Abstract
Over decades, persistent organic pollutants (POPs) and trace metals like mercury (Hg) have reached the remotest areas of the world such as Antarctica by atmospheric transport. Once deposited in polar areas, low temperatures, and limited solar radiation lead to long environmental residence times, allowing the toxic substances to accumulate in biota. We investigated the load of polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDTs) and metabolites (DDEs, DDDs) in embryos from failed eggs of the smallest seabird breeding in Antarctica, the Wilson's storm-petrel (Oceanites oceanicus) at King George Island (Isla 25 de Mayo). We compared samples of different developmental stages collected in 2001, 2003, and 2014 to 2016 to investigate changes in pollutant concentrations over time. We detected eight PCBs including the dioxin-like (dl) congeners PCB 105 and 118 (ΣPCBs: 59-3403 ng g−1 ww) as well as 4,4’-DDE, and 4,4’-DDD (ΣDDX: 19-1035 ng g−1 ww) in the embryos. Samples from the years 2001 and 2003 showed higher concentrations of PCBs than those from 2014 to 2016. Concentrations of DDX was similar in both time intervals. Furthermore, we determined Hg concentrations in egg membranes from 1998 to 2003, and 2014 to 2016. Similar to PCBs, Hg in egg membranes were higher in 1998 than in 2003, and higher in 2003 than in the years 2014 to 2016, suggesting a slow recovery of the pelagic Antarctic environment from the detected legacy pollutants. Embryos showed an increase in pollutant concentrations within the last third of their development. This finding indicates that contaminant concentrations may differ among developmental stages, and it should be taken into account in analyses on toxic impact during embryogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) and trace metals like mercury (Hg) are known to affect the environment and living organisms for a long time (Jacob 2013; Eagles-Smith et al. 2018). This is especially relevant for legacy organochlorine compounds, which are still abundant in many places as a consequence of their low degradation rates, even though they have been banned in most countries for decades (UNEP 2018). Both, POPs and Hg, can travel far distances through global distillation (Wania and Mackay 1996; Choi et al. 2008; Kang et al. 2012), hydrospheric transport (Wania and Mackay 1993; Fuoco et al. 2009), and migrating animals (Braune et al. 2005; Fort et al. 2014). Thus, they can reach pristine polar environments such as Antarctica, where cold temperatures, circumpolar currents, snow, and ice capture them (Wania and Mackay 1996). Additionally, passive air sampling suggests that Arctic and Antarctic stations also emit pollutants to the environment (Choi et al. 2008).
Through food intake and bioconcentration, POPs and Hg accumulate in biota and biomagnify through the food webs (Morel et al. 1998; Langis et al. 1999; Fisk et al. 2001). Top predators are especially prone to concentrate high contaminant loads (Haraguchi et al. 2009; Krahn et al. 2009), and both mammals and birds are at risk of transferring part of their own load to the next generation (Verboven et al. 2009; van de Merwe et al. 2011). One non-invasive method of monitoring POPs in birds is to collect abandoned eggs. Within the egg, the yolk contains the highest amount of the highly lipophilic polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDTs) and the metabolites dichlordiphenyldichlorethen (DDEs) and dichlordiphenyldichlorethan (DDDs; hereafter DDX is used to represent DDTs, DDEs and DDDs together) in an egg is found in the yolk. Only negligible amounts are found in the albumen that contains < 1% of the egg lipids (Drouillard and Norstrom 2001; Russel et al. 1999). The egg yolk, the main nutrient carrier for an embryo, is eventually consumed entirely by the embryo (Noble and Cocchi 1990), and hence, the full load of the yolk’s POPs will be transferred from the egg to the hatchling. On the other hand, Hg accumulates majorly in the albumen proteins of an egg (Wolfe et al. 1998; Grajewska et al. 2015). Still, Hg concentrations of yolk and egg membrane closely correlate with Hg concentrations in albumen, which makes all tissues suitable to monitor Hg concentration in eggs (Brasso et al. 2012; Peterson et al. 2017).
Pollutants like PCBs, DDX, and Hg affect bird fitness and survival. DDX became infamous more than 50 years ago when it was linked to eggshell thinning (e.g., Tucker and Haegele 1970). Resulting eggshell breakage caused severe declines of whole bird populations (WHO 2002; Elliott et al. 1988; Johnstone et al. 1996; Vasseur and Cossu-Leguille 2006). Within the group of PCBs, dioxin-like PCBs (dl-PCBs) have properties similar to dioxins, and can cause endocrine disruptions in animals (Tanabe 2002). They seem to cause mal-development of the lymphatic system and embryo deformation in some bird species (Bosveld and van den Berg 1994). In female little auks (Alle alle), high Hg concentrations caused smaller eggs the following breeding season (Fort et al. 2014), but in mallards also caused deformation of embryos, early mortality, and brain lesions (Wolfe et al. 1998). All these pollutants place a significant threat to wildlife (Goutte et al. 2014). They threaten the stability of bird populations due to their impact on reproductive success, especially of those at the top of the food chain (Fisk et al. 2001; Langis et al. 1999).
The Wilson’s storm-petrel (Oceanites oceanicus) is a common predator breeding in subantarctic and Antarctic regions. Here, we investigated whether POP and Hg contamination for this species changed within the last decades around the Antarctic Peninsula. We collected eggs that were abandoned, e.g., due to prolonged adverse conditions such as snowstorms from the same colony in 2001, 2003, and from 2014 to 2016 (Quillfeldt 2001; Büßer et al. 2004), as well as membranes of hatched eggs in 1998, 2003, and 2014 to 2016. As Wilson’s storm-petrels collect all nutrients needed to form the egg in one location during the pre-laying exodus (Beck et al. 1972), an egg will be influenced by the pollutant concentration of this area. To evaluate differences of pollutant levels among years, it is important to be aware of potentially different nutrient origins. Both, the foraging area and the trophic position of prey species could influence pollutant concentrations in seabird eggs (Braune et al. 2005; Carravieri et al. 2014, 2016; Corsolini and Sará 2017; Cherel et al. 2018; Mills et al. 2020). To evaluate foraging habits and areas among years, we analyzed stable isotope values for carbon and nitrogen. In the Southern Ocean, δ13C values are linked to the latitudes of foraging areas (Cherel and Hobson 2007; Quillfeldt et al. 2005, 2010). Hence, we investigated whether pre-exodus areas of Wilson's storm-petrels were similar among the studied time periods, or whether a transition to more northern or more southern feeding grounds took place. Through enrichment of 15N relative to 14N from prey to predator, δ15N indicates the trophic position of an animal (Post 2002; Weiss et al. 2009). We used this to test whether prey of Wilson’s storm-petrels ranged within similar trophic positions or whether trophic positions changed among the years. Additionally to temporal differences of pollutant exposure, we investigated whether embryos incorporate pollutants at a constant rate, as timing of toxic effects may be crucial for embryonic development, and analyzed different age groups of embryos. Explicitly, we addressed the following questions:
-
1.
What are POP concentrations in embryos, and Hg concentrations in egg membranes of Wilson’s storm-petrels?
-
2.
Do POP and Hg concentrations in embryos and egg membranes, respectively, differ depending on embryo age? And can we, hence, learn when POPs are transferred from the egg to the embryo?
-
3.
Do POP and Hg concentrations change over the years? In addition, do stable isotope analyses indicate differences in pre-laying exodus area and trophic position that may explain differences in pollutants?
We expected to find POP concentrations in the same range as eggs of seabirds with similar foraging preferences from the same area, like Adélie penguins (Pygoscelis adeliae; Cipro et al. 2010; Corsolini et al. 2011). Furthermore, we expected POP transfer to follow lipid transfer from yolk to embryo, but no variation of Hg concentrations in egg membranes through the development. If no change of pre-laying exodus area or trophic feeding level could be detected (Noble and Cocchi 1990), we expected lower POP concentration in samples from more recent years due to the extensive ban of those substances (UNEP 2018), and no change in Hg concentrations among years, due to continuing input into the environment and transport to polar areas (Streets et al. 2017; Soerensen et al. 2012).
Materials and methods
Species and study site
The study took place at the “Tres Hermanos” hill about 1.5 km from Carlini Research Base (Fig. 1) on King George Island (Isla 25 de Mayo), South Shetland Islands (62° 14′ S, 58° 40′ W), where a research project on Antarctic storm-petrel species was set up in 1995. Breeding success and number of established nests were observed for more than 20 years at a colony of Wilson’s storm-petrels. Here, breeding takes place in the scree slopes underneath big rocks, where the depth of accessible nests varies from 20 to 60 cm. Wilson’s storm-petrels are small marine birds weighing on average 38 g (Fig. 1; Beck et al. 1972; Quillfeldt 2006). This common predator feeds mainly on Antarctic krill (Euphausia superba), squid, small fish, and amphipods (Quillfeldt 2002). Wilson’s storm-petrels spend their life at sea when they are not breeding, and can be found in all oceans except for Arctic waters (Quillfeldt 2006). Their breeding sites are in the Antarctic and on subantarctic islands. Breeding pairs establish their nesting burrow in cavities below rocks (Quillfeldt 2006; Roberts 1941), before the females leave again prior to egg-laying on a pre-laying exodus. This foraging trip is used to accumulate nutrients to produce a single egg that contains the recently consumed pollutants, and hence, establishes the burden of pollutants for every surviving hatchling (Beck et al. 1972; Hobson et al. 1997, 2000).
Study area at Carlini Base on King George Island (map data from Quantarctica; Matsuoka et al. 2018), and adult and nestling of the study species Wilson's storm-petrel (Oceanites oceanicus)
Egg and egg membrane collection
In all years, active nests (incubating adult or egg) were identified during the incubation period from mid-December to the end of January; all accessible eggs were measured once in the absence of adult birds (length, width, weight) using calipers and a digital balance. With these measurements, we estimated the chicks’ hatching dates by density loss over time according to Quillfeldt (2001). We looked for hatched chicks 5 days after this estimated date and monitored nestlings regularly afterwards until they fledged, or the end of the season. Eggs that did not hatch until the estimated date were checked at most four more times, approximately every 5 days. Whenever possible nest checks were done at sight to reduce possible disturbance of incubating adults. After a chick hatched, its nest was searched for the eggshell to collect the egg membrane. Membranes were freed from shell parts and dirt by rinsing them with tap water, air dried at the field station, and stored at room temperature in 2003, and from 2014 to 2016. In 1998, eggshells were collected, transported and stored frozen (− 20 °C) until needed. Membranes were then separated from the shell, rinsed under tap water, air dried, and stored at room temperature until further analyses took place.
Snowstorms and the resulting blocking of nest entrances regularly cause failure of hatching of chicks (e.g., Büßer et al. 2004). These eggs and all other abandoned and accessible eggs were collected at the end of the season (i.e., end of March) when no further hatching could be expected. As the inaccessibility of the nests for parents and, hence, hypothermia was the main reason for embryos to die before hatching, we expect pollutant concentrations found in this study to be representative for eggs that hatched. In 2001, embryos were dissected at the research station. In 2003 and 2014 to 2016, collected eggs were preserved whole, and egg membrane and embryo samples were prepared during egg dissection after leaving the field station. All samples were stored at − 20 °C until preparation of samples.
Selection and preparation of samples
We used embryos to analyze POPs, and conducted stable isotope and Hg analyses on egg membranes. To compare pollutant loads from 2014 to 2016 to earlier years, we used available, stored samples from the same colony from 1998 (only egg membranes), 2001 (only embryos), and 2003 (egg membranes and embryos) (Table 1).
Eggs from 2003 and 2014 to 2016 were thawed and opened to extract the embryos. The embryos were weighed, frozen again, and then freeze-dried to mass consistency. Storage time of frozen embryos varied by up to 15 years. Resulting wet weight (ww) was not equally reliable for all samples due to water sublimation. To correct this, ww was calculated backwards from dry weight (dw) using the average percentage of mass loss during drying from the newest samples from 2016 (81.8%, n = 6; compare Leat et al. 2011).
Preliminary trials on other bird material with the below described method, and literature research (compare Online Resource 8) led us to the expectation that POP concentrations in individually analyzed embryos would be below the limit of detection (LOD) or limit of quantification (LOQ) for all but two samples from 2016. All embryos but those two were therefore pooled into eight groups, clustered into two time periods of sampling (2001 and 2003, and 2014 to 2016) and for each time period into four age groups, to learn more about the temporal transfer of organic pollutants to the embryo. We estimated embryo age from ww following the equation for embryonic growth of birds described by Ricklefs (2010). Detailed calculations can be found in Online Resources 1–3. We divided the available embryos as equally as possible into four similar age groups for both time intervals, with the aim of covering a wide range of developmental steps. It is biologically relevant to be able to differentiate between the first two third of development (0–33 days) and the last third of development (34–50 days), as the major amount of lipids and, hence, lipid soluble pollutants are expected to be taken up by the embryo in the last third of development (Noble and Cocchi 1990). Age group one includes embryos with an approximate age up to 25 days, age group two includes embryos aged 26–30 days, age group three includes embryos of the ages 31–33 days, and age group four includes embryos of the ages 34–40 days (Online Resource 3). Group 5 contained only two embryos approximately 1–2 days before hatching from 2016 with an embryonic age of approximately 48 days (Online Resources 3 and 2). No comparable embryos for group 5 were available from 2001 to 2003. Dried embryos were ground as fine as possible first using a stainless steel blade grinder (Severin, 140 W) for approximately 2 min, and then using an agate mortar and pestle.
POP extraction and analysis
Chemicals
All analytical standards were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The indicator congeners PCB 28, 52, 101, 138, 153, and 180 were purchased as standard mix together with PCB 118 in methanol solution (“PCB Mix 3”, 10 µg mL−1). The PCB congeners 49, 77, 99, 105, 110, 156, 170, 183, and 187 as well as the six DDX dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethane (DDD), and dichlorodiphenyldichloroethylene (DDE), each as 2,4’- and 4,4’-chlorinated isomers, as well as 1,2,3,4-tetrachloronaphthalene for use as internal standard, were purchased as pure substances in powder form. The PCB 132 was purchased dissolved in iso-octane. The PCB set contains four dl-PCBs, i.e., PCBs 77, 105, 118, and 156. All substances were prepared and used dissolved in methanol.
Extraction procedure
POP extraction from dried, pulverized, and pooled embryos was performed by microwave assisted extraction (MAE) adapted from Düring and Gäth (2000), followed by sulfuric acid purification adapted from Murphy (1972). Extraction was followed by concentration of extracts by rotary evaporator followed by N2 flow, and then transfer to an aqueous solution for measurement by headspace solid-phase microextraction (HS-SPME) coupled to gas chromatography-mass spectrometry (GC–MS). For extraction, 400 ± 1 mg of dried and pulverized embryos were weighed to polyfluoralkoxy (PFA) MAE vessels. Then, heat transformer disks (Weflon® disks, MLS Corp., Leutkirch, Germany) and 15 mL n-hexane (≥ 99%, p.a. quality, Carl Roth GmbH, Karlsruhe, Germany) were added before closing the vessels. Two types of control vessels were prepared, either with 15 mL n-hexane or with 15 mL n-hexane and 2.5 µL of a 10 ng µL−1 PCB standard solution (PCB Mix 3, Dr. Ehrenstorfer GmbH, Augsburg, Germany). 1,2,3,4-tetrachloronaphthalene was added to all samples as an internal standard (4 µL of a 5 ng µL−1 solution). The MAE was performed at 120 °C with 250 W for 1 min followed by 1000 W for 15 min. After passive cooling and opening of the vessels, aliquots of 12 mL were removed from the supernatant and transferred to 20-mL glass vials. For purification from greasy components, aliquots were treated with 2 mL concentrated sulfuric acid (95.0–98.0%, Sigma-Aldrich, ACS reagent), and shaken by hand for 1 min. After centrifugation (20 min at 210 RCF, Hettich Rotanta 460 R), the acidic solution was carefully removed by glass Pasteur pipettes. This procedure was repeated once with 1 mL of sulfuric acid. Afterwards, 10-mL aliquots of the acid treated n-hexane extracts were transferred to rotary evaporator flasks followed by their careful reduction to volumes of 200 µL. Solvent reduction was accelerated by using a water bath (40 °C), rotating the flask (150 rpm), and by applying negative pressure with a vacuum pump (350–400 mbar). Remaining extracts were transferred to fresh 20-mL headspace vials. The flasks were flushed twice with 150 µL n-hexane each that was also transferred to the 20-mL headspace vials afterwards. The resulting n-hexane volume (~ 500 µL) was largely reduced by a gentle N2 stream. Once only a few drops remained, the vials were passively air dried. The target substances were redissolved with 100 µL of solubilizing methanol (p.a. quality, Carl Roth GmbH, Karlsruhe, Germany), followed by the addition of 10 mL NaCl solution (1 g 10 mL−1) for salting out of POPs to the headspace.
Instrumental and data analysis
Measurement of POPs was performed by HS-SPME coupled to GC–MS. Basics of the performed process are given in Böhm et al. (2017) and Wiltschka et al. (2020). In the present study, the following parameters were used for automated SPME: fiber with 100 µm PDMS coating (Sigma-Aldrich), heating of the fiber prior to extraction to eliminate cross contamination: 10 min, pre-equilibration before extraction: 10 min, extraction: 60 min at 90 °C while shaking the sample vial at 250 rpm. Samples were processed by a CombiPAL autosampler (CTC-Analytics, Zwingen, Switzerland) with thermodesorption of the fiber in the injector of the GC–MS for 3 min at 270 °C. Chromatographic separation and mass detection were performed with a GC-ion trap MS (Trace GC Ultra/ITQ 900, Thermo Fisher Scientific) equipped with a XLB-type capillary column (Thermo TG-XLBMS, 60 m, 0.25 mm, 0.25 µm). Semi-quantitative screening of a sample aliquot in full-scan mode of the MS (m/z 50–500) was used to identify concentration ranges of target PCB congeners as well as to identify further PCB congeners by chlorine pattern. For quantification, the MS was used in selected ion storage (SIS) mode (designated as “selected ion monitoring”, SIM, in Thermo Xcalibur software) with respective segments for the analytes. Quantification was performed based on six-point calibrations for PCBs in the range of 0.01–0.75 µg L−1 and for DDX in the range of 0.01 to 2 µg L−1. For samples that exceeded this range, an extended 12-point calibration from 0.01 to 3.25 µg L−1 was used. For all analytes LODs and LOQs were determined analog to IUPAC (1997), using the numerical factor k = 3 or k = 10, respectively (Online Resource 6). Processing of GC–MS data was performed with the software ‘Xcalibur’ (Thermo Fisher Scientific) combined with a manual verification of peak integration. Data for all analytes were corrected based on TCN as internal standard. Concentrations from SPME–GC–MS measurements were recalculated considering aliquoting and initial sample weight of embryos. Final POP concentrations in embryos are given in ng g−1 dw. Detailed parameters on GC–MS conditions (i.e., oven program and parameters for MS detection) are given in the Online Resources 4 and 5.
Analysis of mercury
We determined total Hg (the sum of inorganic and organic Hg, thereafter called Hg) concentrations in egg membranes from 1998, 2003, and 2014 to 2016 (Table 1). Whenever possible, analysis was conducted with membranes from the same eggs of which embryos were used for POP analysis. Additional samples were used from hatched chicks for all years. Surface contaminants were removed from egg membranes in ultrasonic baths. Each sample was individually sonicated for 3 min in a bath filled with a 2:1 volume ratio of chloroform and methanol. Subsequently, they were rinsed twice in consecutive methanol baths and dried for 48 h at 45 °C. Clean egg membranes were cut with stainless steel scissors into tiny fragments to produce a homogeneous powder. Aliquots of 2–11 mg powdered egg membrane were used to determine Hg concentrations with an Advanced Mercury Analyzer spectrophotometer Altec AMA-254. To evaporate Hg, aliquots were heated progressively until 800 °C under an oxygen atmosphere and Hg was amalgamated on a gold-net during 3 min (Bustamante et al. 2006). Then, the net was heated to liberate the collected Hg, which was finally measured by atomic absorption spectrophotometry (Bustamante et al. 2006). Measurements were repeated two or three times for each sample until having a relative standard deviation (SD) < 10%. Accuracy and reproducibility for each set of samples were tested by analytical blanks and replicate measurements of certified reference material (DOLT-5, dogfish liver, National Research Council of Canada; certified concentration: 0.44 ± 0.18 μg g−1 dw). Measured Hg concentrations for the certified reference material were: 0.33 ± 0.00 μg g−1 dw (n = 7). The detection limit of the AMA was 0.05 ng. Hg concentrations were expressed in µg g−1 dry weight (dw).
Bulk stable isotope analysis
Stable isotope analysis (SIA) was conducted using subsamples of egg membranes from the same eggs that were used for Hg analysis. An amount of 0.32–0.50 mg (mean ± sd: 0.40 mg ± 0.04 mg) of egg membrane was weighed in tin cups. Carbon and nitrogen ratios were determined with a continuous-flow mass spectrometer (Delta V Plus, Thermo Scientific, Bremen, Germany) coupled to an elemental analyzer [Flash EA 1112 (37 samples) or Flash 2000 (16 samples), both Thermo Scientific, Milan, Italy]. Results are expressed in the δ notation as parts per thousand (‰) deviation from the international standards Vienna Pee Dee Belemnite (δ13C) and atmospheric N2 (δ15N). The used formula is δ13C or δ15N = [(Rsample/Rstandard)—1] × 103, where R is 13C/12C or 15 N/14 N, respectively. To assess the quality of the analytical procedures, measurements of internal laboratory standards were conducted using acetanilide (Thermo Scientific) and peptone (Sigma- Aldrich) or two types of caffeine (USGS-61 and USGS-62; Reston stable isotope laboratory, United Stated Geological Survey) and indicated a standard deviation for analyzed samples of < ± 0.06 ‰ for δ13C and < ± 0.09 ‰ for δ15N.
Statistical analyses
Only POPs with concentrations above LOQ (Online Resource 6) were included in the following analyses. Pollutants above LOD but below LOQ were presented in this paper, but were not included in any statistical analyses. Pollutants below LOD are given in the Online Resource 6, but were not considered beyond this. As the small sample size did not meet necessary requirements for non-parametric testing, we used parametric tests for statistical analyses on POPs.
Concentration differences among PCB congeners were tested with a Kruskal–Wallis rank sum test followed by Dunn’s test for post hoc pairwise comparisons with Bonferroni correction (Haynes 2013), concentration differences between DDT metabolites were tested using Wilcoxon rank sum test.
To test concentration differences of PCBs and DDX among age groups in embryos, the parametric Friedman rank sum test was used with data paired for individual pollutants. For post hoc analyses between single age groups, Wilcoxon signed-rank exact tests with paired samples and p-values corrected according to Bonferroni were used. If ties occurred Wilcoxon signed rank tests with continuity correction were used. To test if there was a correlation between Hg concentration of egg membranes and age of the embryo, we used a Spearman’s rank correlation with the embryo’s dw as a proxy for embryo age. To compare Hg concentrations from samples of hatched vs. unhatched eggs, we used a Wilcoxon signed rank sum test with continuity correction.
We analyzed differences of pollutant concentrations for POPs between the available years 2001 and 2003, and 2014 to 2016 using a Wilcoxon signed rank exact test. Samples were paired for pollutants, and differences between early and recent years were tested over all samples, as well as for embryos younger than 34 days or older than 33 days separately. The p-values for these post hoc tests were adjusted using Bonferroni corrections. In analyses using individual age groups, pollutant concentrations in the years 2014 to 2016 were averaged for age group 4 (31–33 days) and 5 (> 33 days) to allow comparisons with years 2001 and 2003 where only 4 age groups could be analyzed. Further, PCB 170 was excluded from age comparisons, as only one sample showed concentrations > LOD for this substance. To test differences of Hg concentrations among the available years 2001, 2003, and 2014 to 2016 we used a Kruskal–Wallis rank sum test followed by pairwise Wilcoxon tests for post hoc pairwise comparisons with Bonferroni correction (Haynes 2013).
The ratio of 4,4’-DDE to the total sum of DDX was calculated as an indicator for differentiating between recent or ancient input (Fries et al. 1969; Borrell and Aguilar 1987; Yogui et al. 2003). Values above 0.7 are considered to show an origin from ancient sources, while values below 0.7 show a more recent input of commercial DDX into the environment.
Stable isotope patterns were analyzed for inter-annual differences (for post hoc analysis with Bonferroni correction) for the years 1998, 2003 and 2014 to 2016 using the Wilks’ Lambda test and a One-way MANOVA (Bartlett Chi2). Trophic positions (TP) for Wilson’s storm-petrels (TPocea) were calculated according to Weiss et al. (2009), using the formula.
where TPprey is 2.3, the estimated TP of the main prey, Antarctic krill (Hodum and Hobson 2000), δ15Nprey is 5.3 ‰, the average δ15N of krill in the northern Antarctic Peninsula (Seyboth et al. 2018), and 3.4 ‰ is the assumed enrichment factor as estimated by Post (2002). Samples that clearly originated from non-Antarctic waters (δ13C > − 21 ‰; 1998: n = 1; 2015: n = 3) were excluded for the calculation of trophic positions. We tested if Hg concentrations were linked to δ13C or δ15N values using Pearson’s product-moment correlations.
All statistical analyses and figures were conducted in R 4.0.5 (R Core Team 2021). Map of study site was composed using QGIS 3.24 (QGIS Development Team 2022) and the Quantarctica data set (Matsuoka et al. 2018).
Results
Pollutant concentrations
In Wilson’s storm-petrel embryos, eight PCBs and two DDT metabolites could be quantified (Table 2; Fig. 2). Concentrations of PCBs differed significantly among congeners (Kruskal–Wallis rank sum test, χ2 = 27.28, p < 0.001). The highest values among the PCBs were reached for PCB 153 with more than 1400 ng g−1 ww. Compared to PCBs 99 and 183, concentrations of PCB 153 were significantly higher (Dunn’s Post hoc test with Bonferroni correction, PCB 99 vs. PCB 153: Z = − 3.44, p = 0.016; PCB 153 vs. PCB 183: Z = 4.57, p < 0.001; results of all pairwise comparisons are presented in Online Resource 7). The sum of all detected PCBs ranged from 59 to 3403 ng g−1 within the samples. Among quantified PCBs, two dl-PCBs (PCBs 105 and 118) were found in sum concentrations between 12 and 549 ng g−1 ww. Among the DDX, 4,4’-DDE reached the highest concentrations with more than 350 ng g−1 ww, and was in general significantly higher than 4,4’-DDD (Wilcoxon rank sum test, W = 11, p = 0.008). The ratio of the 4,4’-DDE concentration to the total concentration of all DDX was on average 0.88 ± 0.21, and only one sample had a ratio below 0.7 with 0.36.
Concentration (ng g−1 ww) for eight quantified PCBs and two DDX in embryo samples of Wilson’s storm-petrels. All PCBs were found in n = 10 samples, except for PCB 170, that was only detected in one sample. Significant differences between congeners are indicated by brackets and asterisks: *p < 0.05; **p < 0.01; ***p < 0.001. Black bars represent median of samples, the box marks 25%, respectively, 75% quantiles. Whiskers show the point closest to but within 1.5-fold distance of interquartiles
Additionally, PCBs 49, 52, 77, 101, 156, and 187 could be qualified, but concentrations were below LOQs (Online Resource 6). Out of these, PCBs 77 and 156 are dl-PCBs. The PCBs 28, 110, and 132 could not be detected in any sample.
Concentrations of Hg in egg membranes ranged from 0.006 to 0.436 µg g−1 dw (Table 2).
Pollutant concentrations and embryo age
The pollutant concentration of PCBs in embryos differed among ages in the years 2001 and 2003 compared to those from 2014 to 2016 (Fig. 3; Friedman rank sum test; 2001 and 2003: χ2 = 20.0; df = 3; p < 0.001; 2014–2016: χ2 = 26.1; df = 3; p < 0.001) with significantly higher concentrations in embryos aged 34 days or older compared to younger embryos (Wilcoxon signed rank exact test for eight pollutants and Bonferroni adjusted p-values; 2001 and 2003: V = 0; p = 0.031; 2014–2016: V = 0; p = 0.031; Fig. 3). No significantly different PCB concentrations could be found in embryos older than 40 days and embryos between 34 and 40 days of age in recent years (Wilcoxon signed rank exact test for eight pollutants and Bonferroni adjusted p-values; 2014–2016: V = 5; p = 0.313). No significant differences among ages could be found for DDT metabolites (Friedman rank sum test for two DDT metabolites, 2001 and 2003: χ2 = 5.4; df = 3; p = 0.145; 2014–2016: χ2 = 6.7; df = 3; p = 0.107). We found no significant correlation of higher Hg concentrations in egg membranes when embryos were older (Spearman’s rank correlation, rs = 1232, p = 0.066, ρ = 0.39), but Hg concentrations of membranes from hatched eggs were significantly higher than those from unhatched eggs (Wilcoxon signed-rank sum test with continuity correction; W = 105.5; p < 0.001).
Concentrations of ΣPCBs and ΣDDX from Wilson storm-petrel embryos for the years 2001 and 2003, as well as for 2014 to 2016 for each pooled age group. Embryo ages and according age groups for both figures are given below the x-axis. Each bar presents one pooled sample, n refers to number of embryos in a pooled sample, which is similar for both figures
Temporal differences of pollutant concentrations and foraging behavior
The overall load of PCBs was higher in 2001 and 2003 than in 2014 to 2016 (Wilcoxon signed rank exact test, V = 50, p < 0.001). For DDT metabolites, no significantly higher concentrations in 2001 and 2003 were found compared to 2014 to 2016 (Wilcoxon signed rank exact test, V = 5, p = 0.078). Also, PCB concentrations were higher in 2001 and 2003 compared to 2014 to 2016 in the group of younger ages (0–33 days; Wilcoxon signed rank exact test for eight PCBs and Bonferroni adjusted p-values, V = 50, p = 0.048) and the group of older ages (≥ 34 days; Wilcoxon signed rank exact test for eight PCBs and Bonferroni adjusted p-values, V = 0, p = 0.031) tested separately. For DDT metabolites, there were no difference in younger or older embryos from 2001 to 2003 compared to 2014 to 2016 (Wilcoxon signed rank exact test for two DDX, 0–33 days: V = 5, p = 0.313; ≥ 34 days: V = 0, p = 0.5).
Concentrations of POPs relative to each other were comparable among age groups (Fig. 4). The pollutants with the highest (PCB 153, 4,4’-DDE) or lowest concentrations (PCB 183) per sample were the same among samples. One exception was embryos aged 34–40 days from 2001 to 2003, which had a very high concentration of 4,4’-DDD. Interestingly, this value was even higher than that of 4,4’-DDE, which is dominating over 4,4’-DDD in all other samples.
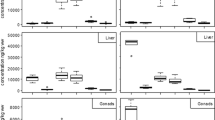
In egg membranes, Hg concentrations were significantly higher in 1998 than in 2003 and in 2014 to 2016, and also higher in 2003 than in 2014 to 2016 (Kruskal Wallis rank sum test: χ2 = 25.1, df = 2, p < 0.001; Fig. 5). Between 1998 and 2003, a significant decrease of medians was observed: 59%, and between 2003 and 2014 to 2016, medians decreased by 66% (Post-hoc Analyses: Wilcoxon rank sum test with Bonferroni correction: 1998 and 2003: W = 66, p = 0.004; 1998 and 2014–2016: W = 349, p < 0.001; 2003 and 2014–2016: W = 203, p = 0.036; Fig. 5). Values of δ13C and δ15N differed significantly among 1998, 2003, and 2014 to 2016, and were significantly different between 2003 and 1998. Between 1998 and 2014 to 2016, or between 2003 and 2014 to 2016, no significant differences were found (1998 and 2003: Wilks' λ = 0.437, χ2 = 11.58, df = 2.0, p = 0.009; 1998 and 2014–2016: Wilks' λ = 0.875, χ2 = 5.76, df = 2.0, p = 0.168; 2003 and 2014–2016: Wilks' λ = 0.864, χ2 = 5.86, df = 2.0, p = 0.160; Fig. 6). Variation within time period of trophic positions was higher than between time periods [medians (ranges) for 1998: 3.2 (3.1–3.4); 2003: 3.0 (2.9–3.2); 2014–2016: 3.2 (2.9—3.4)]. Neither values of δ13C nor of δ15N correlated with Hg loads (Pearson’s product-moment correlation: δ13C: t = − 1.269, df = 51, p = 0.210, r = − 0.17; δ15N: t = 0.734, df = 51, p = 0.466, r = 0.10).
Discussion
Pollutant concentrations
The sum concentrations of all eight PCB congeners quantified in embryos in this study reached values as high as 3403 ng g−1 ww in 2001 and 2003, and 565 ng g−1 ww in 2014 to 2016. In both time periods, these sum PCB concentrations were higher than in other seabirds’ eggs like penguins, gulls or petrels from the Antarctic Peninsula during the austral summer seasons 2003/2004 to 2005/2006 (Cipro et al. 2010; Corsolini et al. 2011; Online resource 8). It is worth to note that some of these studies included from eight and up to 81 PCBs (Cipro et al. 2010; Corsolini et al. 2011; Online resource 8). Similar levels were found only in skua eggs (Stercorarius spp.), for a total of 51 PCB congeners (Online resource 8; Cipro et al. 2013). The underlying reasons for species differences are difficult to determine. Among others, different foraging areas or trophic position of prey items are two possibilities that may explain higher concentrations when averaged for one congener (PCB concentrations/number of congeners) in Wilson’s storm-petrel eggs than in skua eggs (Braune et al. 2005; Carravieri et al. 2014; Corsolini and Sará 2017). However, even compared to Adélie penguins that are similar in habitat and prey preferences, Wilson’s storm-petrel embryos showed higher concentrations (Cipro et al. 2010; Corsolini et al. 2011; Online resource 8). A study on PCB concentrations in Arctic birds suggested that higher metabolic rate, and hence feeding rate, may be a reason for higher pollutant concentrations in birds (Borgå et al. 2005). Wilson's storm-petrels have an almost threefold higher basal, and more than threefold higher field metabolic rate than Adélie penguins (adjusted for body mass). Therefore, a higher food, and hence, pollutant uptake relative to their body mass could explain higher PCB concentrations (Ellis and Gabrielsen 2001).
In the present study, the highest total concentration of PCBs was 3403 ng g−1 ww. Adverse effects of sum concentration of PCBs in other birds’ eggs occurred at 2100 ng g−1 ww, for example, in eggs of the Great blue heron (Ardea herodias), resulting in induced enzymatic reactions (Bosveld and van den Berg 1994). An increase of morphological deformations of embryos appeared in Caspian terns (Hydroprogne caspia) at an egg PCB concentration of 4000 ng g−1 ww compared to normal living embryos with egg PCB concentrations of 3600 ng g−1 ww (Yamashita et al. 1993). Hence, sum concentrations of PCBs in Wilson’s storm-petrel embryos reaching up to 3403 ng g−1 could already pose a threat to the developing bird, especially during the sensitive embryonic phase. Next to 17 analyzed PCBs in the present study, up to 192 other congeners not explicitly looked for could add up to a potentially even higher PCB load. Yet, quantified sum concentrations of the years 2014 to 2016 were lower, with a maximum value of 565 ng g−1 ww, suggesting the toxic threat linked to PCBs was lower in more recent years. However, toxic effects clearly differ among species and some may suffer severe alterations at levels that show no effect in others. No study so far evaluated impacts of PCBs on development or fitness in Wilson’s storm-petrels. While levels of PCBs detected in Wilson's storm-petrels should be observed with caution, more studies evaluating the impact on this species’ health are needed.
Among PCBs, dl-PCBs are especially toxic and can bind to the aryl hydrocarbon-receptor, a ligand dependent transcription factor (Poland et al. 1976; Safe 1994). This binding can cause interference with the immune system, cell migration, or the differentiation of cells of the nervous system (Barouki et al. 2012). In this study, we detected the dl-PCBs 77, 105, 118, and 156 above LOD, of which PCBs 105 and 118 were found above LOQ. Their exact adverse potential in Wilson's storm-petrels without species-specific studies is hard to assess. The fact that in this study even the lowest concentrations of dl-PCBs 105 and 118 together were more than 500-fold higher than European limits for consumable food (eel: 0.010 ng g−1 ww, fish liver: 0.020 ng g−1 ww; European Union 2011) shows how severe those pollutant loads are in Antarctic wildlife.
The patterns of PCB congeners in Wilson's storm-petrel embryos were similar to those found in eggs of other fish- and krill-eating birds (Bosveld and van den Berg 1994; Corsolini et al. 2011; Goutte et al. 2013). Patterns of PCBs differ due to species-specific metabolization of congeners between species and their prey species (Bodin et al. 2008; Koenig et al 2012; Na et al. 2017). Certain PCB congeners with structures like meta-para vicinal H-atoms in at least one phenyl ring could be metabolized and accumulated less in fish liver and fish-eating seabirds than other congeners (Zell et al. 1978; Borlakoglu and Walker 1989). Excretion paths of PCBs for birds include preen oil, feces or maternal transfer to eggs (Barron et al. 1995; Rudolph et al. 2016; Solheim et al. 2016). While patterns of PCBs differed only to small extents in black-legged kittiwakes (Rissa tridactyla) between liver, feather, and preen oil samples, small differences could be seen for certain congeners (Solheim et al. 2016), which could add to species-specific PCB patterns due to differentiation in excretion paths. In this study, observed patterns are in line with those of Adélie penguins or Common terns (Sterna hirundo; Bosveld and van den Berg 1994; Corsolini et al. 2011; Goutte et al. 2013).
The peak level of 4,4’-DDE, a potent endocrine disruptor and androgen receptor antagonist (Xu et al. 2006; Burgos-Aceves et al. 2021) that is even more persistent in animals than its parent compound (Burgos-Aceves et al. 2021), was from a sample from 2001 to 2003 (372 ng g−1 ww). It reached a concentration still well below the critical concentration of reproductive failure in the sensitive brown pelican (Pelecanus occidentalis, 3000 ng g−1; Blus 1982). Several other bird species could withstand higher DDE levels before their ability to reproduce was critically impaired such as the peregrine falcon (Falco peregrinus) and the osprey (Pandion haliaetus; Ratcliffe 1967; Johnson et al. 1975). Hence, we do not expect severe impacts on reproduction on Wilson's storm-petrels due to the comparatively lower DDE concentrations alone.
In all other samples, 4,4’-DDE concentrations were much lower, but with one exception always higher than the other detected DDX, 4,4’-DDD. Neither 2,4’-DDT nor 4,4’-DDT, the main components of commercial DDT mixes, could be detected in our samples. The ratio of 4,4’-DDE concentration to the total concentration of all DDX in those samples was higher than 0.6. DDE is only an impurity of commercial DDT production, and occurs only in low concentrations in the commercial mix. Hence, its occurrence in the wild is almost exclusively due to degradation of DDT. Both, the absence of DDT as well as the DDE/ΣDDX ratios are indicators for old input (Borrell and Aguilar 1987; Yogui et al. 2003). This result is consistent with other studies on Antarctic seabirds such as the Antarctic petrel Thalassoica antarctica whose 4,4’-DDT concentrations in the plasma were very low, or even below LOD (Carravieri et al. 2021). However, at a larger spatial scale, other investigations on seabirds from the Southern Ocean revealed a recent use of DDT, likely for the fight against mosquitos, which are vector of malaria (Carravieri et al. 2014). Such contrasting results reveal the importance of on-going monitoring of contamination in subantarctic and Antarctic environments to evaluate if a new increase of DDX from recent input can be observed.
In one sample, 4,4’-DDD had the highest concentrations compared to the other DDX. To the best of our knowledge, this ratio between 4,4’-DDD and 4,4’-DDE has not yet been reported in biota feeding in Antarctic waters. The reason of this high occurrence of 4,4’-DDD remains unclear. It could be a sign of an uncommon food source, as 4,4’-DDD is the main degradation product of reductive dechlorination reactions of 4,4’-DDT (Yu et al. 2011; Zhang et al. 2015). Hence, prey items like lanternfish (Myctophidae) that spend part of the day at zones of minimal oxygen and use anaerobiosis to cope with these conditions, may explain this finding (Torres et al. 2012). As we did not have stable isotope information for all eggs from this pooled sample, we could not verify whether food items for these eggs were from different trophic positions than other analyzed samples. No 2,4’-DDT, 2,4’-DDD, or 2,4’-DDE was found. This is in accordance with other studies, where these components were below detection levels or had concentrations that were orders of magnitude lower than 4,4’-DDE, which was found in highest concentrations in seabirds (Cipro et al. 2010; Corsolini et al. 2011).
Mean Hg concentrations found in egg membranes of Wilson’s storm-petrels were in the same range as those of Pygoscelis penguins from the South Shetland Islands (Brasso et al. 2012). It is difficult to establish thresholds for lowest observed adverse effect levels for detrimental effects of Hg in eggs, e.g., due to different thresholds among species, and threshold often refer to whole-egg Hg levels (Dietz et al. 2013; Evers et al. 2003). In bird eggs, concentrations of Hg are closely linked to different egg compartments; former studies found that mean Hg concentrations for whole-egg contents are 6.5 to 7.8 times higher than in egg membranes for Gentoo penguins (Pygoscelis papua) or American avocets (Recurvirostra americana), respectively (Brasso et al. 2012; Peterson et al. 2017). When estimating comparable Hg concentrations for whole-eggs (dw) with these values, only one sample from 1998 (0.436 µg g−1 dw for egg membrane, estimate for whole-eggs: 2.8–3.4 µg g−1 dw) is considered to reach a concentration that poses a moderate hazard for reproduction in several bird species (0.6–1.3 µg g−1 ww, equals about 2.8–6.1 µg g−1 dw, Evers et al. 2003). Impacts of high Hg concentrations in eggs include reduced hatchability, malformations of the embryos or reduced nestling survival (Burger and Gochfeld 1997; Heinz et al. 2009; Scheuhammer et al. 2007). However, if nestlings survive long enough, birds will be able to reduce their Hg load during feather growth and molt (Bond and Diamond 2009; Falkowska et al. 2013; Albert et al. 2019). Especially very young birds can benefit from this excretion pathway as the example of common loon chicks (Gavia immer) shows (Fournier et al. 2002).
Pollutant concentrations and embryo age
In our samples, PCB concentrations were relatively constant for the first two thirds of the embryonic development (Fig. 3). After an embryonic age of 34 days, PCB concentrations were significantly higher. This concentration increase happened within the same time frame than in domestic chicken (Gallus gallus domesticus) embryos use yolk lipids for embryonic development; embryos took up only about 20% of lipids stored in the yolk during the first two thirds of their development, but more than another 65% of yolk lipids until briefly before hatching (Noble and Cocchi 1990). The investigated POPs, PCBs and DDX, have a very high affinity to lipids, and are, hence, mainly transferred to the embryos through lipids. While the domestic chicken is considered to belong to the developmental category of precocial birds, storm-petrels are considered by most researchers semi-precocial birds. However, independently of their developmental mode, birds undergo the same structurally defined developmental stages, and growth curves do not differ between precocial and altricial species, once egg size and incubation period is taken into account (Ricklefs and Starck 1998). It is, therefore, likely that also non-precocial birds show the lipid metabolism described by Noble and Cocchi (1990), which largely explains the observed POP increase during embryonic development.
We found a tendency of elevated Hg concentration in egg membranes of more developed embryos. Eggshells, known to become thinner during embryonic development (Karlsson and Lilja 2008; Orłowski and Hałupka 2015), were observed to have decreasing Hg concentrations with increasing embryonic age (Peterson et al. 2017), but no relationship between Hg concentrations in egg membranes and embryonic age was described. As eggshells are thinning, Hg could be transported with calcium to the embryo. This way, part of Hg may not pass through the egg membrane and accumulate here. However, this could only lead to a small increase of Hg concentrations and further studies are needed to evaluate potential pathways of Hg within the egg during embryonic development.
Temporal differences of pollutant concentrations and foraging behavior
Concentrations of PCBs were higher in 2001 and 2003 than in 2014 to 2016. The global ban of many POPs by the Stockholm Convention led to a decline in worldwide, and therefore Antarctic, atmospheric contamination (Pozo et al. 2017). However, melting glaciers represent a potential source of pollutants trapped in the ice for decades (Geisz et al. 2008; Bogdal et al. 2009). Once released into the sea, their accumulation seems currently to primarily take place in benthic environments, where increases in pollutant levels can still be seen (van den Brink et al. 2009, 2011). Whether this will also have an impact on pelagic food webs is yet to be determined. Data from several studies confirm the general trend of pollutant decline in pelagic seabirds, which indicates a lower input for biota feeding in pelagic habitats (van den Brink et al. 2009; van den Brink et al. 2011). While concentrations change, no obvious change in substance relations can be observed. The reduction seen here and in several studies on pelagic seabirds is consistent with known half-life values for organic pollutants (compare Galbán-Malagón et al. 2013).
For DDX, we did not find significantly different concentrations between time periods among all age groups. A study comparing several penguin species showed that DDX concentrations in adult fat tissue were lower around 2010 than they were in the 1980s and 1990s (Ellis et al. 2018). Lower 4,4’-DDT/4,4’-DDE ratios in 2000 to 2009 than in 1960 to 1969 suggest that more recently found DDX concentrations are from DDX that reached the Antarctic through long-distance transportation and is persistent to the environment now (Ellis et al. 2018). In this study, we neither detected 2,4’- or 4,4’-DDT in 2001 and 2003, nor in 2014 to 2016. This is in accordance with the suggestion that detected concentrations do not originate from recent input, and reflect persistent concentrations in the Antarctic environment. Similar concentrations between the two time intervals could hint to an equilibrium between transfer to Antarctic waters and sedimentation of DDX, but additional studies are needed to delineate whether this could be a time trend.
In the present study, egg membrane Hg concentrations were highest in 1998, and were higher in 2003 than in 2014 to 2016. In Adélie penguins, feather Hg concentrations were significantly lower in 2007 than in museum specimen from the 1950s (Carravieri et al. 2016), and prey animals like Southern Ocean squids from subantarctic waters showed decreasing Hg concentrations over a decade of monitoring (Seco et al. 2020). Other species feeding in Antarctic waters like emperor penguins (Aptenodytes forsteri) showed no change of Hg feather concentrations compared to museum samples (Carravieri et al. 2016). However, emissions in the southern hemisphere were at best stable or even increased within the past years (Soerensen et al. 2012; Streets et al. 2017). High emissions originate from artisanal small-scale gold mining that is increasing with rising gold prices. Gold mining is for example found in Ghana or Peru, and hence, no decrease of emissions in the southern hemisphere is expected in the near future (Eagles-Smith et al. 2018). In sub Antarctic regions, increasing atmospheric Hg concentrations are also reflected in Hg concentrations in feathers of several seabird species compared to years or decades earlier (Carravieri et al. 2016; Mills et al. 2020). Interestingly, even with decreasing atmospheric Hg concentrations, animals may not reflect these trends, because several environmental factors, e.g., warming climate, and biogeochemical processes, may promote Hg methylation and its subsequent incorporation in food webs (Wang et al. 2019). Hence, even though our results showed higher Hg concentrations in the early 2000s than in 2014 and 2016, caution is necessary, as due to atmospheric Hg deposition in oceanic waters (Krabbenhoft and Sunderland 2013; Cossa et al. 2011) an increase of Hg concentrations in seabirds like the Wilson’s storm-petrel should not be excluded (Carravieri et al. 2016).
Foraging behavior of wildlife can often explain diverging pollutant concentrations, because of prey from different trophic positions or geographic areas (Braune et al. 2005; Carravieri et al. 2014, 2016; Corsolini and Sará 2017; Cherel et al. 2018; Mills et al. 2020). We used stable isotope δ13C and δ15N values to analyze if differences of pollutant concentrations among time periods could be caused by foraging behavior. Stable isotope values of the years 2014 to 2016 did not differ from 1998 or 2003, while 1998 and 2003 were significantly different from each other. Specifically, there is a high divergence of δ15N values, but a high overlap of δ13C values that can be used as a proxy for latitude (Fig. 5; Cherel and Hobson 2007; Quillfeldt et al. 2010). However, trophic positions calculated from δ15N values differed in all years more within than among years. Additionally, Hg concentrations did not correlate to δ13C or δ15N values. Hence, we think that observed concentration differences in this study of Hg in 1998, 2003 and 2014 to 2016, and of PCBs in 2001 and 2003 compared to 2014 to 2016, are unlikely to be caused by diverging pre-laying exodus latitudes or prey trophic positions, and rather reflect a decrease of the pollutants in the environment over time. However, our available data covered only a small number of breeding seasons, and more data are needed to delineate a significant decreasing trend of POPs and Hg concentrations in Wilson’s storm-petrel eggs (Rigét et al. 2011; Bignert et al. 2004).
Conclusions and outlook
Overall, the pollutants in embryos and egg membranes of Wilson’s storm-petrels were similar to those often reported for biota in Antarctic waters. Concentrations of Hg in the Wilson's storm-petrel egg membranes were in similar ranges than those of seabirds with similar diets like Adélie penguins, but POP concentrations measured in Wilson's storm-petrel embryo samples were higher than those of the larger seabird. In general, concentrations of POPs and Hg decreased over the observed decade, and POP concentrations increased with advancing embryonic development. Our study established a first basis of pollutant concentrations found in embryos and egg membranes of Wilson’s storm-petrels breeding in the Antarctic. Also, our results show the importance of considering the developmental stage of the embryo when analyzing pollutants. Further studies are needed to investigate potential impacts of these pollutants, e.g., on reproductive success and toxicity effects on nestlings. It is important to examine whether the low concentrations during the first weeks of embryonic development are negligible in terms of toxic impact on embryo development compared to higher pollutant loads transferred in later stages of development, or whether even low concentrations could be detrimental in sensitive phases of development. With melting ice masses due to climate change, trapped pollutants may be released into coastal Antarctic waters and incorporated in the food chain, likely increasing the toxicity risks for seabirds.
Data availability
We declare that data archiving is not mandated but data will be made available upon reasonable request.
References
Albert C, Renedo M, Bustamante P, Fort J (2019) Using blood and feathers to investigate large-scale Hg contamination in Arctic seabirds: a review. Environ Res 177:108588. https://doi.org/10.1016/j.envres.2019.108588
Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X (2012) The aryl hydrocarbon receptor system. Drug Metabol Drug Interact 27:3–8. https://doi.org/10.1515/dmdi-2011-0035
Barron MG, Galbraith H, Beltman D (1995) Comparative reproductive and developmental toxicology of PCBs in birds. Comp Biochem Physiol C Comp 112:1–14. https://doi.org/10.1016/0742-8413(95)00074-7
Beck JR, Brown MA, Brown DW (1972) The biology of Wilson’s storm petrel, Oceanites oceanicus (Kuhl), at Signy Island, South Orkney Islands. Scientific Reports (British Antarctic Survey), London
Bignert A, Rigét F, Braune B et al (2004) Recent temporal trend monitoring of mercury in Arctic biota—how powerful are the existing data sets? J Environ Monit 6:351–355. https://doi.org/10.1039/b312118f
Blus LJ (1982) Further interpretation of the relation of organochlorine residues in brown pelican eggs to reproductive success. Environ Pollut (series a) 28:15–33. https://doi.org/10.1016/0143-1471(82)90042-3
Bodin N, Le Loc’h F, Caisey X, et al (2008) Congener-specific accumulation and trophic transfer of polychlorinated biphenyls in spider crab food webs revealed by stable isotope analysis. Environ Pollut 151:252–261. https://doi.org/10.1016/j.envpol.2007.01.051
Bogdal C, Schmid P, Zennegg M et al (2009) Blast from the past: melting glaciers as a relevant source for persistent organic pollutants. Environ Sci Technol 43:8173–8177. https://doi.org/10.1021/es901628x
Böhm L, Düring RA, Bruckert HJ, Schlechtriem C (2017) Can solid-phase microextraction replace solvent extraction for water analysis in fish bioconcentration studies with highly hydrophobic organic chemicals? Environ Toxicol Chem 36:2887–2894. https://doi.org/10.1002/etc.3854
Bond AL, Diamond AW (2009) Total and methyl mercury concentrations in seabird feathers and eggs. Arch Environ Contam Toxicol 56:286–291. https://doi.org/10.1007/s00244-008-9185-7
Borgå K, Wolkers H, Skaare JU et al (2005) Bioaccumulation of PCBs in Arctic seabirds: influence of dietary exposure and congener biotransformation. Environ Pollut 134:397–409. https://doi.org/10.1016/j.envpol.2004.09.016
Borlakoglu JT, Walker CH (1989) Comparative aspects of congener specific PCB metabolism. Eur J Drug Metab Pharmacokinet 14:127–131. https://doi.org/10.1007/BF03190852
Borrell A, Aguilar A (1987) Variations in DDE percentage correlated with total DDT burden in the blubber of fin and sei whales. Mar Pollut Bull 18:70–74. https://doi.org/10.1016/0025-326X(87)90570-4
Bosveld ATC, van den Berg M (1994) Effects of polychlorinated biphenyls, dibenzo-p-dioxins, and dibenzofurans on fish-eating birds. Environ Rev 2:147–166. https://doi.org/10.1139/a94-010
Brasso RL, Polito MJ, Lynch HJ et al (2012) Penguin eggshell membranes reflect homogeneity of mercury in the marine food web surrounding the Antarctic Peninsula. Sci Total Environ 439:165–171. https://doi.org/10.1016/j.scitotenv.2012.09.028
Braune BM, Outridge PM, Fisk AT et al (2005) Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: an overview of spatial and temporal trends. Sci Total Environ 351–352:4–56. https://doi.org/10.1016/j.scitotenv.2004.10.034
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172. https://doi.org/10.1006/enrs.1997.3778
Burgos-Aceves MA, Migliaccio V, Di Gregorio I et al (2021) 1,1,1-trichloro-2,2-bis (p-chlorophenyl)-ethane (DDT) and 1,1-Dichloro-2,2-bis (p, p’-chlorophenyl) ethylene (DDE) as endocrine disruptors in human and wildlife: a possible implication of mitochondria. Environ Toxicol Pharmacol 87:1–12. https://doi.org/10.1016/j.etap.2021.103684
Büßer C, Kahles A, Quillfeldt P (2004) Breeding success and chick provisioning in Wilson’s storm-petrels Oceanites oceanicus over seven years: frequent failures due to food shortage and entombment. Polar Biol 27:613–622. https://doi.org/10.1007/s00300-004-0627-z
Bustamante P, Lahaye V, Durnez C et al (2006) Total and organic Hg concentrations in cephalopods from the north eastern Atlantic waters: influence of geographical origin and feeding ecology. Sci Total Environ 368:585–596. https://doi.org/10.1016/j.scitotenv.2006.01.038
Carravieri A, Bustamante P, Tartu S et al (2014) Wandering albatrosses document latitudinal variations in the transfer of persistent organic pollutants and mercury to southern ocean predators. Environ Sci Technol 48:14746–14755. https://doi.org/10.1021/es504601m
Carravieri A, Cherel Y, Jaeger A et al (2016) Penguins as bioindicators of mercury contamination in the southern Indian ocean: geographical and temporal trends. Environ Pollut 213:195–205. https://doi.org/10.1016/j.envpol.2016.02.010
Carravieri A, Warner NA, Herzke D et al (2021) Trophic and fitness correlates of mercury and organochlorine compound residues in egg-laying Antarctic petrels. Environ Res 193:110518. https://doi.org/10.1016/j.envres.2020.110518
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the southern ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Cherel Y, Barbraud C, Lahournat M et al (2018) Accumulate or eliminate? Seasonal mercury dynamics in albatrosses, the most contaminated family of birds. Environ Pollut 241:124–135. https://doi.org/10.1016/j.envpol.2018.05.048
Choi SD, Baek SY, Chang YS et al (2008) Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: implications for long-range transport and local pollution. Environ Sci Technol 42:7125–7131. https://doi.org/10.1021/es801004p
Cipro CVZ, Taniguchi S, Montone RC (2010) Occurrence of organochlorine compounds in Euphausia superba and unhatched eggs of Pygoscelis genus penguins from Admiralty Bay (King George Island, Antarctica) and estimation of biomagnification factors. Chemosphere 78:767–771. https://doi.org/10.1016/j.chemosphere.2009.10.006
Cipro CVZ, Colabuono FI, Taniguchi S, Montone RC (2013) Persistent organic pollutants in bird, fish and invertebrate samples from King George Island, Antarctica. Antarct Sci 25:545–552. https://doi.org/10.1017/S0954102012001149
Corsolini S, Sarà G (2017) The trophic transfer of persistent pollutants (HCB, DDTs, PCBs) within polar marine food webs. Chemosphere 177:189–199. https://doi.org/10.1016/j.chemosphere.2017.02.116
Corsolini S, Borghesi N, Ademollo N, Focardi S (2011) Chlorinated biphenyls and pesticides in migrating and resident seabirds from east and west Antarctica. Environ Int 37:1329–1335. https://doi.org/10.1016/j.envint.2011.05.017
Cossa D, Heimbürger LE, Lannuzel D et al (2011) Mercury in the southern ocean. Geochim Cosmochim Acta 75:4037–4052. https://doi.org/10.1016/j.gca.2011.05.001
Dietz R, Sonne C, Basu N et al (2013) What are the toxicological effects of mercury in Arctic biota? Sci Total Environ 443:775–790. https://doi.org/10.1016/j.scitotenv.2012.11.046
Drouillard KG, Norstrom RJ (2001) Quantifying maternal and dietary sources of 2,2’4,4’5,5’-hexachlorobiphenyl deposited in eggs of the ring dove (Streptopelia risoria). Environ Toxicol Chem 20:561–567. https://doi.org/10.1002/etc.5620200315
Düring R-A, Gäth S (2000) Microwave assisted methodology for the determination of organic pollutants in organic municipal wastes and soils: extraction of polychlorinated biphenyls using heat transformer disks. Fresenius J Anal Chem 368:684–688. https://doi.org/10.1007/s002160000559
Eagles-Smith CA, Silbergeld EK, Basu N et al (2018) Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47:170–197. https://doi.org/10.1007/s13280-017-1011-x
Elliott JE, Norstrom RJ, Keith JA (1988) Organochlorines and eggshell thinning in northern gannets (Sula bassanus) from Eastern Canada, 1968–1984. Environ Pollut 52:81–102. https://doi.org/10.1016/0269-7491(88)90083-8
Ellis HI, Gabrielsen GW (2001) Energetics of free-ranging seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, FL, pp 359–407
Ellis DS, Cipro CVZ, Ogletree CA et al (2018) A 50-year retrospective of persistent organic pollutants in the fat and eggs of penguins of the southern ocean. Environ Pollut 241:155–163. https://doi.org/10.1016/j.envpol.2018.05.003
Evers DC, Taylor KM, Major A et al (2003) Common loon eggs as indicators of methylmercury availability in North America. Ecotoxicology 12:69–81. https://doi.org/10.1023/A:1022593030009
Falkowska L, Reindl AR, Szumiło E et al (2013) Mercury and chlorinated pesticides on the highest level of the food web as exemplified by herring from the southern Baltic and African penguins from the zoo. Water, Air, Soil Pollut 224:1549. https://doi.org/10.1007/s11270-013-1549-6
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environ Sci Technol 35:732–738. https://doi.org/10.1021/es010719m
Fort J, Robertson GJ, Grémillet D et al (2014) Spatial ecotoxicology: migratory Arctic seabirds are exposed to mercury contamination while overwintering in the northwest Atlantic. Environ Sci Technol 48:11560–11567. https://doi.org/10.1021/es504045g
Fournier F, Karasov WH, Kenow KP et al (2002) The oral bioavailability and toxicokinetics of methylmercury in common loon (Gavia immer) chicks. Comp Biochem Physiol—A Mol Integr Physiol 133:703–714. https://doi.org/10.1016/S1095-6433(02)00140-X
Fries GR, Marrow GS, Gordon CH (1969) Metabolism of o, p’ - and p, p’—DDT by rumen microorganisms. J Agric Food Chem 17:860–862
Fuoco R, Giannarelli S, Wei Y et al (2009) Persistent organic pollutants (POPs) at Ross Sea (Antarctica). Microchem J 92:44–48. https://doi.org/10.1016/j.microc.2008.11.004
Galbán-Malagón CJ, Del Vento S, Berrojalbiz N et al (2013) Polychlorinated biphenyls, hexachlorocyclohexanes and hexachlorobenzene in seawater and phytoplankton from the southern ocean (Weddell, South Scotia, and Bellingshausen Seas). Environ Sci Technol 47:5578–5587. https://doi.org/10.1021/es400030q
Geisz HN, Dickhut RM, Cochran MA et al (2008) Melting glaciers: a probable source of DDT to the Antarctic marine ecosystem. Environ Sci Technol 42:3958–3962. https://doi.org/10.1021/es702919n
Goutte A, Chevreuil M, Alliot F et al (2013) Persistent organic pollutants in benthic and pelagic organisms off Adélie Land, Antarctica. Mar Pollut Bull 77:82–89. https://doi.org/10.1016/j.marpolbul.2013.10.027
Goutte A, Barbraud C, Meillère A et al (2014) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the wandering albatross. Proc R Soc B Biol Sci 281:20133313. https://doi.org/10.1098/rspb.2013.3313
Grajewska A, Falkowska L, Szumiło-Pilarska E et al (2015) Mercury in the eggs of aquatic birds from the Gulf of Gdansk and Wloclawek Dam (Poland). Environ Sci Pollut Res 22:9889–9898. https://doi.org/10.1007/s11356-015-4154-y
Haraguchi K, Hisamichi Y, Endo T (2009) Accumulation and mother-to-calf transfer of anthropogenic and natural organohalogens in killer whales (Orcinus orca) stranded on the Pacific coast of Japan. Sci Total Environ 407:2853–2859. https://doi.org/10.1016/j.scitotenv.2009.01.003
Haynes W (2013) Bonferroni correction. In: Dubitzky W, Wolkenhauer O, Cho K-H, Yokota H (eds) Encyclopedia of systems biology. Springer, New York, New York, NY, p 154
Heinz GH, Hoffman DJ, Klimstra JD et al (2009) Species differences in the sensitivity of avian embryos to methylmercury. Arch Environ Contam Toxicol 56:129–138. https://doi.org/10.1007/s00244-008-9160-3
Hobson KA, Hughes KD, Ewins PJ (1997) Using stable-isotope analysis to identify endogenous and exogenous sources of nutrients in eggs of migratory birds: applications to great lakes contaminants research. Auk 114:467–478
Hobson KA, Sirois J, Gloutney ML (2000) Tracing nutrient allocation to reproduction with stable isotopes: a preliminary investigation using colonial waterbirds of Great Slave Lake. Auk 117:760–774. https://doi.org/10.2307/4089600
Hodum PJ, Hobson KA (2000) Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable-isotope (δ15N and δ13C) analyses. Mar Ecol Prog Ser 198:273–281. https://doi.org/10.1016/S0025-326X(02)00175-3
IUPAC (1997) Compendium of chemical terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford. Online version (2019) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook. Accessed November 2021
Jacob J (2013) A review of the accumulation and distribution of persistent organic pollutants in the environment. Int J Biosci Biochem Bioinform 3:657–661. https://doi.org/10.7763/ijbbb.2013.v3.297
Johnson DR, Melquist WE, Schroeder GJ (1975) DDT and PCB levels in lake Coeur d’Alene, Idaho, osprey eggs. Bull Environ Contam Toxicol 13:401–405. https://doi.org/10.1007/BF01721842
Johnstone RM, Court GS, Fesser AC et al (1996) Long-term trends and sources of organochlorine contamination in Canadian tundra peregrine falcons, Falco peregrinus tundrius. Environ Pollut 93:109–120. https://doi.org/10.1016/0269-7491(96)00037-1
Kang JH, Son MH, Do HS et al (2012) Deposition of organochlorine pesticides into the surface snow of east Antarctica. Sci Total Environ 433:290–295. https://doi.org/10.1016/j.scitotenv.2012.06.037
Karlsson O, Lilja C (2008) Eggshell structure, mode of development and growth rate in birds. Zoology 111:494–502. https://doi.org/10.1016/j.zool.2007.11.005
Koenig S, Fernández P, Solé M (2012) Differences in cytochrome P450 enzyme activities between fish and crustacea: relationship with the bioaccumulation patterns of polychlorobiphenyls (PCBs). Aquat Toxicol 108:11–17. https://doi.org/10.1016/j.aquatox.2011.10.016
Krabbenhoft DP, Sunderland EM (2013) Global change and mercury. Science 341:1457–1458. https://doi.org/10.1126/science.1242838
Krahn MM, Bradley Hanson M, Schorr GS et al (2009) Effects of age, sex and reproductive status on persistent organic pollutant concentrations in “Southern Resident” killer whales. Mar Pollut Bull 58:1522–1529. https://doi.org/10.1016/j.marpolbul.2009.05.014
Langis R, Langlois C, Morneau F (1999) Mercury in birds and mammals. In: Lucotte M, Schetagne R, Thérien N et al (eds) Mercury in the biogeochemical cycle. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 131–144
Leat EHK, Bourgeon S, Borgå K et al (2011) Effects of environmental exposure and diet on levels of persistent organic pollutants (POPs) in eggs of a top predator in the North Atlantic in 1980 and 2008. Environ Pollut 159:1222–1228. https://doi.org/10.1016/j.envpol.2011.01.036
Matsuoka K, Skoglund A, Roth G et al (2018) Quantarctica. Nor Polar Instit. https://doi.org/10.21334/npolar.2018.8516e961
Mills WF, Bustamante P, McGill RAR et al (2020) Mercury exposure in an endangered seabird: long-term changes and relationships with trophic ecology and breeding success. Proc R Soc B Biol Sci 287:20202683. https://doi.org/10.1098/rspb.2020.2683
Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566. https://doi.org/10.1146/annurev.ecolsys.29.1.543
Murphy PG (1972) Sulfuric acid for the cleanup of animal tissues for analysis of acid-stable chlorinated hydrocarbon residues. J Assoc off Anal Chem 55:1360–1362. https://doi.org/10.1093/jaoac/55.6.1360
Na G, Yao Y, Gao H et al (2017) Trophic magnification of dechlorane plus in the marine food webs of Fildes Peninsula in Antarctica. Mar Pollut Bull 117:456–461. https://doi.org/10.1016/j.marpolbul.2017.01.049
Noble RC, Cocchi M (1990) Lipid metabolism and the neonatal chicken. Prog Lipid Res 29:107–140. https://doi.org/10.1016/0163-7827(90)90014-C
Orłowski G, Hałupka L (2015) Embryonic eggshell thickness erosion: a literature survey re-assessing embryo-induced eggshell thinning in birds. Environ Pollut 205:218–224. https://doi.org/10.1016/j.envpol.2015.06.001
Peterson SH, Ackerman JT, Eagles-Smith CA et al (2017) A critical evaluation of the utility of eggshells for estimating mercury concentrations in avian eggs. Environ Toxicol Chem 36:2417–2427. https://doi.org/10.1002/etc.3777
Poland A, Glover E, Kende AS (1976) Stereospecific, high affinity binding of 2,3,7,8 tetrachlorodibenzo p dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 251:4936–4946. https://doi.org/10.1016/s0021-9258(17)33205-2
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Pozo K, Martellini T, Corsolini S et al (2017) Persistent organic pollutants (POPs) in the atmosphere of coastal areas of the Ross Sea, Antarctica: Indications for long-term downward trends. Chemosphere 178:458–465. https://doi.org/10.1016/j.chemosphere.2017.02.118
QGIS Development Team, 2022. QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. Accessed Feb 2022.
Quillfeldt P (2001) Variation in breeding success in Wilson’s storm petrels: influence of environmental factors. Antarct Sci 13:400–409. https://doi.org/10.1017/S0954102001000566
Quillfeldt P (2002) Seasonal and annual variation in the diet of breeding and non-breeding Wilson’s storm-petrels on King George Island, South Shetland Islands. Polar Biol 25:216–221. https://doi.org/10.1007/s00300-001-0332-0
Quillfeldt P (2006) Wilson’s storm-petrel. In: Riffenburgh B (ed) Encyclopedia of the Antarctic, 1st edn. Routledge, London, UK. https://doi.org/10.4324/9780203943182
Quillfeldt P, Masello JF, McGill RAR et al (2010) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:1–11. https://doi.org/10.1186/1742-9994-7-15
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304. https://doi.org/10.3354/meps295295
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed April 2021.
Ratcliffe DA (1967) The peregrine situation in Great Britain 1965–66. Bird Study 14:238–246. https://doi.org/10.1080/00063656709476167
Ricklefs RE (2010) Embryo growth rates in birds and mammals. Funct Ecol 24:588–596. https://doi.org/10.1111/j.1365-2435.2009.01684.x
Ricklefs RE, Starck JM (1998) Embryonic growth and development. In: Starck JM, Ricklefs RE (eds) Avian growth and development. Evolution within the altricial-precocial spectrum. Oxford University Press, New York, pp 31–58
Rigét F, Braune B, Bignert A et al (2011) Temporal trends of Hg in Arctic biota, an update. Sci Total Environ 409:3520–3526. https://doi.org/10.1016/j.scitotenv.2011.05.002
Roberts B (1941) The life cycle of Wilson’s petrel Oceanites oceanicus (Kuhl). British museum (Natural history, ed) British Graham land expedition 1934–37. Scientific Reports, London, pp 141–194
Rudolph I, Chiang G, Galbán-Malagón CJ et al (2016) Persistent organic pollutants and porphyrins biomarkers in penguin faeces from Kopaitic Island and Antarctic Peninsula. Sci Total Environ 573:1390–1396. https://doi.org/10.1016/j.scitotenv.2016.07.091
Russell RW, Gobas FAPC, Haffner GD (1999) Maternal transfer and in ovo exposure of organochlorines in oviparous organisms: a model and field verification. Environ Sci Technol 33:416–420. https://doi.org/10.1021/es9800737
Safe SH (1994) Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87–149. https://doi.org/10.3109/10408449409049308
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18. https://doi.org/10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2
Seco J, Xavier JC, Brierley AS et al (2020) Mercury levels in southern ocean squid: variability over the last decade. Chemosphere 239:124785. https://doi.org/10.1016/j.chemosphere.2019.124785
Seyboth E, Botta S, Mendes CRB et al (2018) Isotopic evidence of the effect of warming on the northern Antarctic Peninsula ecosystem. Deep Sea Res Part II Oceanogr 149:218–228. https://doi.org/10.1016/j.dsr2.2017.12.020
Soerensen AL, Jacob DJ, Streets DG et al (2012) Multi-decadal decline of mercury in the North Atlantic atmosphere explained by changing subsurface seawater concentrations. Geophys Res Lett 39:1–6. https://doi.org/10.1029/2012GL053736
Solheim SA, Sagerup K, Huber S et al (2016) The black-legged kittiwake preen gland—an overlooked organ for depuration of fat-soluble contaminants? Polar Res 35:29651. https://doi.org/10.3402/polar.v35.29651
Streets DG, Horowitz HM, Jacob DJ et al (2017) Total mercury released to the environment by human activities. Environ Sci Technol 51:5969–5977. https://doi.org/10.1021/acs.est.7b00451
Tanabe S (2002) Contamination and toxic effects of persistent endocrine disrupters in marine mammals and birds. Mar Pollut Bull 45:69–77
Torres JJ, Grigsby MD, Elizabeth Clarke M (2012) Aerobic and anaerobic metabolism in oxygen minimum layer fishes: the role of alcohol dehydrogenase. J Exp Biol 215:1905–1914. https://doi.org/10.1242/jeb.060236
Tucker RK, Haegele HA (1970) Eggshell thinning as influenced by method of DDT exposure. Bull Environ Contam Toxicol 5:191–194. https://doi.org/10.1007/BF01558308
UNEP (2018) Stockholm convention on persistent organic pollutants (POPs). Revised in 2017
Union E (2011) Commission regulation no 1259/2011. Off J Eur Union 54:18–23. https://doi.org/10.3000/19770677.L_2011.320.eng
van de Merwe JP, Chan AKY, Lei ENY et al (2011) Bioaccumulation and maternal transfer of PBDE 47 in the marine medaka (Oryzias melastigma) following dietary exposure. Aquat Toxicol 103:199–204. https://doi.org/10.1016/j.aquatox.2011.02.021
van den Brink N, Riddle M, van den Heuvel-Greve M et al (2009) Correspondence on Geisz et al/ melting glaciers: a probable source of DDT to the Antarctic marine ecosystem. Environ Sci Technol 43:3976–3977. https://doi.org/10.1021/es8034494
van den Brink NW, Riddle MJ, van den Heuvel-Greve M, van Franeker JA (2011) Contrasting time trends of organic contaminants in Antarctic pelagic and benthic food webs. Mar Pollut Bull 62:128–132. https://doi.org/10.1016/j.marpolbul.2010.09.002
Vasseur P, Cossu-Leguille C (2006) Linking molecular interactions to consequent effects of persistent organic pollutants (POPs) upon populations. Chemosphere 62:1033–1042. https://doi.org/10.1016/j.chemosphere.2005.05.043
Verboven N, Verreault J, Letcher RJ et al (2009) Differential investment in eggs by Arctic breeding glaucous gulls (Larus hyperboreus) exposed to persistent organic pollutants. Auk 126:123–133. https://doi.org/10.1525/auk.2009.08039
Wang F, Outridge PM, Feng X et al (2019) How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? – Implications for evaluating the effectiveness of the Minamata convention. Sci Total Environ 674:58–70. https://doi.org/10.1016/j.scitotenv.2019.04.101
Wania F, Mackay D (1993) Global fractionation and cold condensation of low volatility organochlorine compounds in polar regions. Ambio 22:10–18. https://doi.org/10.2307/4314030
Wania F, Mackay D (1996) Tracking the distribution of persistent organic pollutants. Environ Sci Technol 30:390–396. https://doi.org/10.1016/s0926-3373(97)80026-4
Weiss F, Furness RW, McGill RAR et al (2009) Trophic segregation of Falkland Islands seabirds: insights from stable isotope analysis. Polar Biol 32:1753–1763. https://doi.org/10.1007/s00300-009-0674-6
Wiltschka K, Neumann L, Werheid M et al (2020) Hydrodechlorination of hexachlorobenzene in a miniaturized nano-Pd(0) reaction system combined with the simultaneous extraction of all dechlorination products. Appl Catal B Environ 275:119100. https://doi.org/10.1016/j.apcatb.2020.119100
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146. https://doi.org/10.1897/1551-5028(1998)017%3c0146:EOMOWA%3e2.3.CO;2
World Health Organization (WHO, 2002) Global assessment of the state-of-the-science of endocrine disruptors. In Damstra T, Barlow S, Bergman A, Kavlock R, van der Kraak G (eds) International Programme on Chemical Safety. WHO/PCS/EDC/02.2, Geneva, Switzerland.
Xu LC, Sun H, Chen JF et al (2006) Androgen receptor activities of p, p′-DDE, fenvalerate and phoxim detected by androgen receptor reporter gene assay. Toxicol Lett 160:151–157. https://doi.org/10.1016/j.toxlet.2005.06.016
Yamashita N, Tanabe S, Ludwig JP et al (1993) Embryonic abnormalities and organochlorine contamination in double-crested cormorants (Phalacrocoras auritus) and Caspian terns (Hydroprogne caspia) from the upper Great Lakes in 1988. Environ Pollut 79:163–173
Yogui GT, De Oliveira Santos MC, Montone RC (2003) Chlorinated pesticides and polychlorinated biphenyls in marine tucuxi dolphins (Sotalia fluviatilis) from the Cananéia estuary, southeastern Brazil. Sci Total Environ 312:67–78. https://doi.org/10.1016/S0048-9697(03)00198-0
Yu HY, Bao LJ, Liang Y, Zeng EY (2011) Field validation of anaerobic degradation pathways for dichlorodiphenyltrichloroethane (DDT) and 13 metabolites in marine sediment cores from China. Environ Sci Technol 45:5245–5252. https://doi.org/10.1021/es2006397
Zell M, Neu HJ, Ballschmiter K (1978) Single component analysis of polychlorinated biphenyl (PCB) and chlorinated pesticide residues in marine fish samples. Fresenius Zeitschrift Für Anal Chemie Labor Und Betriebsverfahren 292:97–107. https://doi.org/10.1007/BF00680118
Zhang Q, Chen Z, Li Y et al (2015) Occurrence of organochlorine pesticides in the environmental matrices from King George Island, West Antarctica. Environ Pollut 206:142–149. https://doi.org/10.1016/j.envpol.2015.06.025
Acknowledgements
We would like to thank Dr. Néstor Coria who, with his permanent predisposition and enthusiasm, supported the realization of the collaboration between Argentina and Germany from the very beginning. Your Antarctic colleagues wish you a speedy recovery. We would like to thank all people involved in collecting samples, especially Marcela J. Nabte, and Benjamin Richter. For advice and assistance in the lab, we would like to thank Marcel P. Simon, Leoni König, and Justine Thébault. The authors are grateful to Carine Churlaud and Maud Brault-Favrou from the Plateforme Analyses Elémentaires of LIENSs for their support during Hg analysis and to G. Guillou from the Plateforme Analyses Isotopiques of LIENSs for running the stable isotope analysis. Thanks to the CPER (Contrat de Projet Etat-Région) and the FEDER (Fonds Européen de Développement Régional) for funding the AMA and the IRMS of LIENSs laboratory. The IUF (Institut Universitaire de France) is acknowledged for its support to PB as a Senior Member. We are grateful for logistic support by the Alfred Wegener Institute, and the Instituto Antártico Argentino (Buenos Aires) that additionally supported the project via a grant to Dr. Néstor Coria (PICTA-2010-0111) by the "Agencia Nacional de Promoción Científica y Tecnológica". Many thanks to Marco Plebani, Ingrid Pollent, the associate Editor Barbara Wienecke and two anonymous reviewers, whose helpful comments improved the manuscript. This study was funded by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority program SPP1154 “Antarctic Research with comparative investigations in Arctic ice areas” by grants to PQ (Qu148/12 and Qu148/18). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
NK, PQ, LB, and RD conceived and designed the research. NK, LB, and PB conducted or were involved in pollutant analyses. ML facilitated permits and access to the field station in the framework of our collaboration between Argentina and Germany. CB, ML, NK, and PQ collected samples. NK and LB analyzed data. NK wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuepper, N.D., Böhm, L., Braun, C. et al. Persistent organic pollutants and mercury in a colony of Antarctic seabirds: higher concentrations in 1998, 2001, and 2003 compared to 2014 to 2016. Polar Biol 45, 1229–1245 (2022). https://doi.org/10.1007/s00300-022-03065-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03065-w