inoue Y, Filella I (2011) The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: a review and meta-analysis. Remote Sens Environ 115:281–297
García-Plazaola JI, Becerril JM (1999) A rapid HPLC method to measure lipophilic antioxidants in stressed plants: simultaneous determination of carotenoids and tocopherols. Phytochem Anal 10:307–313
García-Plazaola JI, Becerril JM, Hernández A, Niinemets U, Kollist H (2004) Acclimation of antioxidant pools to the light environment in a natural forest canopy. New Phytol 163:87–97
García-Plazaola JI, Esteban R, Fernández-Marín B, Kranner I, Porcar-Castell A (2012) Thermal energy dissipation and xanthophyll cycles beyond the Arabidopsis model. Photosynth Res 113:89–103
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354:857–861
Lovelock CE, Robinson SA (2002) Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ 25:1239–1250
Lovelock CE, Jackson AE, Melick DR, Seppelt RD (1995) Reversible photoinhibition in antarctic moss during freezing and thawing. Plant Physiol 109:955–961
Magney T, Logan BA, Reblin JS, Boelman NT, Eitel JUH, Greaves HE, Griffin KL, Prager CM, Vierling LA (2017) Xanthophyll cycle activity in two prominent arctic shrub species. Arct Antarct Alp Res 49:277–289
Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K (2009) Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol 36:20–36
Matsubara S, Chen YC, Caliandro R, Govindjee CRM (2011) Photosystem II fluorescence lifetime imaging in avocado leaves: contributions of the lutein epoxide and violaxanthin cycles to fluorescence quenching. J Photochem Photobiol B 104:271–284
Molina-Montenegro MA, Galleguillos C, Oses R, Acuña-Rodríguez IS, Lavín P, Gallardo-Cerda J, Torres-Díaz C, Diez B, Pizarro GE, Atala C (2016) Adaptive phenotypic plasticity and competitive ability deployed under a climate change scenario may promote the invasion of Poa annua in Antarctica. Biol Invasions 18:603–618
Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Niinemets Ü, Kollist H, García-Plazaola JI, Hernández A, Becerril JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate tress? Plant Cell Environ 26:1787–1801
Ochyra R, Lewis Smith RI, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Oliver MJ, Guo L, Alexander DC, Ryals JA, Wone BWM, Cushman JC (2011) A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 23:1231–1248
Øvstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia. Cambridge University Press, Cambridge
Peat HJ, Clarke A, Convey P (2007) Diversity and biogeography of the Antarctic flora. J Biogeogr 34:132–146
Peñuelas J, Filella I, Gamon JA (1995) Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol 131:291–296
Perera-Castro AV, Waterman MJ, Turnbull JD, Ashcroft MB, McKinley E, Watling JR, Bramley-Alves J, Casanova-Katny A, Zuniga G, Flexas J, Robinson SA (2020a) It is hot in the sun: Antarctic mosses have high temperature optima for photosynthesis despite cold climate. Front Plant Sci 11:1178. https://doi.org/10.3389/fpls.2020.01178
Perera-Castro AV, Flexas J, González-Rodríguez ÁM, Fernández-Marín B (2020b) Photosynthesis on the edge: photoinhibition, desiccation and freezing tolerance of Antarctic bryophytes. Photosynth Res 149:135–153
Pérez-Torres E, García A, Dinamarca J, Alberdi M, Gutiérrez A, Gidekel M, Ivanov AG, Hüner NPA, Corcuera LJ, Bravo LA (2004) The role of photochemical quenching and antioxidants in photoprotection of Deschampsia antarctica. Funct Plant Biol 31:731–741
Pfündel EE, Dilley RA (1993) The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol 101:65–71
Pinnola A, Bassi R (2018) Molecular mechanisms involved in plant photoprotection. Biochem Soc Trans 46:467–482
Post A (1990) Photoprotective pigment as an adaptive strategy in the Antarctic moss Ceratodon purpuerus. Polar Biol 10:241–245
Robinson S, Wasley J, Tobin AK (2003) Living on the edge—plants and global change in continental and maritime Antarctica. Glob Change Biol 9:1681–1717
Sáez P, Rivera BK, Ramírez CF, Vallejos V, Cavieres LA, Corcuera LJ, Bravo LA (2019) Effects of temperature and water availability on light energy utilization in photosynthetic processes of Deschampsia antárctica. Physiol Plant 165:511–523
Schroeter B, Green TGA, Kulle D, Pannewitz S, Schlensog M, Sancho LG (2012) The moss Bryum argenteum var. Muticum Brid. is well adapted to cope with high light in continental Antarctica. Antarct Sci 24:281–291
Streb P, Aubert S, Bligny R (2003) High temperature effects on light sensitivity in the two high mountain plant species Soldanella alpina (L.) and Rannunculus glacialis (L.). Plant Biol 5:432–440
Trewavas A (2003) Aspects of plant intelligence. Ann Bot 92:1–20
Valladares F, Zaragoza-Castells J, Sánchez-Gómez D, Matesanz S, Alonso B, Portsmuth A, Delgado A, Atkin OK (2008) Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Ann Bot 102:923–933
Verhoeven A (2014) Sustained energy dissipation in winter evergreens. New Phytol 201:57–65
Acknowledgements
We would like to thank José Vicente Alberto for kindly providing meteorological data from Livingston Island. We deeply thank the extraordinary support received at the Spanish Antarctic Research Station BAE JCI from all the staff of the “Unidad de Tecnología Marina-UTM” and to Joan Riba with particular emphasis. Javier Martinez-Abaigar (University of La Rioja), for helpful assistance on the taxonomic identification of Polytrichum species. We also thank prof. Alexander G. Ivanov and one anonymous reviewer for helpful comments on the manuscript. The Spanish Ministry of Science, Innovation and Universities (MICIU/FEDER, EU) and the Basque Government funded this research through the projects CTM2014-53902-C2-2-P, PGC2018-093824-B-C44 and UPV/EHU IT-1018-16.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Conceptualisation: J.I.G.-P and B.F.-M.; Methodology: J.I.G.-P, M.L.-P. and B.F.-M.; Investigation: J.I.G.-P, M.L.-P. and B.F.-M.; Writing: J.I.G.-P and B.F.-M. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Consent for publications
All authors agree.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Plazaola, J.I., López-Pozo, M. & Fernández-Marín, B. Xanthophyll cycles in the juniper haircap moss (Polytrichum juniperinum) and Antarctic hair grass (Deschampsia antarctica) on Livingston Island (South Shetland Islands, Maritime Antarctica). Polar Biol 45, 1247–1256 (2022). https://doi.org/10.1007/s00300-022-03068-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03068-7